Insecticidal Activity of Plant Powders against the Parasitoid, Pteromalus venustus, and Its Host, the Alfalfa Leafcutting Bee
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing of ALBs
2.2. Rearing of Parasitoids
2.3. Plant Powders
2.4. Acute Contact Toxicity—Adult P. venustus
2.4.1. Low Contact with Plant Powders
2.4.2. High Contact with Plant Powders
2.5. Reproductive Toxicity—P. venustus
2.6. Acute Contact Toxicity—Adult ALBs
2.6.1. Low Contact with Plant Powders
2.6.2. High Contact with Plant Powders
2.7. Statistical Analysis
3. Results
3.1. Acute Contact Toxicity—Adult P. venustus
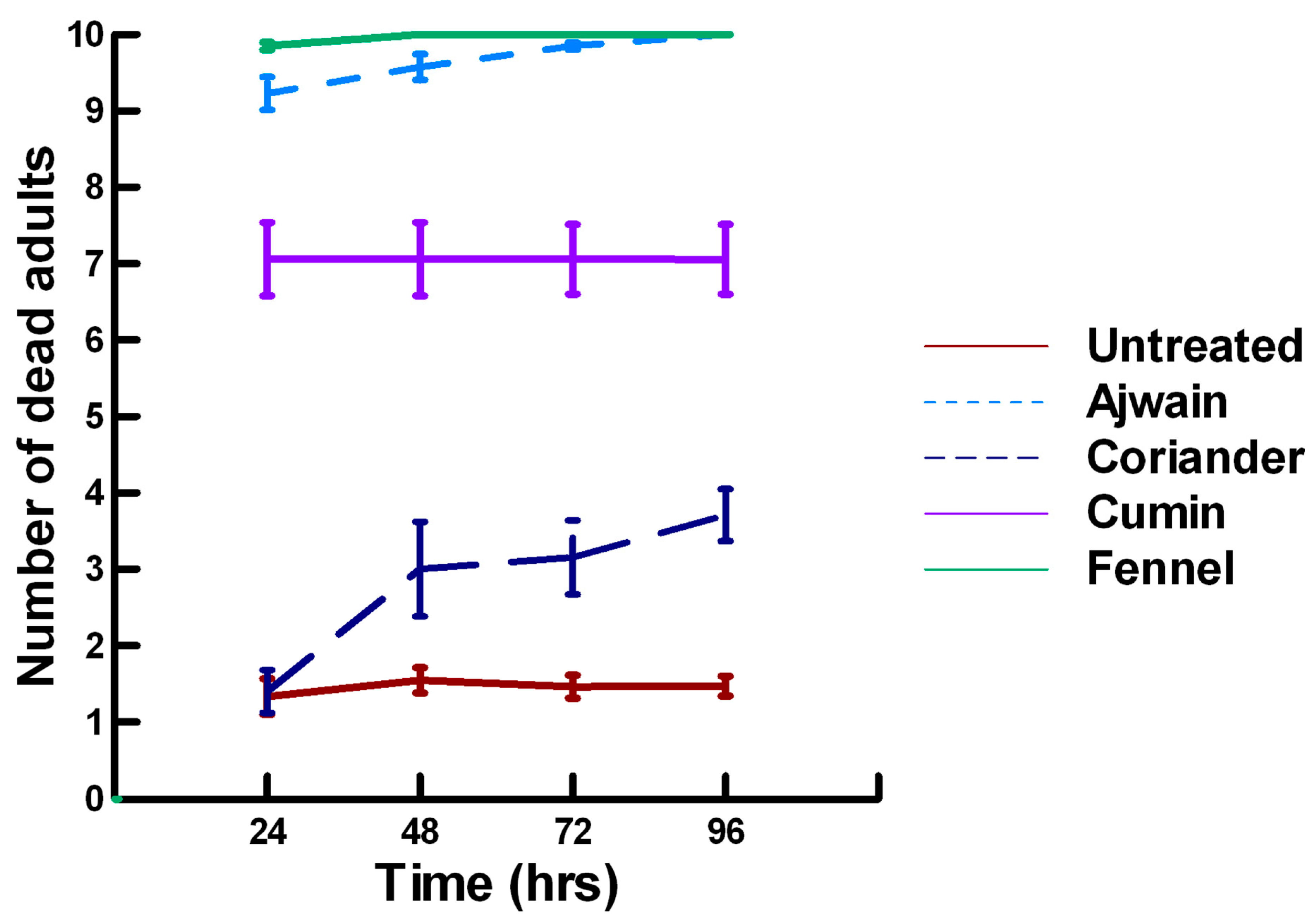
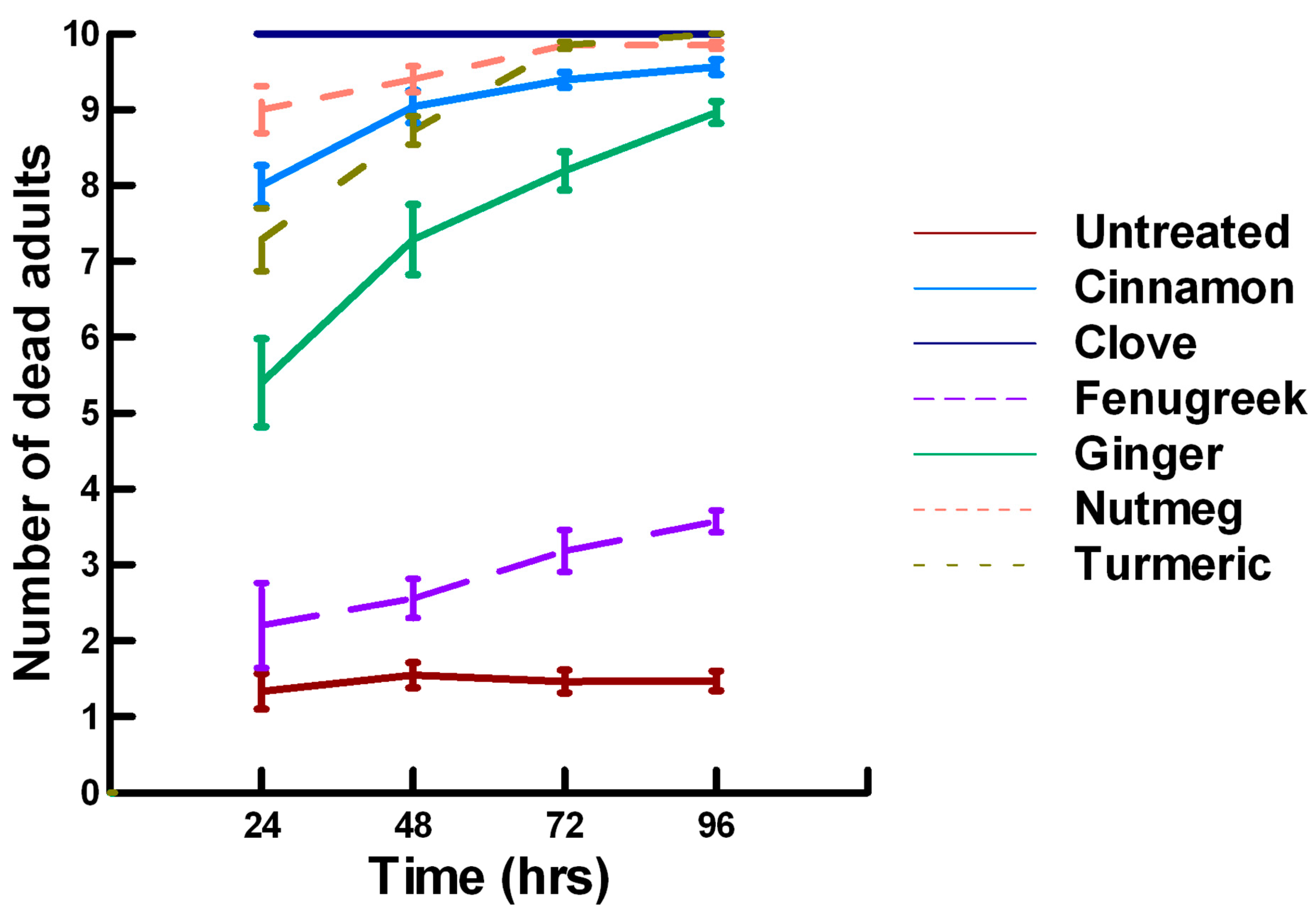
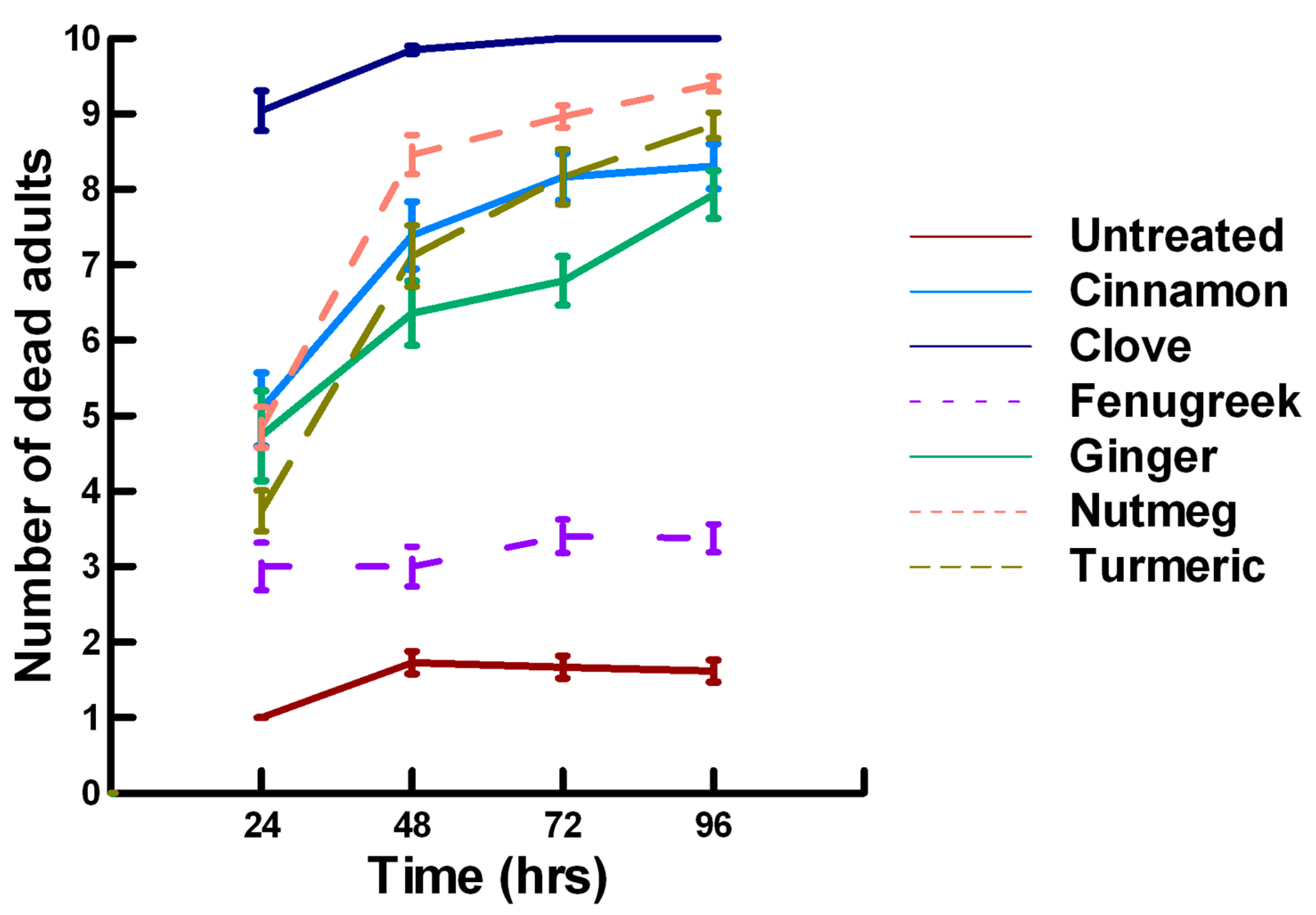
3.1.1. Low Contact with Plant Powders (19 to 79 mg/cm2)
3.1.2. High Contact with Plant Powders (589 mg/cm2)
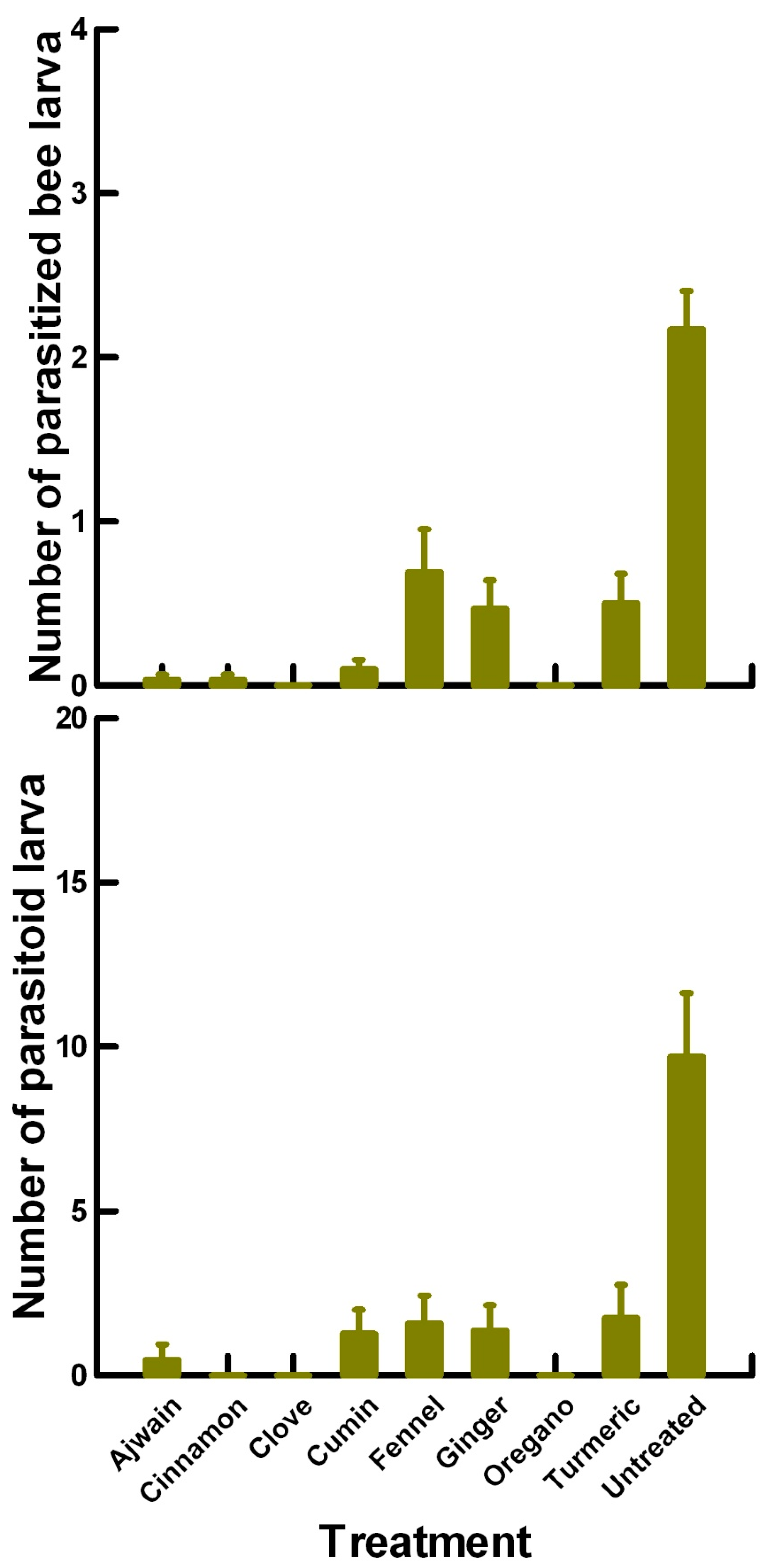
3.2. Reproductive Toxicity (4.75 to 40 mg/cm2)—P. venustus
