Nest Turrets of Acromyrmex Grass-Cutting Ants: Micromorphology Reveals Building Techniques and Construction Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Area Description
2.2. Sampling, Morphological, and Micromorphological Analysis
3. Results
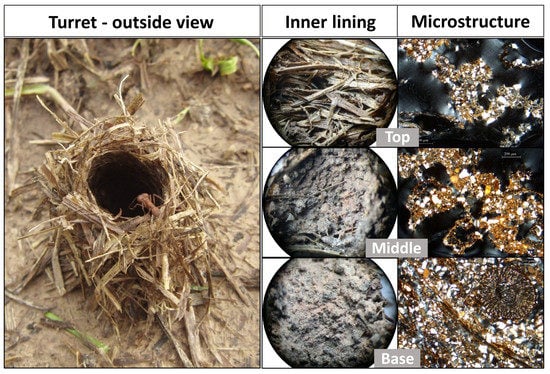
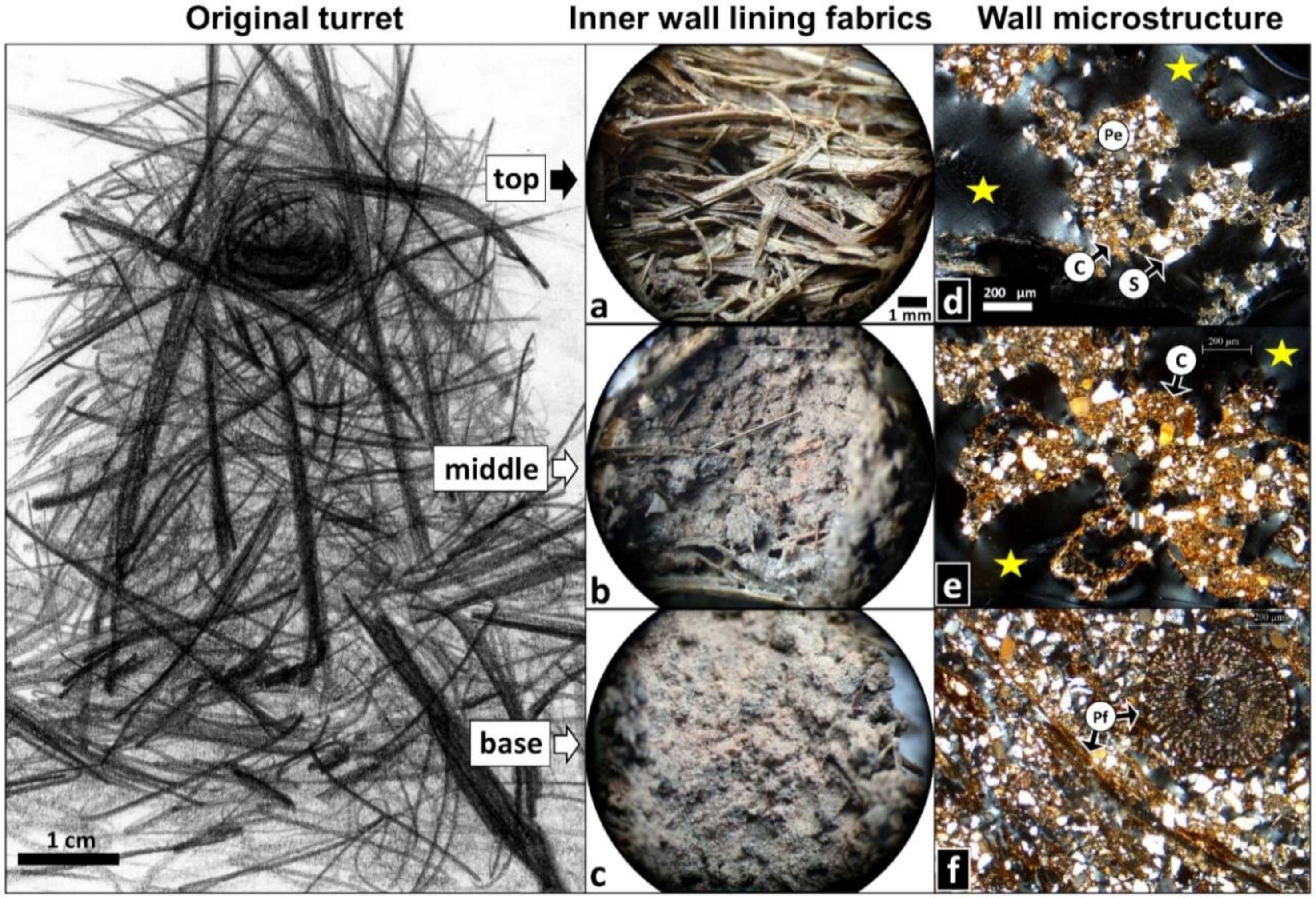
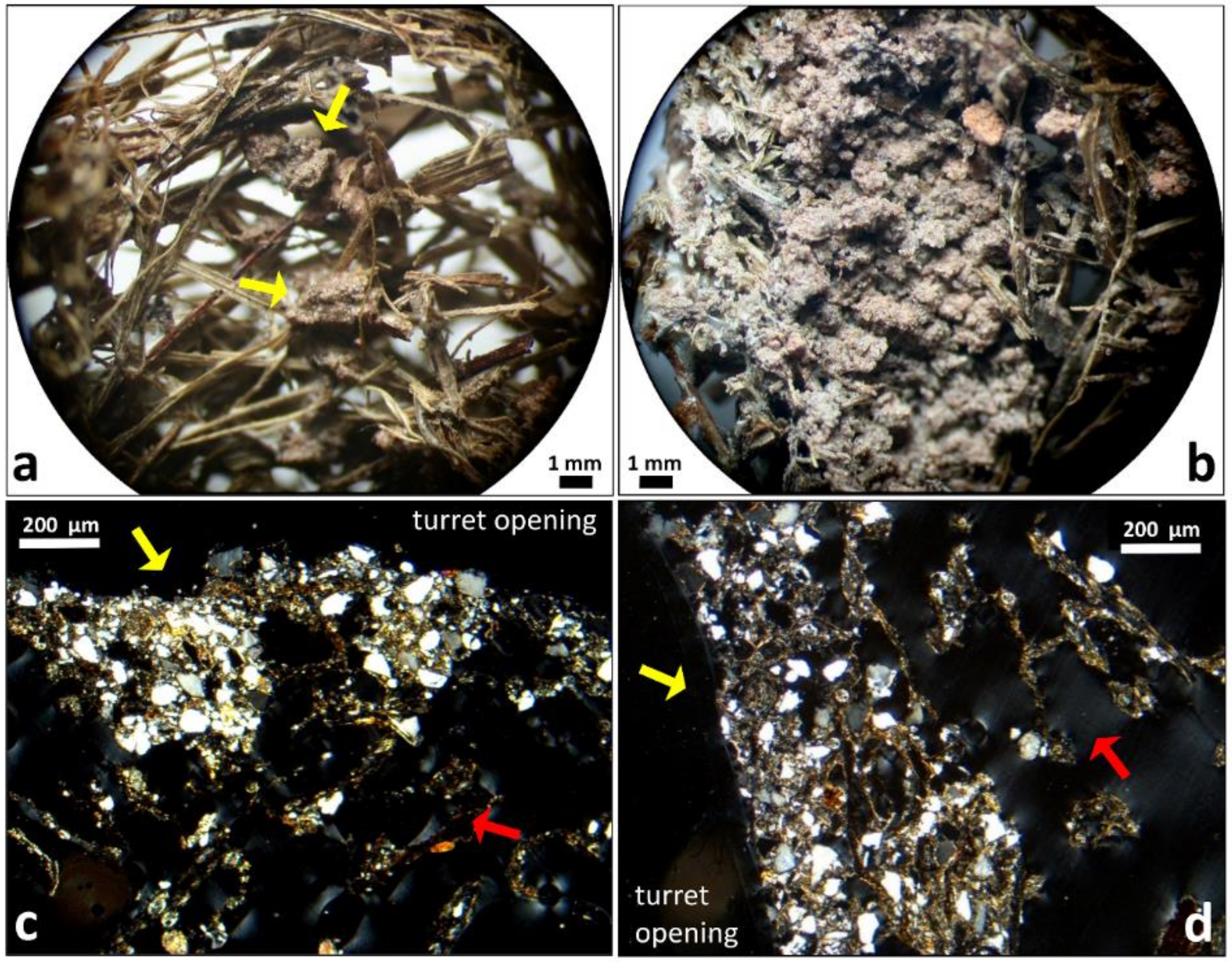
3.1. Original Nest Turrets: External Morphology, Inner Walls, and Microstructures
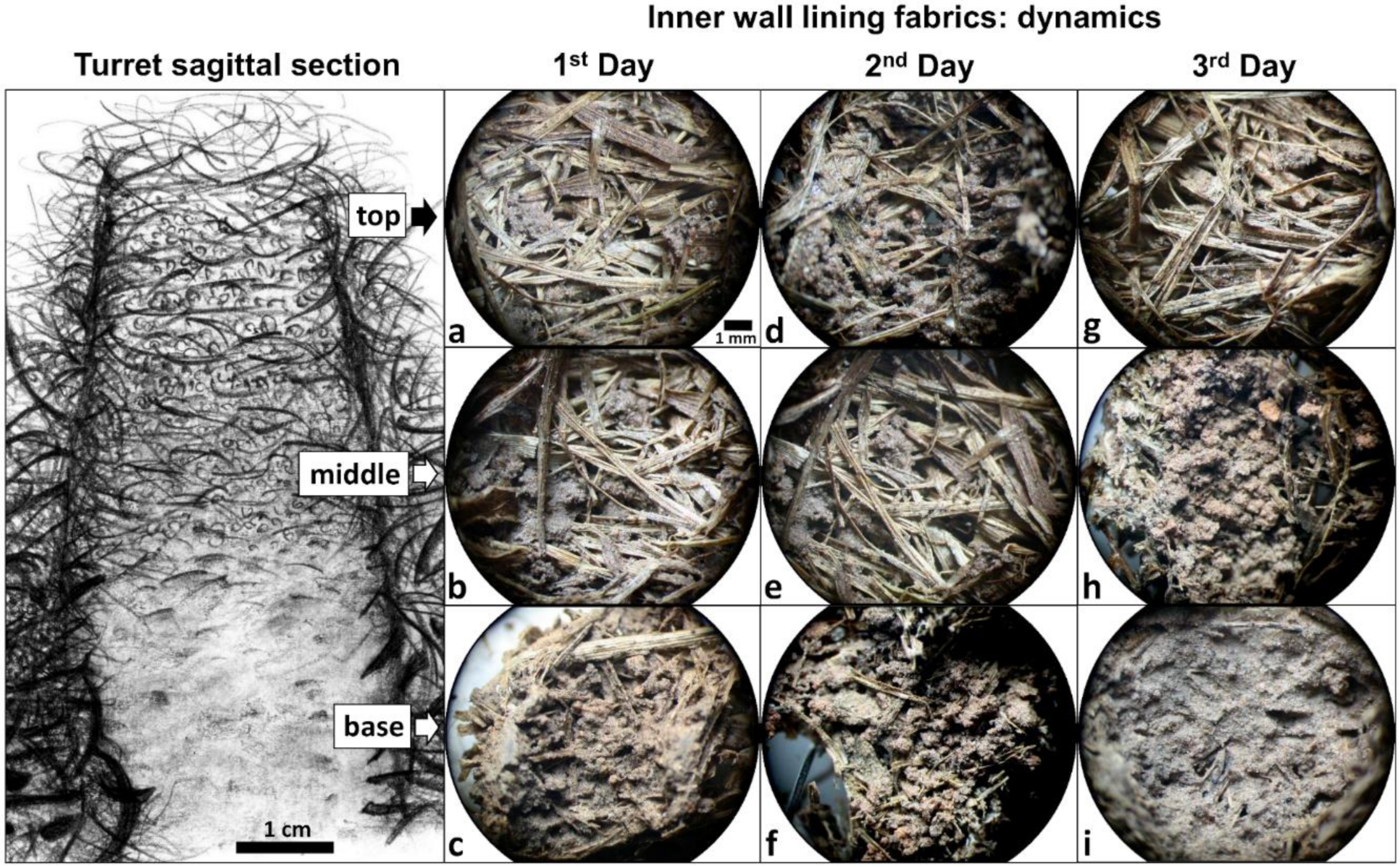
3.2. Rebuilt Turrets: Dynamics of Inner Wall Lining Fabrics
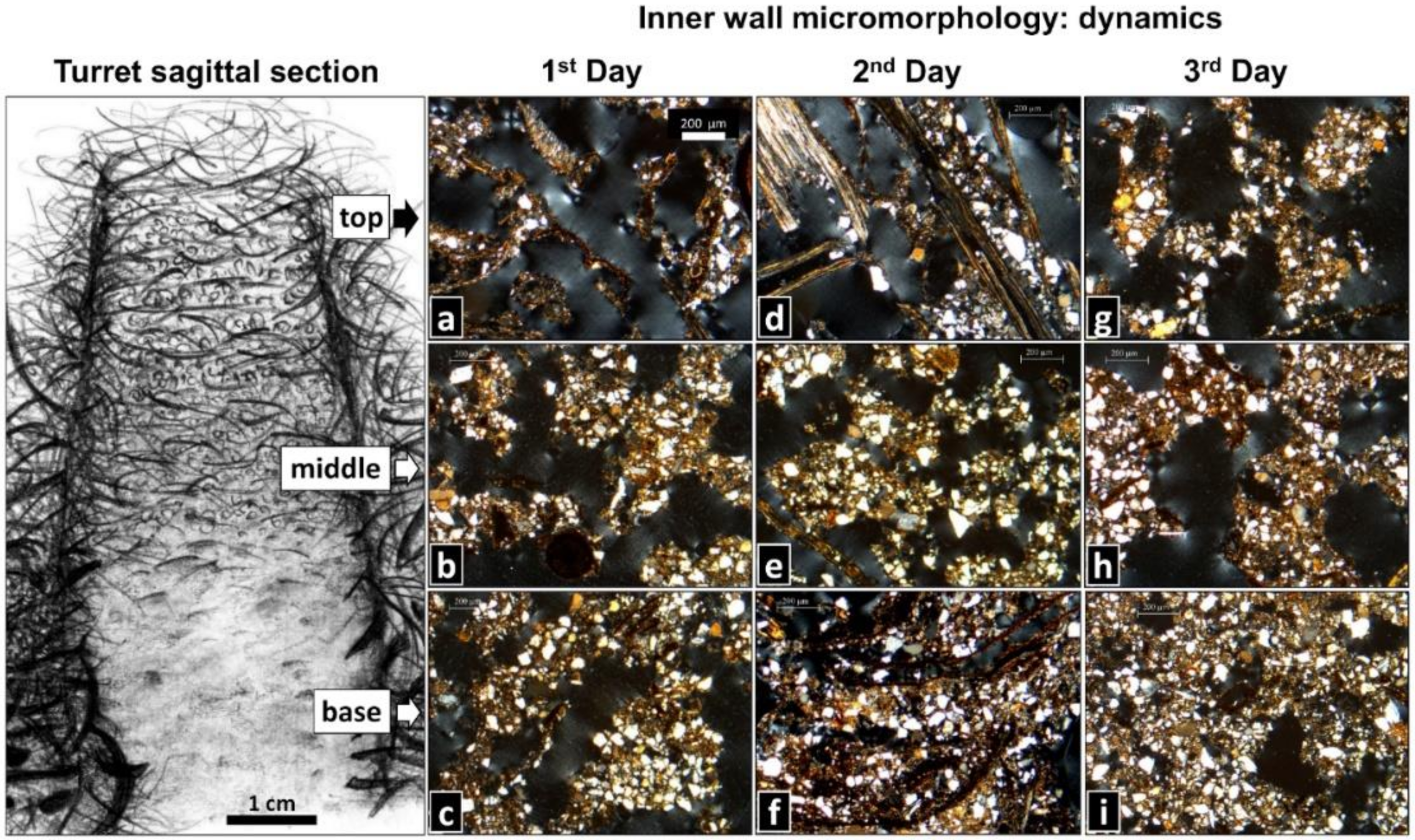
3.3. Rebuilt Turrets: Dynamics of Inner Wall Micromorphology
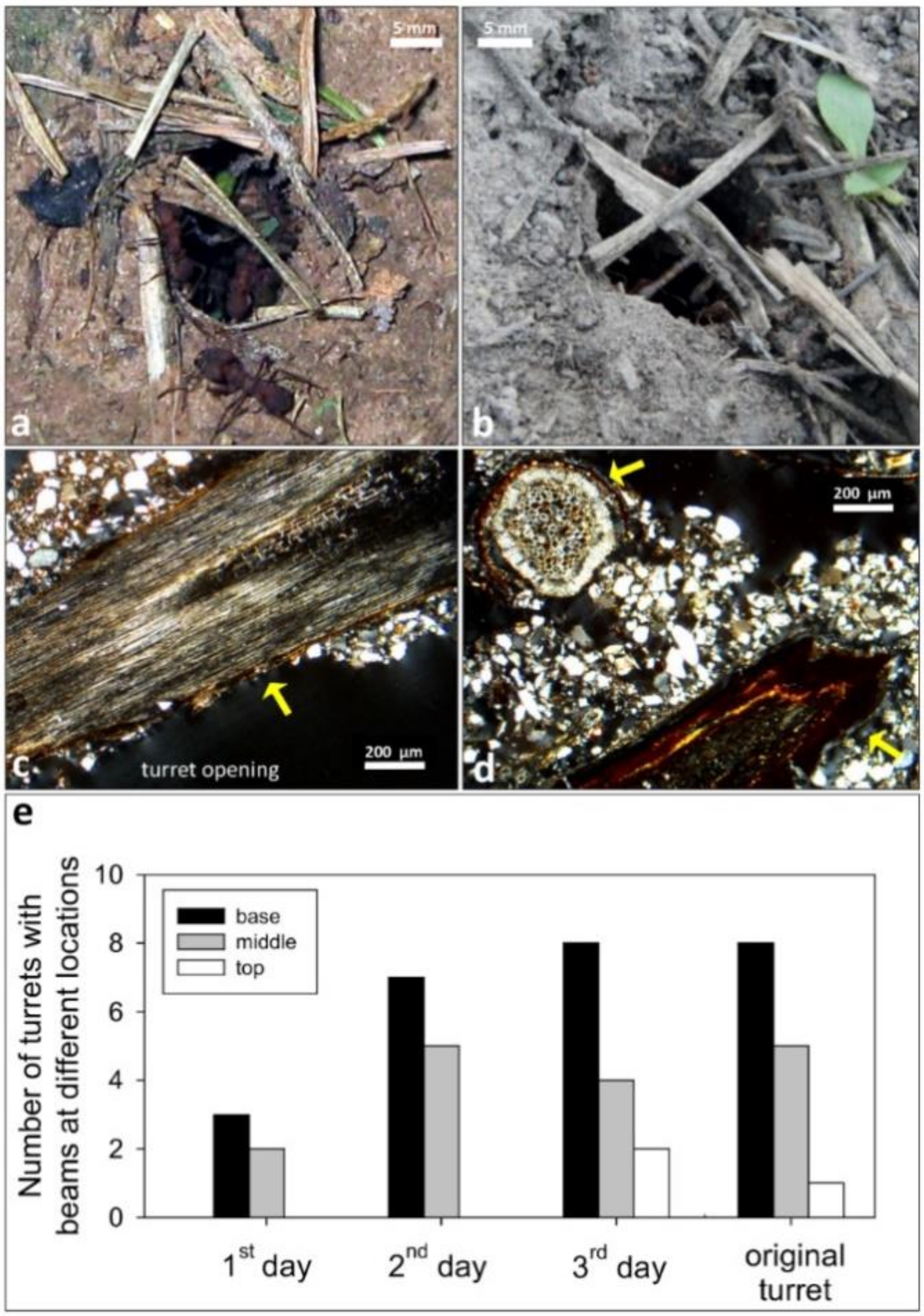
3.4. Onset of Turret Building: Use of Beams
4. Discussion
4.1. Intermeshing Grass Fragments and Twigs around the Nest Opening
4.2. Cementing the Vegetal Mesh with Soil Pellets
4.3. Lining the Inner Gallery Wall with Soil Pellets
4.4. Comparative Aspects of Turret Building
