Molecular Characterization and Expression Profiling of Nuclear Receptor Gene Families in Oriental Fruit Fly, Bactrocera Dorsalis (Hendel)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Cultures
2.2. Total RNA Extraction and cDNA Synthesis
2.3. Identification and Annotation of NRs
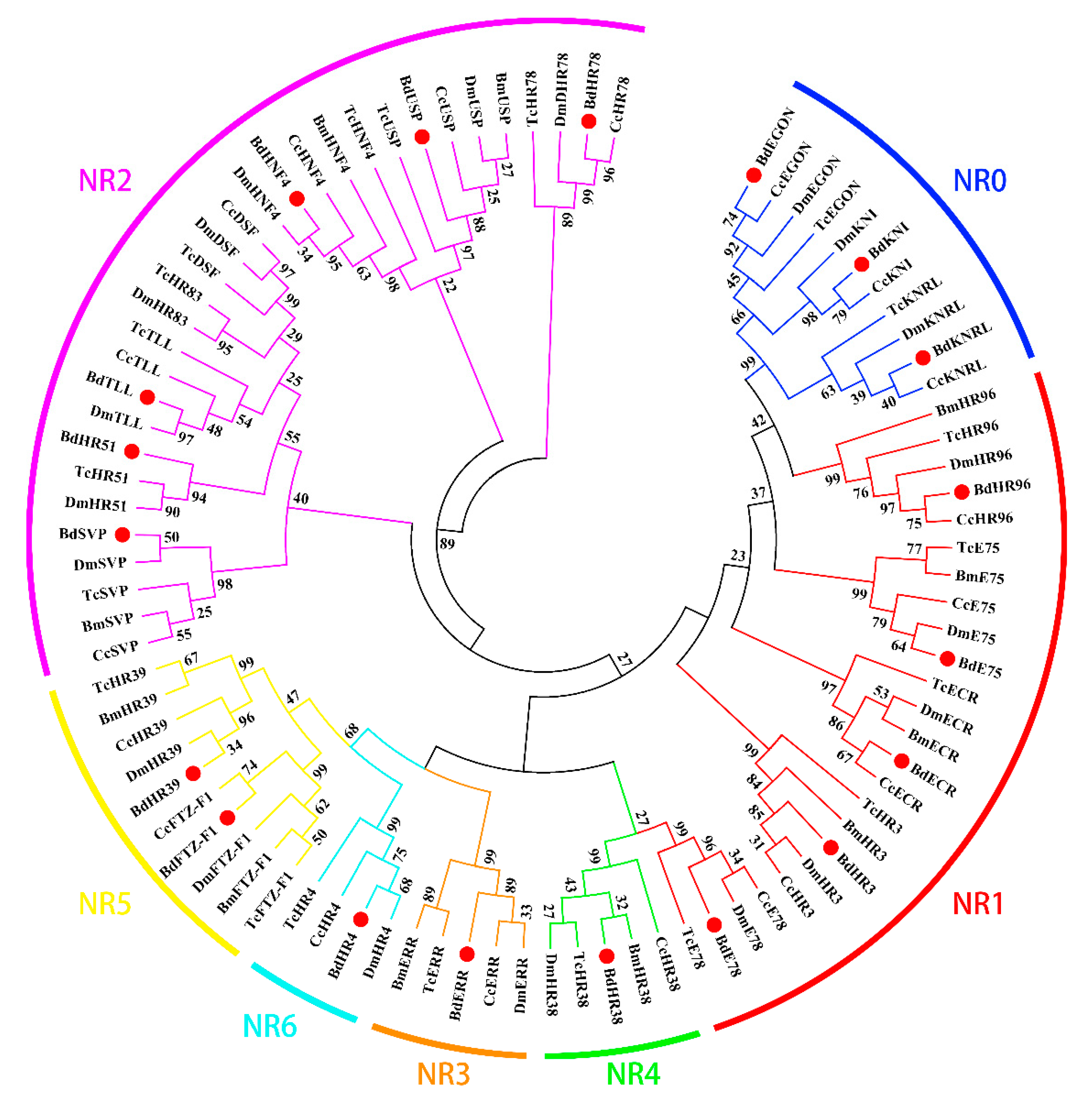
2.4. Phylogenetic Analysis
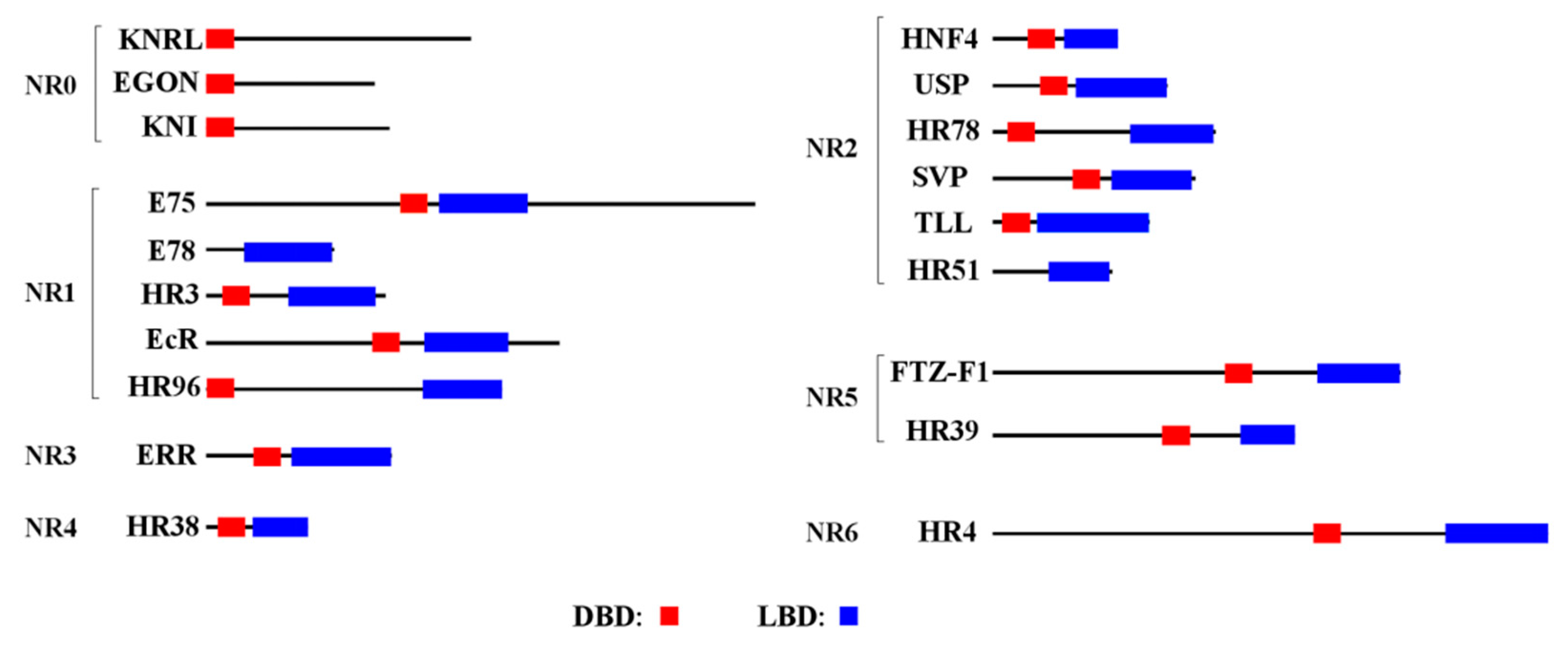
2.5. Domain Analysis
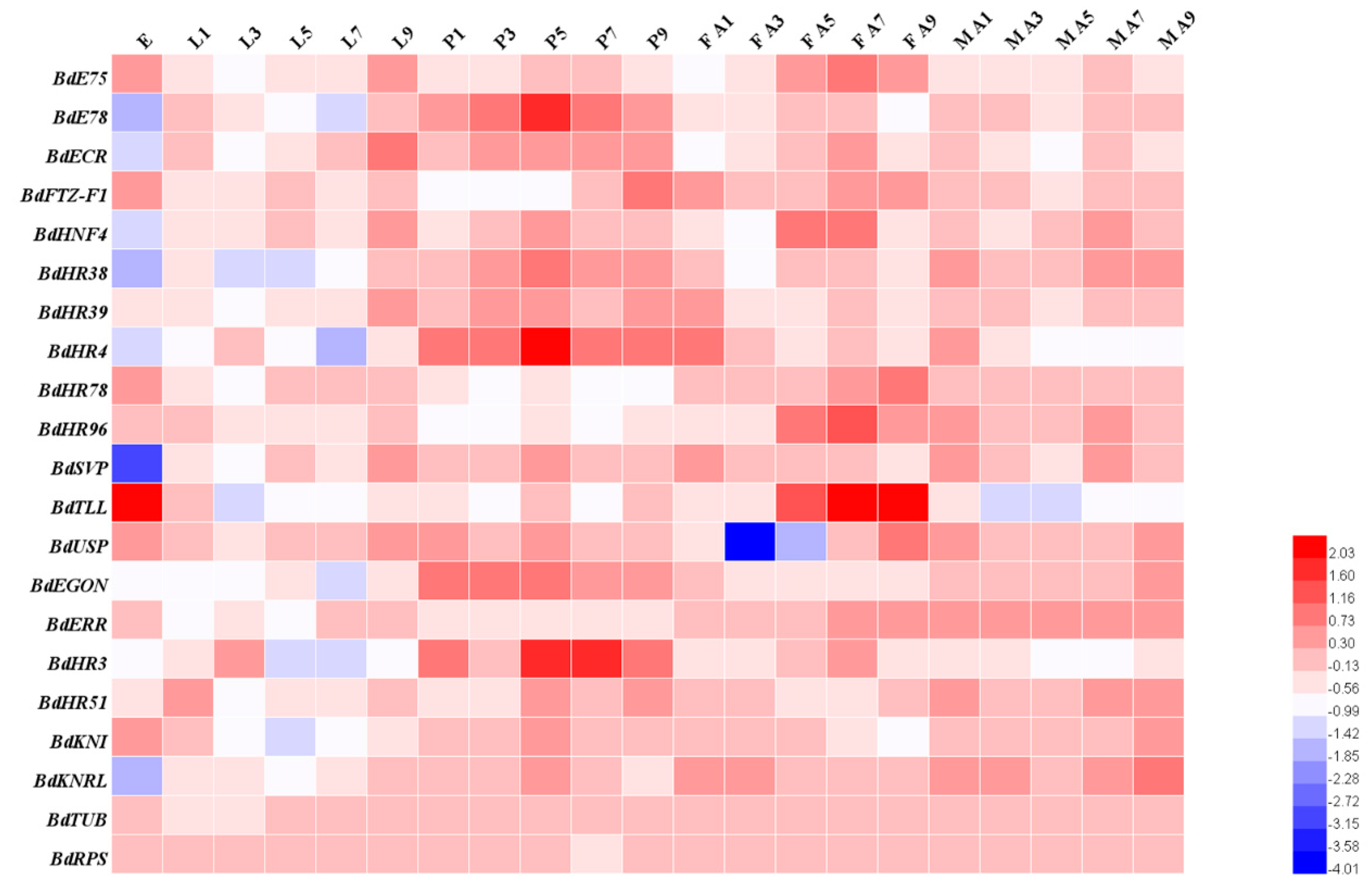
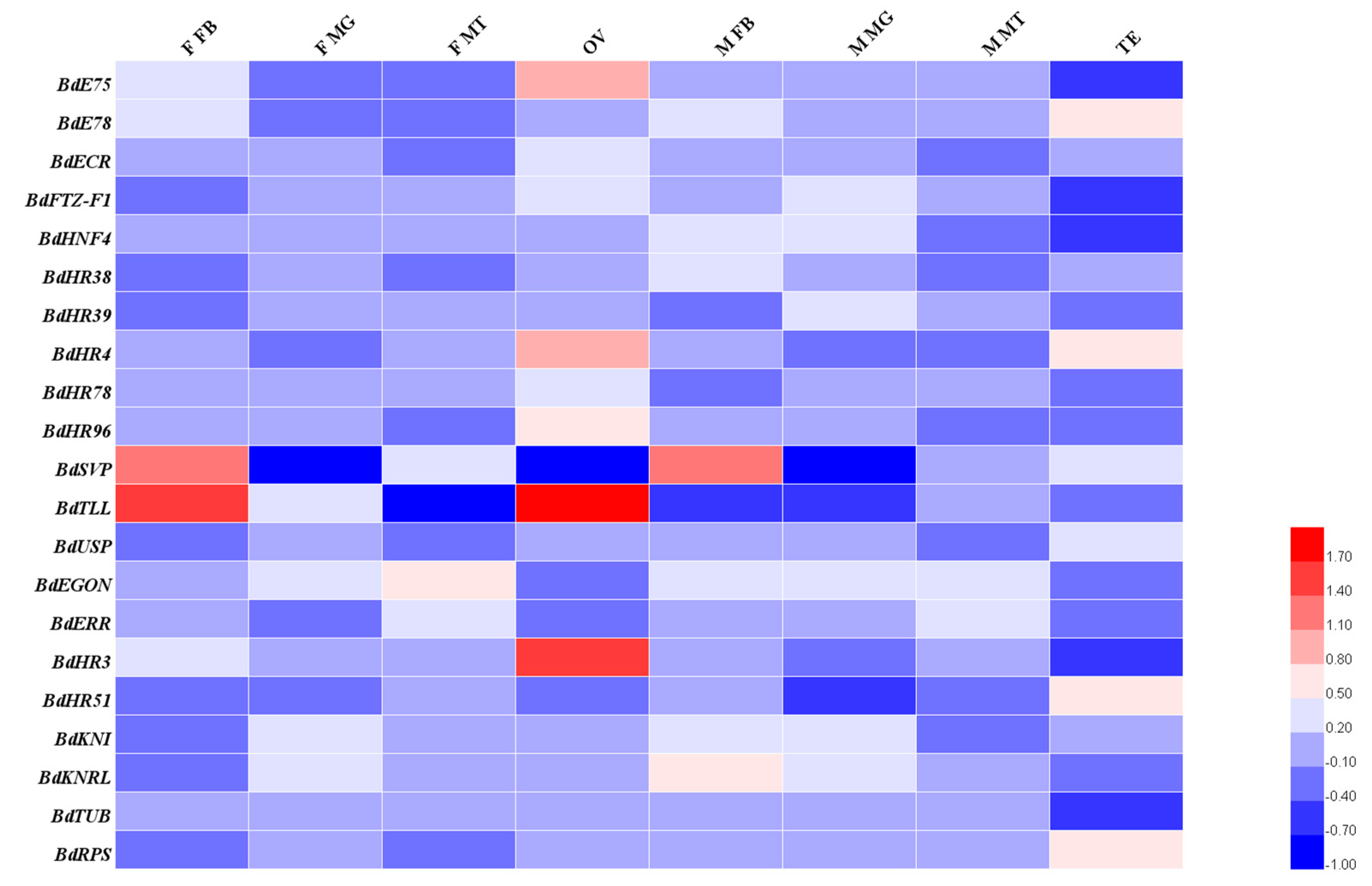