3.3. Acute Contact Toxicity—Adult Male ALB
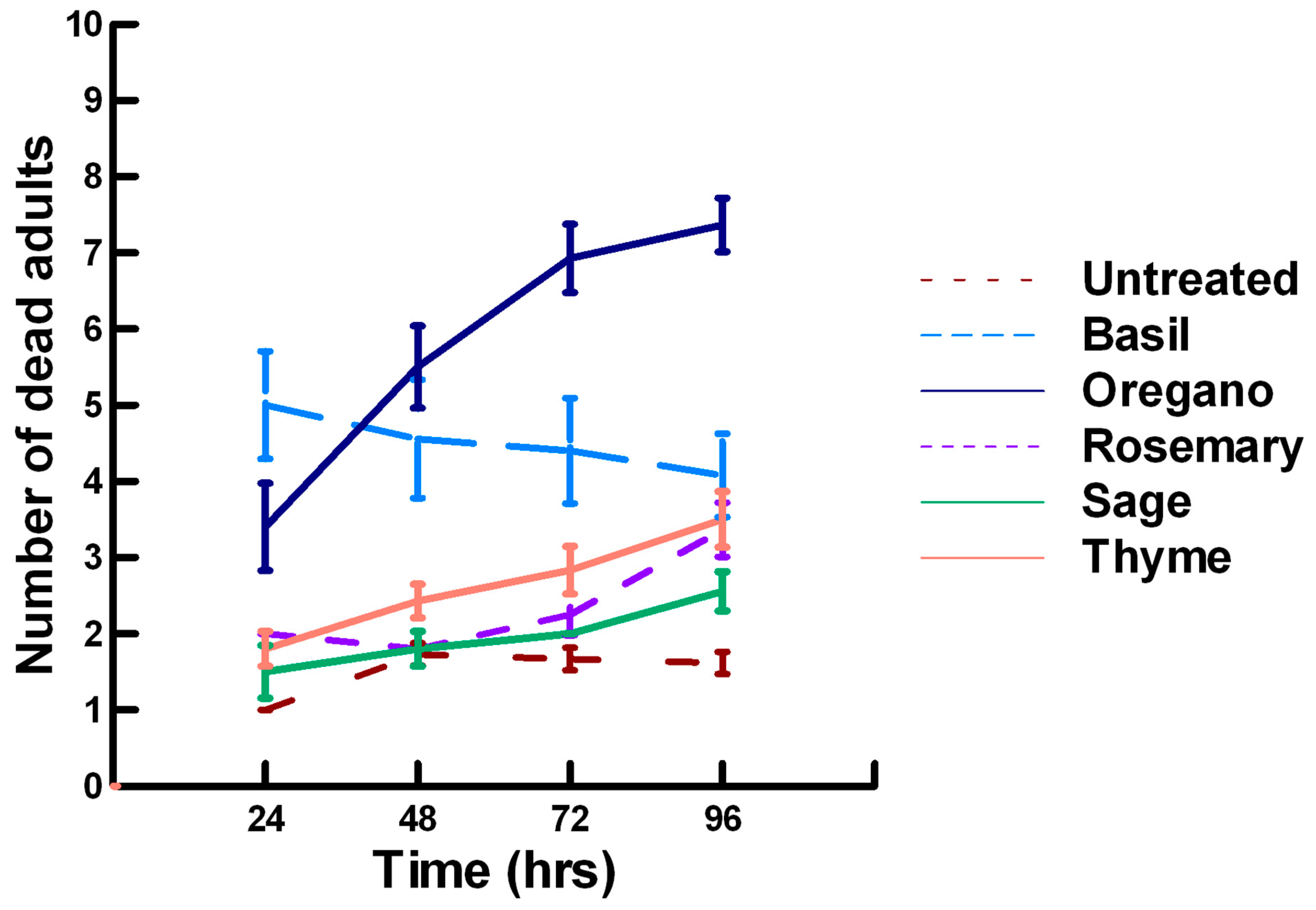
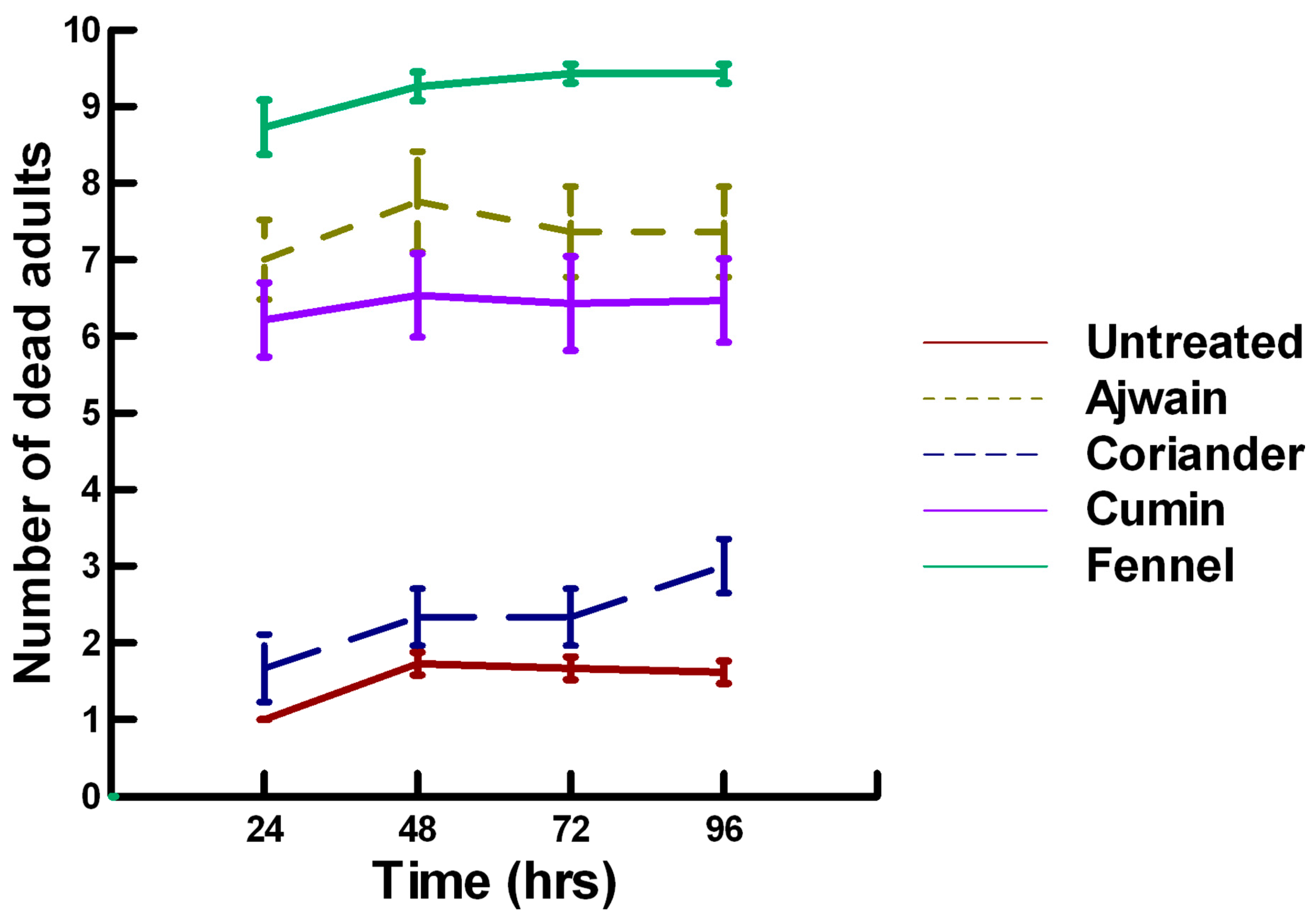
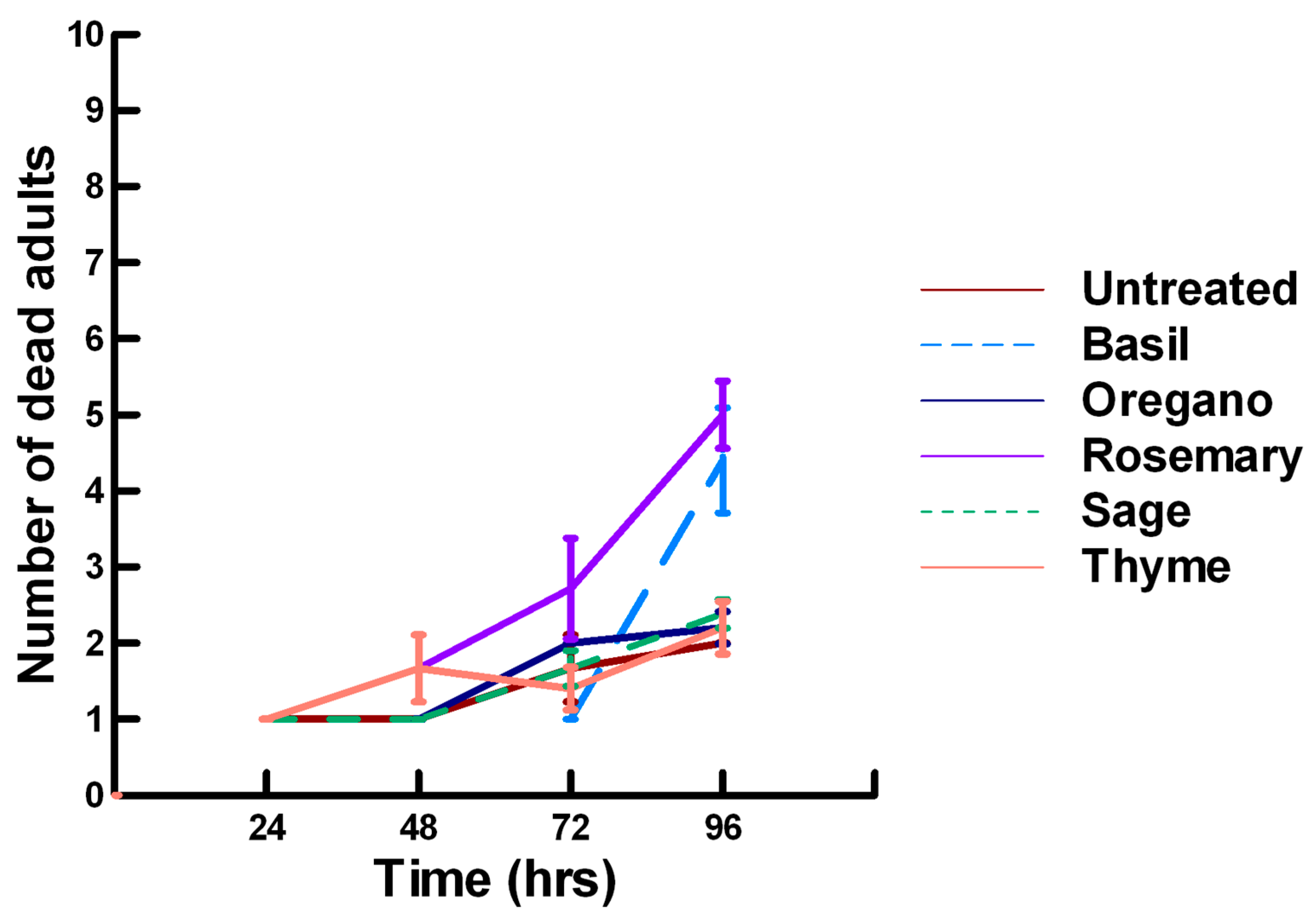
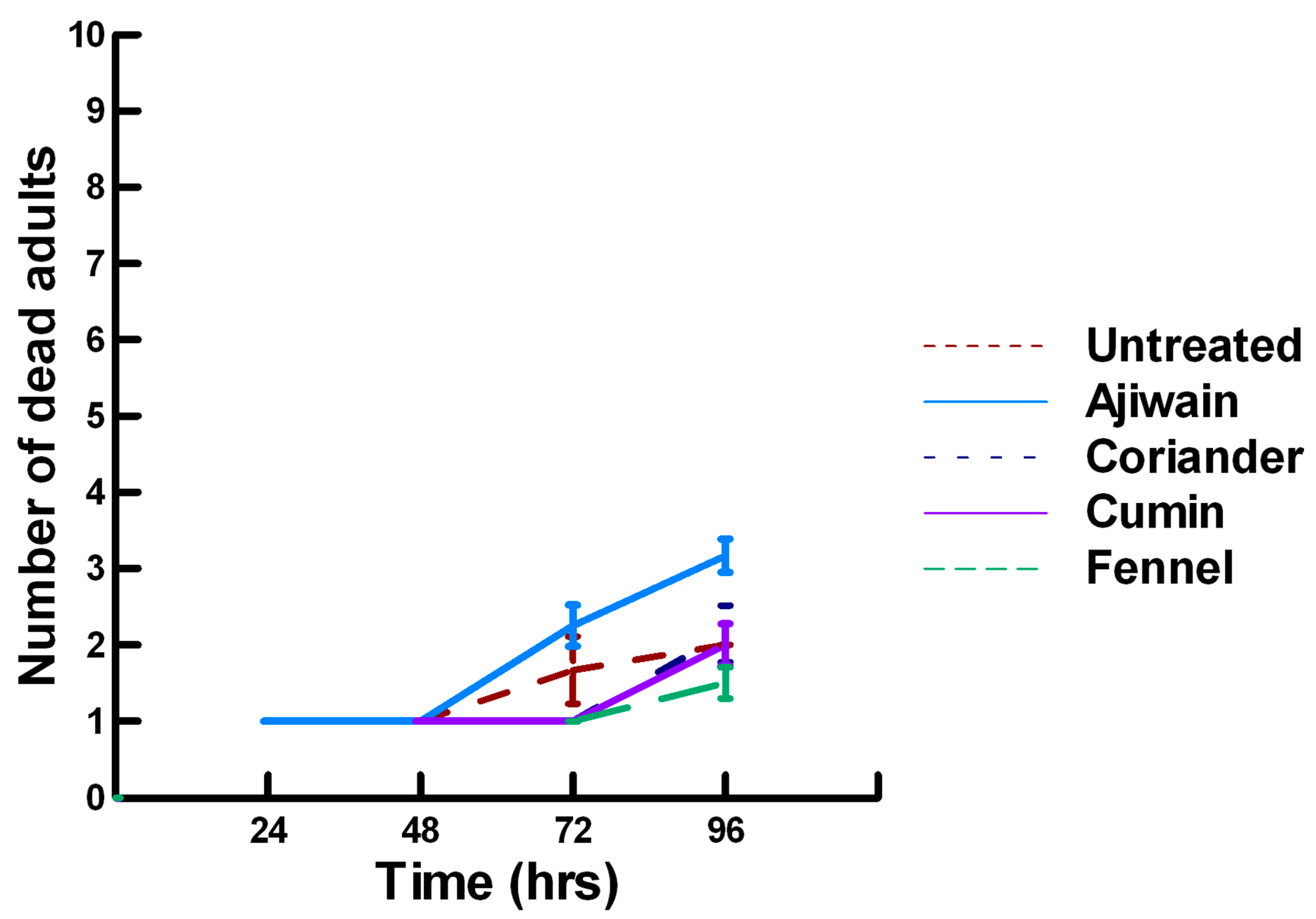
3.3.1. Low Contact with Plant Powders (32 to 65 µg/cm2)
3.3.2. High Contact with Plant Powders (0.20 to 0.33 mg/cm2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richards, K.W. Alfalfa Leafcutter bee management in Canada. Bee World 2015, 68, 168–178. [Google Scholar] [CrossRef]
- Pitts-Singer, T.L.; Cane, J.H. The alfalfa leafcutting bee, Megachile rotundata: The world’s most intensively managed solitary bee. Annu. Rev. Entomol. 2011, 56, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Goerzen, D.W. Alfalfa Leafcutting Bee Incubation Calendar—2015. Available online: http://saspa.com/PDF/Alfalfa%20leafcutting%20bee%20incubation%20calendar%20-%20May%202019.pdf (accessed on 20 May 2019).
