The Time–Concentration–Mortality Responses of Western Flower Thrips, Frankliniella occidentalis, to the Synergistic Interaction of Entomopathogenic Fungus Metarhizium flavoviride, Insecticides, and Diatomaceous Earth
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing Protocols for WFT
2.2. Fungal Strains and Preparation
2.3. DE and Insecticide Formulations
2.4. Radial Hyphal Growth Test in the Presence of Insecticides
2.5. Conidia Production Measure in vitro in the Presence of Insecticides
2.6. Bioassays
2.7. Quantification of Spore Production on Mycotized WFT Carcasses
2.8. Statistical Analysis
3. Results
3.1. Radial Hyphal Growth in the Presence of Insecticides
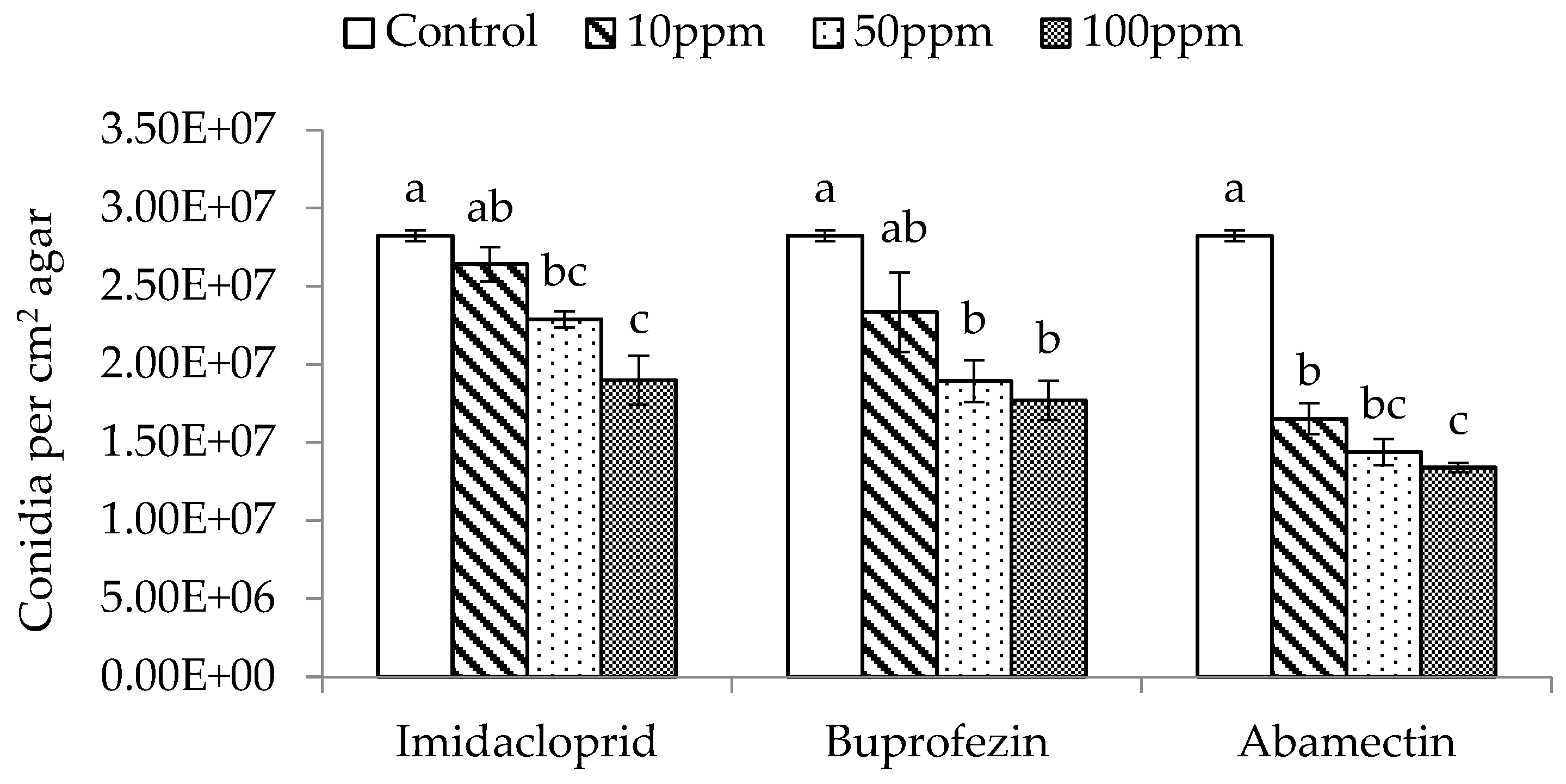
3.2. Conidia Production Measure in vitro in the Presence of Insecticides
3.3. Bioassays
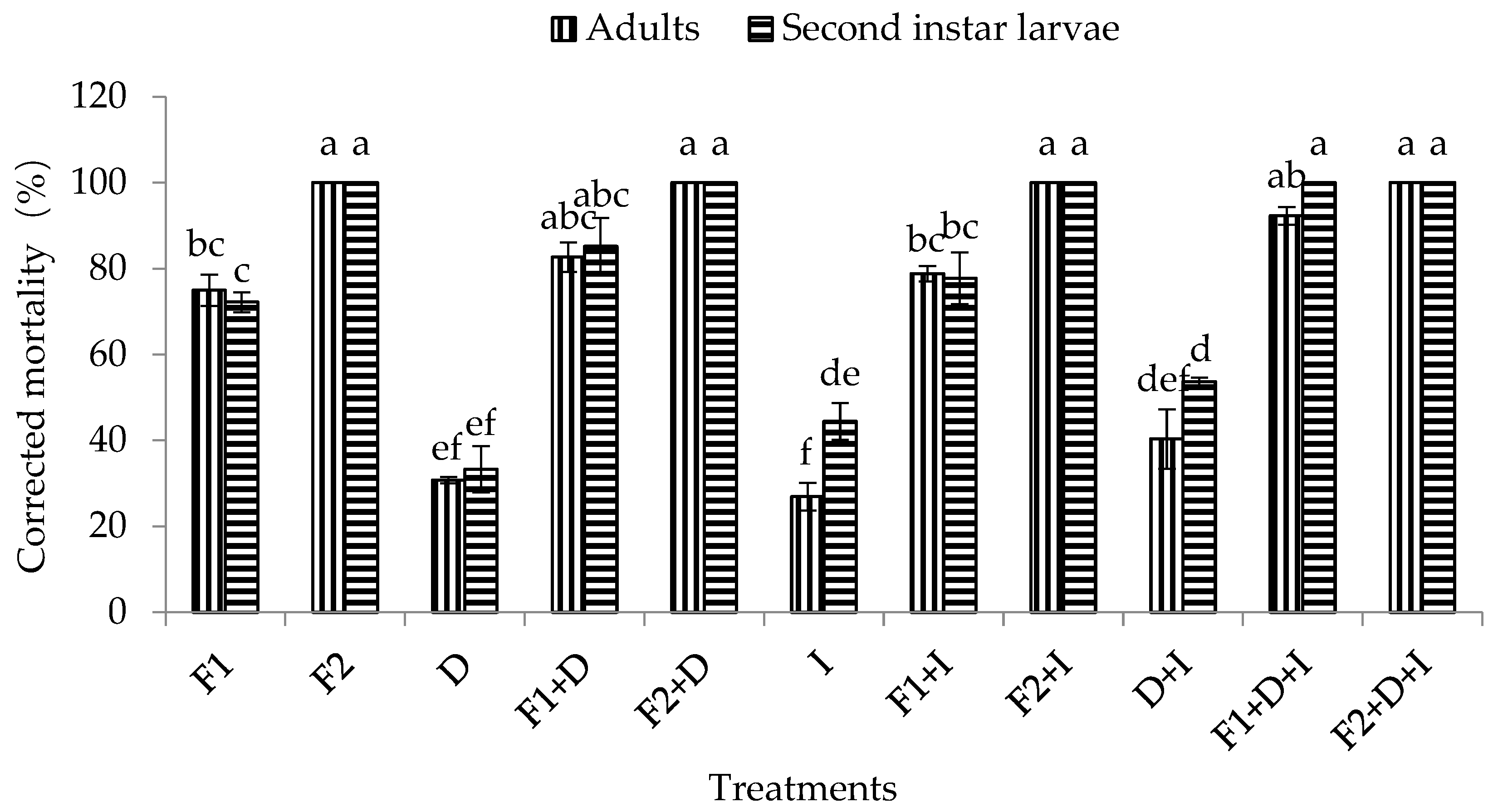
3.3.1. Effect of Different Bioassays on Mortalities with Adults and Second Instar Larvae of WFT
3.3.2. Fitted TCM Relationships
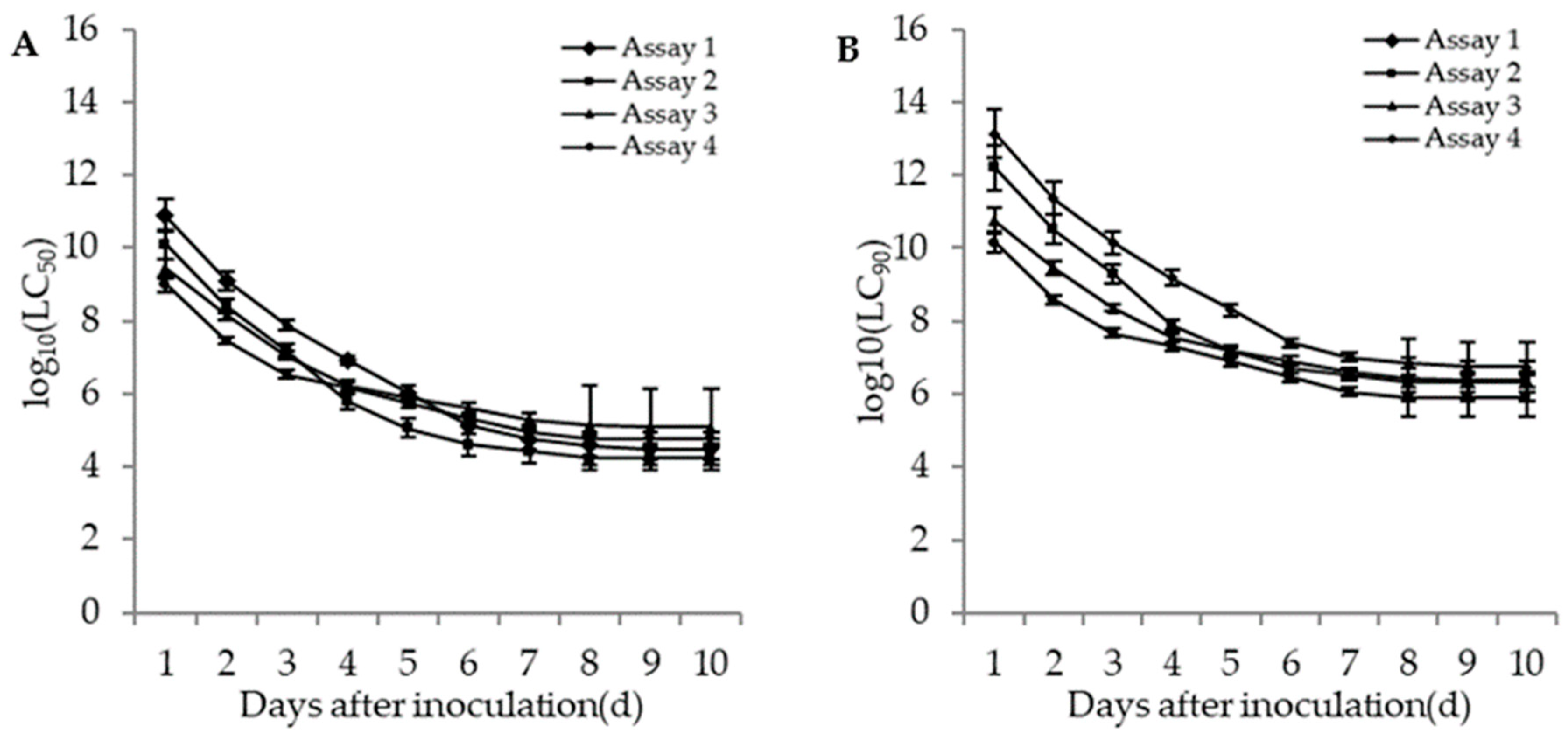
3.3.3. Lethal Concentrations (LC50 and LC90) of M. flavoviride against the Adults and Second Instar Larvae of WFT under Different Bioassays
3.3.4. Lethal Times (LT50 and LT90) of M. flavoviride against the Adults and Second Instar Larvae of WFT under Different Bioassays
3.4. Conidia Production from WFT Carcasses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lewis, T. Pest thrips in perspective. In Thrips as Crop Pests; Lewis, T., Ed.; CAB International: Wallingford, UK, 1997; pp. 1–13. [Google Scholar]
- Gao, Y.L.; Reitz, S.R.; Wang, J.; Xu, X.N.; Lei, Z.R. Potential of a strain of the entomopathogenic fungus Beauveria bassiana (Hypocreales: Cordycipitaceae) as a biological control agent against western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Biocontrol Sci. Technol. 2012, 22, 491–495. [Google Scholar] [CrossRef]
- Otieno, J.A.; Pallmann, P.; Poehling, H.M. The combined effect of soil-applied azadirachtin with entomopathogens for integrated management of western flower thrips. J. Appl. Entomol. 2015, 140, 174–186. [Google Scholar] [CrossRef]
- Morse, J.G.; Hoddle, M.S. Invasion Biology of Thrips. Annu. Rev. Entomol. 2006, 51, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.G.; Pappu, H.R. Tactics for management of thrips (Thysanoptera: Thripidae) and tomato spotted wilt virus in tomato. J. Econ. Entomol. 2004, 97, 1648–1658. [Google Scholar] [CrossRef]
- Kivett, J.M.; Cloyd, R.A.; Bello, N.M. Evaluation of Entomopathogenic Fungi Against the Western Flower Thrips (Thysanoptera: Thripidae) Under Laboratory Conditions. J. Entomol. Sci. 2016, 51, 274–291. [Google Scholar] [CrossRef]
- Espinosa, P.J.; Bielza, P.; Contreras, J.; Lacasa, A. Insecticide resistance in field populations of Frankliniella occidentalis (Pergande) in Murcia (south-east Spain). Pest Manag. Sci. 2002, 58, 967–971. [Google Scholar] [CrossRef]
- Wu, S.Y.; Tang, L.D.; Zhang, X.R.; Xing, Z.L.; Lei, Z.R.; Gao, Y.L. A decade of a thrips invasion in China: Lessons learned. Ecotoxicology 2018, 27, 1032–1038. [Google Scholar] [CrossRef]
- Ansari, M.A.; Shah, F.A.; Whittaker, M.; Prasad, M.; Butt, T.M. Control of western flower thrips (Frankliniella occidentalis) pupae with Metarhizium anisopliae in peat and peat alternative growing media. Biol. Control 2007, 40, 293–297. [Google Scholar] [CrossRef]