2.6. Temporal and Developmental Expression Profiles
3. Results
3.1. NR Identification and Characterization
3.2. Phylogenetic and Structural Analysis
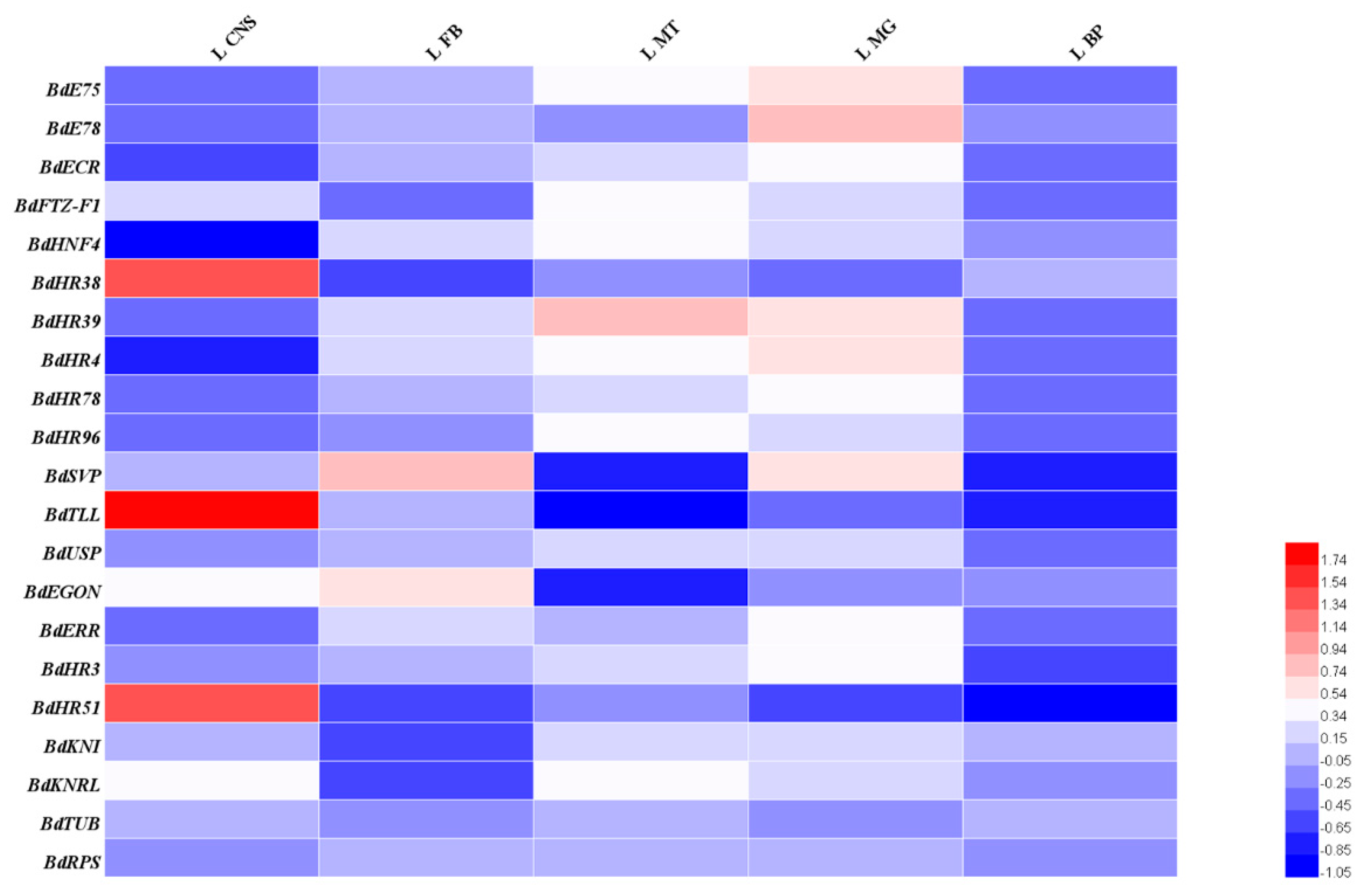
3.3. Spatiotemporal Expression Patterns