- Hobbs, G.A.; Krunic, M.D. Comparative behaviour of three chalcidoid (Hymenoptera) parasites of the alfalfa Leafcutting bee, Megachile rotundata, in the laboratory. Can. Entomol. 1971, 103, 674–685. [Google Scholar] [CrossRef]
- Whitfield, G.; Richards, K. Postdiapause development and adult emergence of Pteromalus venustus (Hymenoptera: Pteromalidae) during alfalfa Leafcutting bee incubation. Can. Entomol. 1987, 119, 491–493. [Google Scholar] [CrossRef]
- Whitfield, G.; Richards, K. Influence of temperature on survival and rate of development of Pteromalus venustus (Hymenoptera: Pteromalidae), a parasite of the alfalfa Leafcutting bee (Hymenoptera: Megachilidae). Can. Entomol. 1985, 117, 811–818. [Google Scholar] [CrossRef]
- Goerzen, D.W. Parasite Control in Alfalfa Leafcutting Bee Populations—2015. Available online: http://www.saspa.com/PDF/alfalfa%20leafcutting%20parasite%20control%20-%20may%202015.pdf (accessed on 25 September 2019).
- Hill, B.D.; Richards, K.W.; Schaalje, G.B. Use of dichlorvos resin strips to reduce parasitism of alfalfa Leafcutting bee (Hymenoptera: Megachilidae) cocoons during incubation. J. Econ. Ent. 1984, 77, 1307–1312. [Google Scholar] [CrossRef]
- Okoroiwu, H.U.; Iwara, I.A. Dichlorvos toxicity: A public health perspective. Interdiscip. Toxicol. 2018, 11, 129–137. [Google Scholar] [CrossRef]
- Clinch, P.G. Effect on honey bees of combs exposed to vapour from dichlorvos slow-release strips. N. Z. J. Agric. Res. 1970, 13, 448–452. [Google Scholar] [CrossRef]
- Yang, R.Z.; Tang, C.S. Plants used for pest control in China: A literature review. Econ. Bot. 1988, 42, 376–406. [Google Scholar] [CrossRef]
- Boeke, S.J.; Van Loon, J.J.A.; Van Huis, A.; Kossou, D.K.; Dicke, M. The Use of Plant Materials to Protect Stored Leguminous Seeds against Seed Beetles: A Review; Backhuys Publishers: Wageningen, The Netherlands, 2001; pp. 1–108. ISBN 978-905-782-100-4. [Google Scholar]
- Nenaah, G.E.; Ibrahim, S.I.A. Chemical composition and the insecticidal activity of certain plants applied as powders and essential oils against two stored-products coleopteran beetles. J. Pest. Sci. 2011, 84, 393. [Google Scholar] [CrossRef]
- Tapondjou, L.A.; Adler, C.; Bouda, H.; Fontem, D.A. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Prod. Res. 2002, 38, 395–402. [Google Scholar] [CrossRef]
- Kéita, S.M.; Vincent, C.; Schmit, J.P.; Arnason, J.T.; Bélanger, A. Efficacy of essential oil of Ocimum basilicum L. and O. gratissimum L. applied as an insecticidal fumigant and powder to control Callosobruchus maculatus (Fab.) [Coleoptera: Bruchidae]. J. Stored Prod. Res. 2001, 37, 339–349. [Google Scholar] [CrossRef]
- Devi, K.C.; Devi, S.S. Insecticidal and oviposition deterrent properties of some spices against coleopteran beetle, Sitophilus oryzae. J. Food Sci. Technol. 2013, 50, 600–604. [Google Scholar] [CrossRef]
- Farhana, K.; Islam, H.; Emran, E.H.; Islam, N. Toxicity and repellant activity of three spice materials on Tribolium castaneum (Herbst) adults. J. Bio-Sci. 2006, 14, 131–134. [Google Scholar] [CrossRef]
- Mahdi, S.; Rahman, M. Insecticidal effect of some spices on Callosobruchus maculatus (Fabricius) in black gram seeds. Univ. J. Zool. Rajshahi Univ. 2008, 27, 47–50. [Google Scholar] [CrossRef]
- Rajendran, S.; Sriranjini, V. Plant products as fumigants for stored-product insect control. J. Stored Prod. Res. 2008, 44, 126–135. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, L. Essential oils in stored product insect pest control. J. Food Qual. 2018, 2018, 1–18. [Google Scholar] [CrossRef]
- Beneli, G.; Canale, A.; Flamini, G.; Coini, P.L.; Demi, F.; Ceccarini, L.; Macchia, M.; Conti, B. Biotoxicity of Melaleuca alternifolia (Myrtaceae) essential oil against the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), and its parasitoid Psyttalia concolor (Hymenoptera: Braconidae). Ind. Crops Prod. 2013, 50, 595–603. [Google Scholar] [CrossRef]
- Ketoh, G.K.; Glitho, A.I.; Huignard, J. Susceptibility of the bruchid Callosobruchus maculatus (Coleoptera: Bruchidae) and its Parasitoid Dinarmus basalis (Hymenoptera: Pteromalidae) to Three Essential Oils. J. Econ. Entomol. 2002, 95, 174–182. [Google Scholar] [CrossRef]
- Suthisut, D.; Fields, P.G.; Chandrapatya, A. Fumigant toxicity of essential oils from three Thai plants (Zingiberaceae) and their major compounds against Sitophilus zeamais, Tribolium castaneum and two parasitoids. J. Stored Prod. Res. 2011, 47, 222–230. [Google Scholar] [CrossRef]