- Ansari, M.A.; Brownbridge, M.; Shah, F.A.; Butt, T.M. Efficacy of entomopathogenic fungi against soil-dwelling life stages of western flower thrips, Frankliniella occidentalis, in plant-growing media. Entomol. Exp. Appl. 2008, 127, 80–87. [Google Scholar] [CrossRef]
- Jensen, S.E. Insecticide resistance in the western flower thrips, Frankliniella occidentalis. Integr. Pest Manag. Rev. 2000, 5, 131–146. [Google Scholar] [CrossRef]
- Saito, T.; Brownbridge, M. Compatibility of foliage-dwelling predatory mites and mycoinsecticides, and their combined efficacy against western flower thrips Frankliniella occidentalis. J. Pest Sci. 2018, 91, 1291–1300. [Google Scholar] [CrossRef]
- Wu, S.Y.; Gao, Y.L.; Xu, X.N.; Goettel, M.S.; Lei, Z.R. Compatibility of Beauveria bassiana with Neoseiulus barkeri for Control of Frankliniella occidentalis. J. Integr. Agric. 2015, 14, 98–105. [Google Scholar] [CrossRef]
- Mouden, S.; Sarmiento, K.F.; Klinkhamer, P.G.; Leiss, K.A. Integrated pest management in western flower thrips: Past, present and future. Pest Manag. Sci. 2017, 73, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Rumbos, C.I.; Sakka, M.K.; Vayias, B.J.; Stephou, V.K.; Nakas, C.N. Insecticidal effect of the combined application of spinosad, Beauveria bassiana and diatomaceous earth for the control of Tribolium confusum. Biocontrol Sci. Technol. 2016, 26, 809–819. [Google Scholar] [CrossRef]
- Hajek, A.E.; St. Leger, R.J. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef]
- Sohrabi, F.; Jamali, F.; Morammazi, S.; Saber, M.; Kamita, S.G. Evaluation of the compatibility of entomopathogenic fungi and two botanical insecticides tondexir and palizin for controlling Galleria mellonella L. (Lepidoptera: Pyralidae). Crop Prot. 2019, 117, 20–25. [Google Scholar] [CrossRef]
- Duarte, R.T.; Gonçalves, K.C.; Espinosa, D.J.L.; Moreira, L.F.; Bortoli, S.A.D.; Humber, R.A.; Polanczyk, R.A. Potential of Entomopathogenic Fungi as Biological Control Agents of Diamondback Moth (Lepidoptera: Plutellidae) and Compatibility with Chemical Insecticides. J. Econ. Entomol. 2016, 109, 594–601. [Google Scholar] [CrossRef]
- Vestergaard, S.; Gillespie, A.T.; Butt, T.M.; Schreiter, G.; Eilenberg, J. Pathogenicity of the hyphomycete fungi Verticillium lecanii and Metarhizium anisopliae to the western flower thrips, Frankliniella occidentalis. Biocontrol Sci. Technol. 1995, 5, 185–192. [Google Scholar] [CrossRef]
- Wu, S.Y.; Gao, Y.L.; Smagghe, G.; Xu, X.N.; Lei, Z.R. Interactions between the entomopathogenic fungus Beauveria bassiana and the predatory mite Neoseiulus barkeri and biological control of their shared prey/host Frankliniella occidentalis. Biol. Control 2016, 17, 43–51. [Google Scholar] [CrossRef]
- Ugine, T.A.; Wraight, S.P.; Brownbridge, M.; Sanderson, J.P. Development of a novel bioassay for estimation of median lethal concentrations (LC50) and doses (LD50) of the entomopathogenic fungus Beauveria bassiana against western flower thrips, Frankliniella occidentalis. J. Invertebr. Pathol. 2005, 89, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Lei, Z.R.; Reitz, S.R.; Wu, S.Y.; Gao, Y.L. Laboratory and Greenhouse Evaluation of a Granular Formulation of Beauveria bassiana for Control of Western Flower Thrips, Frankliniella occidentalis. Insects 2019, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Niassy, S.; Maniania, N.K.; Subramanian, S.; Gitonga, L.M.; Mburu, D.M.; Masiga, D.; Ekesi, E. Selection of promising fungal biological control agent of the western flower thrips Frankliniella occidentalis (Pergande). Lett. Appl. Microbiol. 2012, 54, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Wraight, S.P.; Ugine, T.A.; Ramos, M.E.; Sanderson, J.P. Efficacy of spray applications of entomopathogenic fungi against western flower thrips infesting greenhouse impatients under variable moisture conditions. Biol. Control 2016, 97, 31–47. [Google Scholar] [CrossRef]