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, W.P.; Pettry, D.E.; Switzer, R.E. Structure and hydrology of mounds of the imported fire ants in the southeastern United States. Geoderma 1999, 93, 1–17. [Google Scholar] [CrossRef]
- Frouz, J.; Jilková, V. The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrmecol. News 2008, 11, 191–199. [Google Scholar]
- Whitford, W.G.; Eldridge, D.J. Effects of Ants and Termites on Soil and Geomorphological Processes; Shroder, J., Butler, D.R., Hupp, C.R., Eds.; Academic Press: San Diego, CA, USA, 2013; Volume 12. [Google Scholar]
- Bonetto, A.A.; Manzi, R.; Pignalberi, C. Los “tacurúes” de Camponotus punctulatus (Mayr). Physis 1960, 12, 217–224. [Google Scholar]
- Cosarinsky, M.I. Nest micromorphology of the neotropical mound building ants Camponotus punctulatus and Solenopsis sp. Sociobiology 2006, 47, 329–344. [Google Scholar]
- Gorosito, N.B.; Curmi, P.; Hallaire, V.; Folgarait, P.J.; Lavelle, P.M. Morphological changes in Camponotus punctulatus (Mayr) anthills of different ages. Geoderma 2006, 132, 249–260. [Google Scholar] [CrossRef]
- Bruch, C. Contribución al estudio de las hormigas de la provincia de San Luis. Rev. Mus. La Plata 1916, 23, 291–357. [Google Scholar]
- Cassill, D.; Tschinkel, W.R.; Vinson, S.B. Nest complexity, group size and brood rearing in the fire ant, Solenopsis invicta. Insectes Soc. 2002, 49, 158–163. [Google Scholar] [CrossRef]
- Jonkman, J.C.M. The external and internal structure and growth of nests of the leaf-cutting ant Atta vollenweideri Forel, 1893 (Hym.: Formicidae): Part I. Z. Angew. Entomol. 1980, 89, 158–173. [Google Scholar] [CrossRef]
- Jonkman, J.C.M. The external and internal structure and growth of nests of the leaf-cutting ant Atta vollenweideri Forel, 1893 (Hym.: Formicidae): Part II. Z. Angew. Entomol. 1980, 89, 217–246. [Google Scholar] [CrossRef]