- Ketoh, G.K.; Koumaglo, H.K.; Glitho, I.A. Inhibition of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) development with essential oil extracted from Cymbopogon schoenanthus L. Spreng. (Poaceae), and the wasp Dinarmus basalis (Rondani) (Hymenoptera: Pteromalidae). J. Stored Prod. Res. 2005, 41, 363–371. [Google Scholar] [CrossRef]
- Boateng, B.A.; Kusi, F. Toxicity of Jatropha seed oil to Callosobruchus maculatus (Coleoptera: Bruchidae) and its parasitoid, Dinarmus basalis (Hymenoptera: Pteromalidae). J. Appl. Sci. Res. 2008, 4, 945–951. [Google Scholar]
- Shaaya, E.; Ravid, U.; Paster, N.; Juven, B.; Zisman, U.; Pissarev, V. Fumigant toxicity of essential oils against four major stored product insects. J. Chem. Ecol. 1991, 17, 499–504. [Google Scholar] [CrossRef]
- Ashouri, S.; Shayesteh, N.; Maroufpoor, M.; Ebadollahi, A.; Ghasemzadeh, S. Toxicity and progeny reduction potency of two powdered spices, turmeric and cinnamon on adults of Rhyzopertha dominica (F.) and Sitophilus granaries (L.). Mun. Ent. Zool. 2010, 5, 1096–1103. [Google Scholar]
- Shayesteh, N.; Ashouri, S. Effect of four powdered spices as repellants against adults of Rhyzopertha dominica (F.), Sitophilus granaries (L.) and Tribolium castaneum (Herbst) in laboratory conditions. Julius-Kuhn-Archiv. 2010, 425, 799–804. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Poorjavad, N.; Goldansaz, S.H.; Dadpour, H.; Khajehali, J. Effect of Ferula assafoetida essential oil on some biological and behavioral traits of Trichogramma embryophagum and T. evanescens. BioControl 2014, 59, 403–413. [Google Scholar] [CrossRef]
- Sanon, A.; Dabire, C.; Huignard, J.; Monge, J.P. Influence of Hyptis suaveolens (Lamiaceae) on the host location behavior of the parasitoid Dinarmus basalis (Hymenoptera: Pteromalidae). Environ. Entomol. 2006, 35, 718–724. [Google Scholar] [CrossRef]
- Sanon, A.; Ba, M.N.; Dabiré, L.C.B.; Nébié, R.C.H.; Monge, J.P. Side effects of grain protectants on biological control agents: How Hyptis plant extracts affect parasitism and larval development of Dinarmus basalis. Phytoparasitica 2011, 39, 215–222. [Google Scholar] [CrossRef]
- Boeke, S.J.; Sinzogan, A.A.; De Almeida, R.P.; De Boer, P.W.; Jeong, G.; Kossou, D.K.; Van Loon, J.J. Side-effects of cowpea treatment with botanical insecticides on two parasitoids of Callosobruchus maculatus. Entomol. Exp. Appl. 2003, 108, 43–51. [Google Scholar] [CrossRef]
- González, J.O.W.; Laumann, R.A.; da Silveira, S.; Moraes, M.C.B.; Borges, M.; Ferrero, A.A. Lethal and sublethal effects of four essential oils on the egg parasitoids Trissolcus basalis. Chemosphere 2013, 92, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Parreira, D.S.; Alcántara-de la Cruz, R.; Leite, G.L.D.; Ramalho, F.D.S.; Zanuncio, J.C.; Serrão, J.E. Quantifying the harmful potential of ten essential oils on immature Trichogramma pretiosum stages. Chemosphere 2018, 199, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Baines, D.; Wilton, E.; Pawluk, A.; Gorter, M.D.; Chomistek, N. Neonicotinoids act like endocrine disrupting chemicals in newly-emerged bees and winter bees. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Ney, R.E., Jr. Chapter 1: Fundamentals. In Fate and Transport of Organic Chemicals in the Environment, A Practical Guide, 2nd ed.; Government Institutes, Inc.: Rockville, MD, USA, 1995; pp. 1, 2, 18, 174, 182. [Google Scholar]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, M.; Chomistek, N.; Dayment, H.; Goerzen, W.; Baines, D. Insecticidal Activity of Plant Powders against the Parasitoid, Pteromalus venustus, and Its Host, the Alfalfa Leafcutting Bee. Insects 2020, 11, 359. https://doi.org/10.3390/insects11060359
Ong M, Chomistek N, Dayment H, Goerzen W, Baines D. Insecticidal Activity of Plant Powders against the Parasitoid, Pteromalus venustus, and Its Host, the Alfalfa Leafcutting Bee. Insects. 2020; 11(6):359. https://doi.org/10.3390/insects11060359
Chicago/Turabian StyleOng, Mikhaela, Nora Chomistek, Hanna Dayment, Wayne Goerzen, and Danica Baines. 2020. "Insecticidal Activity of Plant Powders against the Parasitoid, Pteromalus venustus, and Its Host, the Alfalfa Leafcutting Bee" Insects 11, no. 6: 359. https://doi.org/10.3390/insects11060359
APA StyleOng, M., Chomistek, N., Dayment, H., Goerzen, W., & Baines, D. (2020). Insecticidal Activity of Plant Powders against the Parasitoid, Pteromalus venustus, and Its Host, the Alfalfa Leafcutting Bee. Insects, 11(6), 359. https://doi.org/10.3390/insects11060359