- Ge, W.C.; Luo, W.C.; Chen, B.; Zhang, H.R.; Gu, X.H.; Zhang, L.M. Virulence of three hyphomycetes entomopathogenic fungi against the adult of Frankliniella occidentalis (Pergande). J. South. Agric. 2019, 50, 1735–1741. [Google Scholar]
- Montserrat, M.; Castañé, C.; Santamaria, S. Neozygites parvispora Zygomycotina: Entomophthorales causing an epizootic in Frankliniella occidentalis (Thysanoptera: Thripidae) on cucumber in Spain. J. Invertebr. Pathol. 1998, 71, 165–168. [Google Scholar] [CrossRef]
- Stepanycheva, E.; Petrova, M.; Chermenskaya, T.; Pavela, R. Fumigant effect of essential oils on mortality and fertility of thrips Frankliniella occidentalis Perg. Environ. Sci. Pollut. Res. 2019, 26, 30885–30892. [Google Scholar] [CrossRef]
- Koschier, E.H. Essential oil compounds for thrips control—A review. Nat. Prod. Commun. 2008, 3, 1171–1182. [Google Scholar] [CrossRef]
- Ebeling, W. Sorptive dust for pest control. Annu. Rev. Entomol. 1971, 16, 123–158. [Google Scholar] [CrossRef]
- Michalaki, M.P.; Athanassiou, C.G.; Steenberg, T.; Buchelos, C.T. Effect of Paecilomyces fumosoroseus (Wise) Brown and Smith (Ascomycota: Hypocreales) alone or in combination with diatomaceous earth against Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae) and Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Biol. Control 2007, 40, 280–286. [Google Scholar]
- Wakil, W.; Riasat, T.; Ashfaq, M. Residual efficacy of thiamethoxam, Beauveria bassiana (Balsamo) Vuillemin, and diatomaceous earth formulation against Rhyzopertha dominica F. (Coleoptera: Bostrychidae). J. Pest Sci. 2012, 85, 341–350. [Google Scholar] [CrossRef]
- Wakil, W.; Schmitt, T. Field trials on the efficacy of Beauveria bassiana, diatomaceous earth and Imidacloprid for the protection of wheat grains from four major stored grain insect pests. J. Stored Prod. Res. 2014, 64, 160–167. [Google Scholar] [CrossRef]
- Akbar, W.; Lord, J.C.; Nechols, J.R.; Howard, R.W. Diatomaceous earth increases the efficacy of Beauveria bassiana against Tribolium castaneum larvae and increases conidia attachment. J. Econ. Entomol. 2004, 97, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Farooq, M.; Shakeel, M.; Din, N.; Hussain, S.; Saeed, N.; Shakeel, Q.; Rajput, A. Influence of entomopathogenic fungus, Metarhizium anisopliae, alone and in combination with diatomaceous earth and thiamethoxam on mortality, progeny production, mycosis, and sporulation of the stored grain insect pests. Environ. Sci. Pollut. Res. 2017, 24, 28165–28174. [Google Scholar] [CrossRef]
- Nwaubani, S.I.; Opit, G.P.; Otitodun, G.O.; Adesida, M.A. Efficacy of two Nigeria-derived diatomaceous earths against Sitophilus oryzae (Coleoptera: Curculionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) on wheat. J. Stored Prod. Res. 2014, 59, 9–16. [Google Scholar] [CrossRef]
- Korunic, Z. Review Diatomaceous Earths, a Group of Natural Insecticides. J. Stored Prod. Res. 1998, 34, 87–97. [Google Scholar] [CrossRef]