- Moreira, A.A.; Forti, L.C.; Andrade, A.P.P.; Boaretto, M.A.C.; Lopes, J.F.S. Nest architecture of Atta laevigata (F. Smith, 1858) (Hymenoptera: Formicidae). Stud. Neotrop. Fauna Environ. 2004, 39, 109–116. [Google Scholar] [CrossRef]
- Moreira, A.A.; Forti, L.C.; Boaretto, M.A.C.; Andrade, A.P.P.; Lopes, J.F.S.; Ramos, V.M. External and internal structure of Atta bisphaerica Forel (Hymenoptera: Formicidae) nests. J. Appl. Entomol. 2004, 128, 204–211. [Google Scholar] [CrossRef]
- Bollazzi, M.; Forti, L.C.; Roces, F. Ventilation of the giant nests of Atta leaf-cutting ants: Does underground circulating air enter the fungus chambers? Insectes Soc. 2012, 59, 487–498. [Google Scholar] [CrossRef]
- Cosarinsky, M.I.; Roces, F. The construction of turrets for nest ventilation in the grass-cutting ant Atta vollenweideri: Import and assembly of building materials. J. Insect Behav. 2012, 25, 222–241. [Google Scholar] [CrossRef]
- Fröhle, K.; Roces, F. The determination of nest depth in founding queens of leaf-cutting ants (Atta vollenweideri): Idiothetic and temporal control. J. Exp. Biol. 2012, 215, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Pielström, S.; Roces, F. Vibrational communication in the spatial organization of collective digging in the leaf-cutting ant Atta vollenweideri. Anim. Behav. 2012, 84, 743–752. [Google Scholar] [CrossRef]
- Halboth, F.; Roces, F. Underground anemotactic orientation in leaf-cutting ants: Perception of airflow and experience-dependent choice of airflow direction during digging. Naturwissenschaften 2017, 104, 82. [Google Scholar] [CrossRef]
- Daguerre, J.B. Hormigas del género Atta Fabricius de la Argentina (Hymenoptera, Formicidae). Rev. Soc. Entomol. Argent. 1945, 12, 438–460. [Google Scholar]
- Kleineidam, C.; Ernst, R.; Roces, F. Wind-induced ventilation of the giant nests of the leaf-cutting ant Atta vollenweideri. Naturwissenschaften 2001, 88, 301–305. [Google Scholar] [CrossRef]
- Cosarinsky, M.I.; Roces, F. Neighbor leaf-cutting ants and mound-building termites: Comparative nest micromorphology. Geoderma 2007, 141, 224–234. [Google Scholar] [CrossRef]
- Halboth, F.; Roces, F. The construction of ventilation turrets in Atta vollenweideri leaf-cutting ants: Carbon dioxide levels in the nest tunnels, but not airflow or air humidity, influence turret structure. PLoS ONE 2017, 12, e0188162. [Google Scholar] [CrossRef]
- Bollazzi, M.; Kronenbitter, J.; Roces, F. Soil temperature, digging behaviour, and the adaptive value of nest depth in South American species of Acromyrmex leaf-cutting ants. Oecologia 2008, 158, 165–175. [Google Scholar] [CrossRef] [PubMed]
- De Santis, L. Las Principales Hormigas Dañinas de la Provincia de Buenos Aires; Ministerio de Obras Públicas de la Provincia de Buenos Aires, Dirección de Agricultura, Ganadería e Industrias: La Plata, Argentina, 1941; p. 40.