- Brinkman, M.A.; Gardner, W.A. Use of diatomaceous earth and entomopathogen combinations against the red imported fire ant (Hymenoptera: Formicidae). Fla. Entomol. 2001, 84, 740–741. [Google Scholar] [CrossRef]
- Riasat, T.; Wakil, W.; Ashfaq, M.; Sahi, S.-T. Effect of Beauveria bassiana mixed with diatomaceous earth on mortality, mycosis and sporulation of Rhyzopertha dominica on stored wheat. Phytoparasitica 2011, 39, 325–331. [Google Scholar] [CrossRef]
- Vayias, B.J.; Athanassiou, C.G. Factors affecting the insecticidal efficacy of the diatomaceous earth formulation SilicoSec against adults and larvae of the confused flour beetle, Tribolium confusum Du Val (Coleoptera: Tenebrionidae). Crop Prot. 2004, 23, 565–573. [Google Scholar] [CrossRef]
- Vayias, B.J.; Athanassiou, C.G.; Kavallieratos, N.G.; Tsesmeli, C.D.; Buchelos, C.T. Persistence and efficacy of two diatomaceous earth formulations and a mixture of diatomaceous earth with natural pyrethrum against Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae) on wheat and maize. Pest Manag. Sci. 2006, 62, 456–464. [Google Scholar] [CrossRef]
- Ziaee, M.; Khashaveh, A. Effect of five diatomaceous earth formulations against Tribolium castaneum (Coleoptera: Tenebrionidae), Oryzaephilus surinamensis (Coleoptera: Silvanidae) and Rhyzopertha dominica (Coleoptera: Bostrychidae). Insect Sci. 2007, 14, 359–365. [Google Scholar] [CrossRef]
- Ziaee, M.; Moharramipour, S.; Francikowski, J. The synergistic effects of Carum copticum essential oil on diatomaceous earth against Sitophilus granarius and Tribolium confusum. J. Asia Pac. Entomol. 2014, 17, 817–822. [Google Scholar] [CrossRef]
- Feng, M.G.; Pu, X.Y. Time-concentration-mortality modeling of the synergistic interaction of Beauveria bassiana and imidacloprid against Nilaparvata lugens. Pest Manag. Sci. 2005, 61, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Feng, M.G. Evaluation of the time-concentration-mortality responses of Plutella xylostella larvae to the interaction of Beauveria bassiana with a nereistoxin analogue insecticide. Pest Manag. Sci. 2006, 62, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.D.; Dun, Y.H.; Feng, M.G. Time and concentration dependent interactions of Beauveria bassiana with sublethal rates of imidacloprid against the aphid pests Macrosiphoniella sanborni and Myzus persicae. Ann. Appl. Biol. 2005, 146, 459–468. [Google Scholar] [CrossRef]
- Rivero-Borja, M.; Guzmán-Franco, A.W.; Rodríguez-Leyva, E.; Santillán-Ortega, C.; Pérez-Panduro, A. Interaction of Beauveria bassiana and Metarhizium anisopliae with chlorpyrifos ethyl and spinosad in Spodoptera frugiperda larvae. Pest Manag. Sci. 2018, 74, 2047–2052. [Google Scholar] [CrossRef]
- Russell, C.W.; Ugine, T.A.; Hajek, A.E. Interactions between imidacloprid and Metarhizium brunneum on adult Asian longhorned beetles (Anoplophora glabripennis (Motschulsky)) (Coleoptera: Cerambycidae). J. Invertebr. Pathol. 2010, 105, 305–311. [Google Scholar] [CrossRef]
- Nian, X.G.; He, Y.R.; Lu, L.H.; Zhao, R. Evaluation of the time-concentration-mortality responses of Plutella xylostella larvae to the interaction of Isaria fumosorosea with the insecticides beta-cypermethrin and Bacillus thuringiensis. Pest Manag. Sci. 2014, 71, 216–224. [Google Scholar] [CrossRef]
- Tang, J.F.; Liu, X.Y.; Ding, Y.C.; Jiang, W.J.; Xie, J.Q. Evaluation of Metarhizium anisopliae for rice planthopper control and its synergy with selected insecticides. Crop Prot. 2019, 121, 132–138. [Google Scholar] [CrossRef]
- Sabbour, M.M.; Abd-El-Aziz, S.E.; Sherief, M.A. Efficacy of three entomopathogenic fungi alone or in combination with diatomaceous earth modifications for the control of three pyralid moths in stored grains. J. Plant Prot. Res. 2012, 52, 359–363. [Google Scholar] [CrossRef]
- Arooni-Hesari, M.; Talaei-Hassanloui, R.; Sabahi, Q. Simultaneous Use of Entomopathogenic Fungus Beauveria bassiana and Diatomaceous Earth against the Larvae of Indian Meal Moth, Plodia interpunctella. Adv. Biosci. Biotechnol. 2015, 6, 501–507. [Google Scholar] [CrossRef]
- Jacobson, R.J.; Chandler, D.; Fenlon, J.; Russell, K.M. Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae) to control Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on cucumber plants. Biocontrol Sci. Technol. 2001, 11, 391–400. [Google Scholar] [CrossRef]
- Goettel, S.; Inglis, G.D. Fungi: Hyphomycetes. In Manual of Techniques in Insect Pathology; Elsevier: Amsterdam, The Netherlands, 1997; pp. 213–249. [Google Scholar]
- Rodrigues, J.; Borges, P.R.; Fernandes, É.K.K.; Luz, C. Activity of additives and their effect in formulations of Metarhizium anisopliae s.l. IP 46 against Aedes aegypti adults and on post mortem conidiogenesis. Acta Trop. 2019, 193, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Y.; Feng, M.G. DPS Data Processing System for Practical Statistics; Science Press: Beijing, China, 2012. [Google Scholar]
- Nowierski, R.M.; Zheng, Z.; Jaronski, S.; Delgado, F.; Swearingen, W. Analysis and modeling of time-dose-mortality of Melanoplus sanguinipes, Locusta migratoria migratorioides, and Schistocerca gregaria (Orthoptera: Acrididae) from Beauveria, Metarhizium, and Paecilomyces isolates from Madagascar. J. Invertebr. Pathol. 1996, 67, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.G.; Liu, C.L.; Xu, J.H.; Xu, Q. Modeling and biological implication of time-dose-mortality data for the entomophthoralean fungus, Zoophthora anhuiensis, on the green peach aphid Myzus persicae. J. Invertebr. Pathol. 1998, 72, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Feng, M.G. The time-dose-mortality modeling and virulence indices for two entomophthoralean species, Pandora delphacis, and P. Neoaphidis, against the green peach aphid, Myzus persicae. Biol. Control 2000, 17, 29–34. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; He, S.Q.; Chen, B.; Su, Z.T.; Wang, W.Q.; Zhang, L.Y.; Li, Z.Y.; Xiao, G.L. Time-concentration-mortality response of potato tuber moth pupae to the biocontrol agent Cordyceps tenuipes. Biocontrol Sci. Technol. 2019, 29, 965–978. [Google Scholar] [CrossRef]
- Shi, W.B.; Zhang, L.; Feng, M.G. Time-concentration-mortality responses of carmine spider mite (Acari: Tetranychidae) females to three hypocrealean fungi as biocontrol agents. Biol. Control 2008, 46, 495–501. [Google Scholar] [CrossRef]
- Qiu, J.Z.; Song, F.F.; Mao, L.H.; Tu, J.; Guan, X. Time-dose-mortality data and modeling for the entomopathogenic fungus Aschersonia placenta against the whitefly Bemisia tabaci. Can. J. Microbiol. 2013, 59, 97–101. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; You, M.; Liu, B. Time-dose-mortality modelling and virulence indices for six strains of Verticillium lecanii against sweetpotato whitefly, Bemisia tabaci (Gennadius). J. Appl. Entomol. 2004, 128, 494–500. [Google Scholar] [CrossRef]