- Bonetto, A.A. Las Hormigas “Cortadoras” de la Provincia de Santa Fé (Géneros: Atta y Acromyrmex); Ministerio de Agricultura y Ganadería MAG: Santa Fe, Argentina, 1959; pp. 1–77.
- Zolessi, L.; Abenante, Y. Nidificación y mesoetología de Acromyrmex en el Uruguay III, Acromyrmex (A.) hispidus Santschi 1925 (Hymenoptera: Formicidae). Rev. Biol. Urug. 1973, 1, 151–165. [Google Scholar]
- Zolessi, L.; González, L. Nidificación y mesoetología de Acromyrmex en el Uruguay II, Acromyrmex (Acromyrmex) lobicornis (Emery, 1887) (Hymenoptera: Formicidae). Rev. Biol. Urug. 1974, 1, 37–57. [Google Scholar]
- Quirán, E.; Pilati, A. Estructura de los hormigueros de Acromyrmex lobicornis (Hymenoptera: Formicidae) en un sitio natural semiárido de La Pampa, Argentina. Rev. Soc. Entomol. Argent. 1998, 57, 45–48. [Google Scholar]
- Farji-Brener, A.G. Leaf-cutting ant nests in temperate environments: Mounds, mound damages and nest mortality rate in Acromyrmex lobicornis. Stud. Neotrop. Fauna Environ. 2000, 35, 131–138. [Google Scholar] [CrossRef]
- Bollazzi, M.; Roces, F. The thermoregulatory function of thatched nests in the South American grass-cutting ant, Acromyrmex heyeri. J. Insect Sci. 2010, 10, 1–17. [Google Scholar] [CrossRef]
- Espina, E.R.; Timaure, A. Características de los nidos de Acromyrmex landolti (Forel) en el oeste de Venezuela. Rev. Fac. Agron. Univ. Zulia 1977, 4, 53–62. [Google Scholar]
- Moreira, I.J.S.; Santos, M.F.; Madureira, M.S. Why do Acromyrmex nests have thatched entrance structures? Evidence for use as a visual homing cue. Insectes Soc. 2019, 66, 165–170. [Google Scholar] [CrossRef]
- Sousa-Souto, L.; Viana Junior, A.B.; Nascimento, E.S. Spatial distribution of Acromyrmex balzani (Emery) (Hymenoptera: Formicidae: Attini) nests using two sampling methods. Sociobiology 2013, 60, 162–168. [Google Scholar] [CrossRef]
- Silva Junior, M.; Castellani, M.; Moreira, A.A.; D’Esquivel, M.D.; Forti, L.C.; Lacau, S. Spatial distribution and architecture of Acromyrmex landolti Forel (Hymenoptera, Formicidae) nests in pastures of Southwestern Bahia, Brazil. Sociobiology 2013, 60, 20–29. [Google Scholar] [CrossRef]
- Verza, S.S.; Gervásio, R.C.R.G.; Alves e Silva, O.M.; Gomes, M.O.; Souza, S.A.; Mussury, R.M. Nest structure engineering of the leaf-cutting ant, Acromyrmex landolti, in the semiarid Caatinga biome. Insectes Soc. 2019. [Google Scholar] [CrossRef]
- Navarro, J.G.; Jaffé, K. On the adaptive value of nest features in the grass-cutting ant Acromyrmex landolti. Biotropica 1985, 17, 347–348. [Google Scholar] [CrossRef]
- Antonialli, W.F.; Giannotti, E. Nest architecture and population dynamics of the ponerine ant Ectatomma opaciventre Roger (Hymenoptera, Formicidae). J. Adv. Zool. 1997, 18, 64–71. [Google Scholar]