- Shan, L.T.; Feng, M.G. Evaluation of the biocontrol potential of various Metarhizium isolates against green peach aphid Myzus persicae (Homoptera: Aphididae). Pest Manag. Sci. 2010, 66, 669–675. [Google Scholar] [PubMed]
- Shi, Y.W.; Qin, X.W.; Wang, Z.Y.; Wang, M.; Liu, J.; Chen, Y.; Zhang, R.J.; Liu, Y.D. Analysis of the time-concentration-mortality response of Nilaparvata lugens (Hemiptera: Delphacidae) to Paichongding. J. Econ. Entomol. 2015, 108, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.; Eilenberg, J. Time-concentration mortality of Pieris brassicae (Lepidoptera: Pieridae) and Agrotis segetum (Lepidoptera: Noctuidae) larvae from different destruxins. Environ. Entomol. 2000, 29, 1041–1047. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Qin, X.W.; Yuan, F.H.; Zhang, R.J. Analysis of Time-and Concentration-Mortality Relationship of Nitenpyram Against Different Larval Stages of Nilaparvata lugens (Hemiptera: Delphacidae). J. Econ. Entomol. 2010, 103, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B.; Wood, S.N.; Lomer, C.J. Biological control of locusts and grasshoppers using a fungal pathogen: The importance of secondary cycling. Proc. R. Soc. B Biol. Sci. 1995, 259, 265–270. [Google Scholar]


| Assay | Metarhizium flavoviride (conidia/mL) | Diatomaceous Earth (ppm) | Imidacloprid (ppm) |
|---|---|---|---|
| A 1 | 1.2 × 106 | 0 | 0 |
| 1.2 × 108 | 0 | 0 | |
| A 2 | 1.2 × 106 | 50 | 0 |
| 1.2 × 108 | 50 | 0 | |
| A 3 | 1.2 × 106 | 0 | 200 |
| 1.2 × 108 | 0 | 200 | |
| A 4 | 1.2 × 106 | 50 | 200 |
| 1.2 × 108 | 50 | 200 |
| Method | Conditional Mortality Model | Cumulative Mortality Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter a | Value | S.E. | tb | Parameter a | Value | var (τj) | cov (β,τj) | |
| Assay 1 | β | 0.53 | 0.11 | 4.94 | β | 0.53 | 0.00 | 0.00 |
| γ1 | −6.13 | 0.86 | 7.16 | τ1 | −6.13 | 0.19 | −0.02 | |
| γ2 | −5.68 | 0.84 | 6.78 | τ2 | −5.19 | 0.18 | −0.02 | |
| γ3 | −5.28 | 0.83 | 6.38 | τ3 | −4.54 | 0.17 | −0.02 | |
| γ4 | −4.95 | 0.82 | 6.04 | τ4 | −4.03 | 0.17 | −0.02 | |
| γ5 | −4.54 | 0.80 | 5.68 | τ5 | −3.56 | 0.16 | −0.02 | |
| γ6 | −4.06 | 0.77 | 5.30 | τ6 | −3.09 | 0.15 | −0.02 | |
| γ7 | −4.57 | 0.82 | 5.58 | τ7 | −2.88 | 0.15 | −0.02 | |
| γ8 | −5.23 | 0.98 | 5.37 | τ8 | −2.79 | 0.15 | −0.02 | |
| γ9 | −5.82 | 1.28 | 4.54 | τ9 | −2.74 | 0.14 | −0.02 | |
| γ10 | −15.01 | 0.00 | 1501224821.11 | τ10 | −2.74 | 0.14 | −0.02 | |
| H-L test c | C8 = 3.73, p = 0.88 | |||||||
| Assay 2 | β | 0.57 | 0.11 | 5.08 | β | 0.57 | 0.01 | 0.01 |
| γ1 | −6.13 | 0.88 | 6.98 | τ1 | −6.13 | 0.28 | −0.04 | |
| γ2 | −5.65 | 0.87 | 6.52 | τ2 | −5.17 | 0.27 | −0.04 | |
| γ3 | −5.17 | 0.86 | 6.04 | τ3 | −4.48 | 0.26 | −0.04 | |
| γ4 | −4.26 | 0.81 | 5.24 | τ4 | −3.67 | 0.25 | −0.03 | |
| γ5 | −4.35 | 0.78 | 5.61 | τ5 | −3.26 | 0.23 | −0.03 | |
| γ6 | −4.44 | 0.80 | 5.57 | τ6 | −2.99 | 0.22 | −0.03 | |
| γ7 | −5.26 | 1.00 | 5.26 | τ7 | −2.89 | 0.21 | −0.03 | |
| γ8 | −5.08 | 1.04 | 4.91 | τ8 | −2.79 | 0.21 | −0.03 | |
| γ9 | −15.26 | 0.00 | 1526174665.79 | τ9 | −2.77 | 0.21 | −0.03 | |
| γ10 | −15.26 | 0.00 | 1526170593.00 | τ10 | −2.79 | 0.21 | −0.03 | |
| H-L test c | C8 = 4.79, p = 0.78 | |||||||
| Assay 3 | β | 0.92 | 0.14 | 6.78 | β | 0.92 | 0.01 | 0.01 |
| γ1 | −9.01 | 1.07 | 8.41 | τ1 | −9.01 | 0.44 | −0.05 | |
| γ2 | −8.20 | 1.06 | 7.74 | τ2 | −7.84 | 0.42 | −0.05 | |
| γ3 | −7.29 | 1.04 | 7.03 | τ3 | −6.83 | 0.41 | −0.05 | |
| γ4 | −6.72 | 0.92 | 7.27 | τ4 | −6.08 | 0.36 | −0.05 | |
| γ5 | −7.04 | 0.91 | 7.78 | τ5 | −5.75 | 0.33 | −0.05 | |
| γ6 | −6.95 | 0.97 | 7.20 | τ6 | −5.49 | 0.32 | −0.05 | |
| γ7 | −6.66 | 0.97 | 6.91 | τ7 | −5.22 | 0.31 | −0.05 | |
| γ8 | −7.07 | 11.72 | 0.60 | τ8 | −5.07 | 1.26 | −0.05 | |
| γ9 | −8.01 | 1.37 | 5.86 | τ9 | −5.02 | 1.17 | −0.05 | |
| γ10 | −17.36 | 0.00 | 1736162872.06 | τ10 | −5.02 | 1.17 | −0.05 | |
| H-L test c | C8 = 12.38, p = 0.14 | |||||||
| Assay 4 | β | 1.06 | 0.15 | 7.12 | β | 1.06 | 0.01 | 0.01 |
| γ1 | −9.90 | 1.17 | 8.43 | τ1 | −9.90 | 0.63 | −0.08 | |
| γ2 | −8.47 | 1.16 | 7.31 | τ2 | −8.25 | 0.61 | −0.08 | |
| γ3 | −7.76 | 1.04 | 7.45 | τ3 | −7.28 | 0.53 | −0.07 | |
| γ4 | −8.08 | 0.98 | 8.25 | τ4 | −6.91 | 0.48 | −0.07 | |
| γ5 | −7.46 | 0.96 | 7.78 | τ5 | −6.45 | 0.45 | −0.07 | |
| γ6 | −7.00 | 0.96 | 7.29 | τ6 | −6.00 | 0.42 | −0.07 | |
| γ7 | −6.69 | 0.98 | 6.80 | τ7 | −5.59 | 0.41 | −0.06 | |
| γ8 | −7.34 | 1.17 | 6.26 | τ8 | −5.43 | 0.41 | −0.06 | |
| γ9 | −18.23 | 0.00 | 1822796044.24 | τ9 | −5.43 | 0.41 | −0.06 | |
| γ10 | −18.23 | 0.00 | 1822790822.24 | τ10 | −5.43 | 0.41 | −0.06 | |
| H-L test c | C8 = 11.96, p = 0.15 | |||||||
| Method | Conditional Mortality Model | Cumulative Mortality Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter a | Value | S.E. | tb | Parameter a | Value | var (τj) | cov (β,τj) | |
| Assay 1 | β | 0.46 | 0.10 | 4.48 | β | 0.46 | 0.00 | 0.00 |
| γ1 | −5.54 | 0.82 | 6.73 | τ1 | −5.54 | 0.20 | −0.02 | |
| γ2 | −5.31 | 0.81 | 6.53 | τ2 | −4.73 | 0.19 | −0.02 | |
| γ3 | −4.99 | 0.80 | 6.22 | τ3 | −4.16 | 0.18 | −0.02 | |
| γ4 | −4.81 | 0.80 | 5.99 | τ4 | −3.74 | 0.18 | −0.02 | |
| γ5 | −4.60 | 0.79 | 5.86 | τ5 | −3.38 | 0.18 | −0.02 | |
| γ6 | −4.08 | 0.78 | 5.27 | τ6 | −2.98 | 0.17 | −0.02 | |
| γ7 | −4.70 | 0.82 | 5.71 | τ7 | −2.82 | 0.17 | −0.02 | |
| γ8 | −4.01 | 0.80 | 5.01 | τ8 | −2.55 | 0.17 | −0.02 | |
| γ9 | −4.56 | 0.89 | 5.10 | τ9 | −2.43 | 0.16 | −0.02 | |
| γ10 | −14.61 | 0.00 | 1460589375.39 | τ10 | −2.43 | 0.16 | −0.02 | |
| H-L test c | C8 = 2.26, p = 0.97 | |||||||
| Assay 2 | β | 0.46 | 0.10 | 4.43 | β | 0.46 | 0.00 | 0.00 |
| γ1 | −5.10 | 0.81 | 6.33 | τ1 | −5.10 | 0.25 | −0.03 | |
| γ2 | −4.94 | 0.81 | 6.11 | τ2 | −4.32 | 0.24 | −0.03 | |
| γ3 | −4.68 | 0.80 | 5.88 | τ3 | −3.79 | 0.24 | −0.03 | |
| γ4 | −4.21 | 0.78 | 5.40 | τ4 | −3.29 | 0.23 | −0.03 | |
| γ5 | −3.78 | 0.76 | 4.95 | τ5 | −2.81 | 0.22 | −0.03 | |
| γ6 | −3.61 | 0.75 | 4.80 | τ6 | −2.44 | 0.21 | −0.03 | |
| γ7 | −3.82 | 0.81 | 4.71 | τ7 | −2.21 | 0.21 | −0.03 | |
| γ8 | −4.40 | 0.97 | 4.56 | τ8 | −2.11 | 0.20 | −0.03 | |
| γ9 | −4.93 | 1.21 | 4.07 | τ9 | −2.05 | 0.20 | −0.03 | |
| γ10 | −14.58 | 0.00 | 1458324483.35 | τ10 | −2.05 | 0.20 | −0.03 | |
| H-L test c | C7 = 4.67, p = 0.70 | |||||||
| Assay 3 | β | 1.04 | 0.14 | 7.36 | β | 1.04 | 0.01 | 0.01 |
| γ1 | −9.80 | 1.12 | 8.78 | τ1 | −9.80 | 0.48 | −0.06 | |
| γ2 | −8.68 | 1.12 | 7.78 | τ2 | −8.40 | 0.48 | −0.06 | |
| γ3 | −7.73 | 1.04 | 7.46 | τ3 | −7.32 | 0.43 | −0.06 | |
| γ4 | −8.09 | 0.98 | 8.28 | τ4 | −6.94 | 0.40 | −0.06 | |
| γ5 | −8.20 | 0.97 | 8.48 | τ5 | −6.69 | 0.37 | −0.05 | |
| γ6 | −7.84 | 0.95 | 8.26 | τ6 | −6.41 | 0.35 | −0.05 | |
| γ7 | −7.26 | 0.93 | 7.82 | τ7 | −6.06 | 0.34 | −0.05 | |
| γ8 | −7.62 | 0.99 | 7.67 | τ8 | −5.87 | 0.33 | −0.05 | |
| γ9 | −8.83 | 1.37 | 6.46 | τ9 | −5.81 | 0.33 | −0.05 | |