- LeBrun, E.G.; Moffett, M.; Holway, D.A. Convergent evolution of levee building behavior among distantly related ant species in a floodplain ant assemblage. Insectes Soc. 2011, 58, 263–269. [Google Scholar] [CrossRef]
- Murphy, C.P. Thin Section Preparation of Soil and Sediments; AB Academic Publishers: Berkhamsted, UK, 1986. [Google Scholar]
- Stoops, G. Application of some pedological methods to the analysis of termite mounds. In Études sur les Termites Africains; Bouillon, A., Ed.; Université Lovanium: Léopoldville, Congo, 1964; pp. 379–398. [Google Scholar]
- FitzPatrick, E.A. Micromorphology of Soils; Chapman and Hall: London, UK, 1984. [Google Scholar]
- Bullock, P.; Federoff, N.; Jongerius, A.; Tursina, T. Handbook for Soil Thin Section Description; Waine Research: Wolverhampton, UK, 1985. [Google Scholar]
- Fowler, H.G. Acromyrmex (Moellerius) landolti Forel en el Paraguay: Las subspecies balzani (Emery) y fracticornis (Forel) (Insecta: Hymenoptera). Neotropica 1977, 23, 39–44. [Google Scholar]
- Gonçalves, C.R. O gênero Acromyrmex no Brasil (Hym. Formicidae). Stud. Entomol. 1961, 4, 113–180. [Google Scholar]
- Kleineidam, C.; Roces, F. Carbon dioxide concentrations and nest ventilation in nests of the leaf-cutting ant Atta vollenweideri. Insectes Soc. 2000, 47, 241–248. [Google Scholar] [CrossRef]
- Mueller, U.G.; Wcislo, W.T. Nesting biology of the fungus-growing ant Cyphomyrmex longiscapus. Insectes Soc. 1998, 45, 181–189. [Google Scholar] [CrossRef]
- Smith, A.P. An investigation of the mechanisms underlying nest construction in the mud wasp Paralastor sp. (Hymenoptera: Eumenidae). Anim. Behav. 1978, 26, 232–240. [Google Scholar] [CrossRef]
- Schaber, B.D. Observations of nesting behavior and turret construction by Odynerus dilectus (Hymenoptera: Eumenidae). Can. Entomol. 1985, 117, 1159–1161. [Google Scholar] [CrossRef]
- McCook, H.C. Notes on the intelligence of the American turret spider. Proc. Acad. Natl. Sci. Phila. 1883, 35, 131–132. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosarinsky, M.I.; Römer, D.; Roces, F. Nest Turrets of Acromyrmex Grass-Cutting Ants: Micromorphology Reveals Building Techniques and Construction Dynamics. Insects 2020, 11, 140. https://doi.org/10.3390/insects11020140
Cosarinsky MI, Römer D, Roces F. Nest Turrets of Acromyrmex Grass-Cutting Ants: Micromorphology Reveals Building Techniques and Construction Dynamics. Insects. 2020; 11(2):140. https://doi.org/10.3390/insects11020140
Chicago/Turabian StyleCosarinsky, Marcela I., Daniela Römer, and Flavio Roces. 2020. "Nest Turrets of Acromyrmex Grass-Cutting Ants: Micromorphology Reveals Building Techniques and Construction Dynamics" Insects 11, no. 2: 140. https://doi.org/10.3390/insects11020140
APA StyleCosarinsky, M. I., Römer, D., & Roces, F. (2020). Nest Turrets of Acromyrmex Grass-Cutting Ants: Micromorphology Reveals Building Techniques and Construction Dynamics. Insects, 11(2), 140. https://doi.org/10.3390/insects11020140