| γ10 | −18.09 | 0.00 | 1809361422.42 | τ10 | −5.81 | 0.33 | −0.05 | |
| H-L test c | C8 = 15.49, p = 0.05 | |||||||
| Assay 4 | β | 0.64 | 0.12 | 5.45 | β | 0.64 | 0.01 | 0.01 |
| γ1 | −6.35 | 0.91 | 7.00 | τ1 | −6.35 | 0.39 | −0.05 | |
| γ2 | −4.71 | 0.87 | 5.39 | τ2 | −4.53 | 0.36 | 0.05 | |
| γ3 | −4.73 | 0.82 | 5.80 | τ3 | −3.93 | 0.33 | −0.05 | |
| γ4 | −5.17 | 0.82 | 6.30 | τ4 | −3.68 | 0.31 | −0.04 | |
| γ5 | −4.66 | 0.81 | 5.76 | τ5 | −3.36 | 0.30 | −0.04 | |
| γ6 | −4.50 | 0.85 | 5.32 | τ6 | −3.08 | 0.29 | −0.04 | |
| γ7 | −4.46 | 0.96 | 4.66 | τ7 | −2.86 | 0.28 | −0.04 | |
| γ8 | −5.13 | 1.29 | 3.97 | τ8 | −2.76 | 0.28 | −0.04 | |
| γ9 | −3.79 | 1.06 | 3.59 | τ9 | −2.45 | 0.29 | −0.04 | |
| γ10 | −1.42 | 0.00 | 142261625.14 | τ10 | −1.12 | 0.28 | −0.01 | |
| H-L test c | C8 = 7.11, p = 0.53 | |||||||
| Developmental Stages | LT50/LT90 (d) | Bioassay and Conidia Concentration (conidia/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Assay 1 | Assay 2 | Assay 3 | Assay 4 | ||||||
| 1.2 × 106 | 1.2 × 108 | 1.2 × 106 | 1.2 × 108 | 1.2 × 106 | 1.2 × 108 | 1.2 × 106 | 1.2 × 108 | ||
| Adults | LT50 | 4.94 | 2.82 | 3.77 | 2.26 | 4.42 | 2.06 | 4.23 | 1.53 |
| LT90 | — | 5.31 | — | 3.90 | — | 3.48 | 6.98 | 2.69 | |
| Second instar larvae | LT50 | 5.49 | 3.11 | 4.26 | 2.45 | 5.07 | 1.70 | 2.45 | 1.41 |
| LT90 | — | 6.47 | — | 4.89 | — | 2.86 | 6.18 | 2.45 | |
| Heading | Treatments of Additive or Formulation | Number of Conidia/Adult × 105 | Number of Conidia/Second Instar Larva × 105 |
|---|---|---|---|
| T 1 | F1 | 3.63 ± 0.18b | 2.60 ± 0.17d |
| F2 | 5.28 ± 0.13a | 4.10 ± 0.35a | |
| T 2 | D + F1 | 3.32 ± 0.04b | 2.50 ± 0.06d |
| D + F2 | 4.62 ± 0.04a | 3.67 ± 0.09ab | |
| T 3 | I + F1 | 3.57 ± 0.19b | 2.87 ± 0.19bcd |
| I + F2 | 4.87 ± 0.12a | 3.73 ± 0.09a | |
| T 4 | D + I + F1 | 3.60 ± 0.32b | 2.77 ± 0.09cd |
| D + I + F2 | 5.17 ± 0.22a | 3.50 ± 0.06abc | |
| F7,23 20.70 | F7,23 13.38 | ||
| p = 0.001 < 0.01 | p = 0.001 < 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, W.; Du, G.; Zhang, L.; Li, Z.; Xiao, G.; Chen, B. The Time–Concentration–Mortality Responses of Western Flower Thrips, Frankliniella occidentalis, to the Synergistic Interaction of Entomopathogenic Fungus Metarhizium flavoviride, Insecticides, and Diatomaceous Earth. Insects 2020, 11, 93. https://doi.org/10.3390/insects11020093
Ge W, Du G, Zhang L, Li Z, Xiao G, Chen B. The Time–Concentration–Mortality Responses of Western Flower Thrips, Frankliniella occidentalis, to the Synergistic Interaction of Entomopathogenic Fungus Metarhizium flavoviride, Insecticides, and Diatomaceous Earth. Insects. 2020; 11(2):93. https://doi.org/10.3390/insects11020093
Chicago/Turabian StyleGe, Wenchao, Guangzu Du, Limin Zhang, Zhengyue Li, Guanli Xiao, and Bin Chen. 2020. "The Time–Concentration–Mortality Responses of Western Flower Thrips, Frankliniella occidentalis, to the Synergistic Interaction of Entomopathogenic Fungus Metarhizium flavoviride, Insecticides, and Diatomaceous Earth" Insects 11, no. 2: 93. https://doi.org/10.3390/insects11020093
APA StyleGe, W., Du, G., Zhang, L., Li, Z., Xiao, G., & Chen, B. (2020). The Time–Concentration–Mortality Responses of Western Flower Thrips, Frankliniella occidentalis, to the Synergistic Interaction of Entomopathogenic Fungus Metarhizium flavoviride, Insecticides, and Diatomaceous Earth. Insects, 11(2), 93. https://doi.org/10.3390/insects11020093