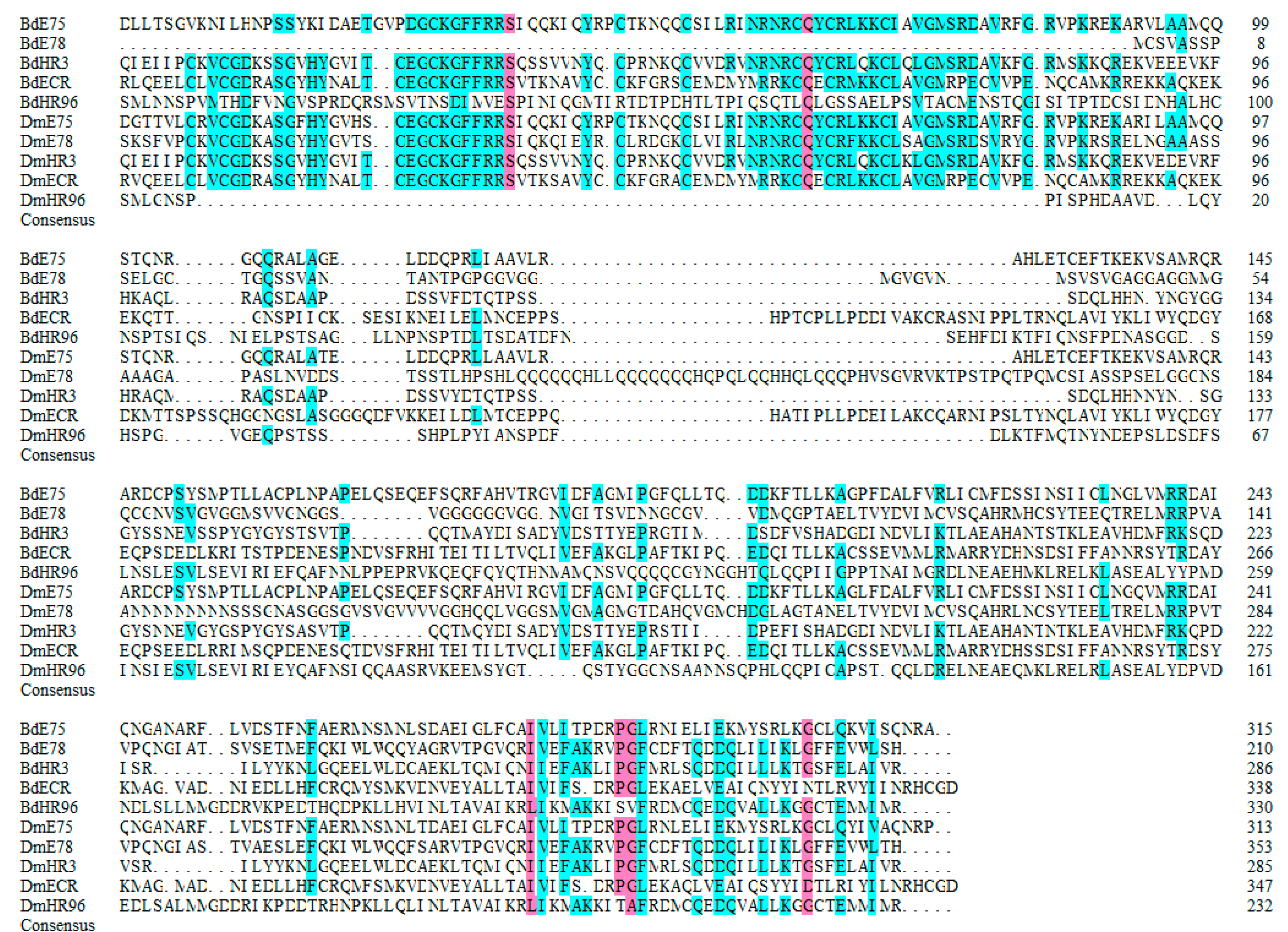
3.4. Multisequence Alignment
4. Discussion
4.1. NR0 Subfamily
4.2. NR1 Subfamily
4.3. NR2 Subfamily
4.4. NR3 Subfamily
4.5. NR4 Subfamily
4.6. NR5 Subfamily
4.7. NR6 Subfamily
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, R.M. The nuclear receptor superfamily: A Rosetta Stone for physiology. Mol. Endocrinol. 2005, 19, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Benoit, G.; Cooney, A.; Giguere, V.; Ingraham, H.; Lazar, M.; Muscat, G.; Perlmann, T.; Renaud, J.P.; Schwabe, J.; Sladek, F.; et al. International Union of Pharmacology. LXVI. Orphan nuclear receptors. Pharmacol. Rev. 2006, 58, 798–836. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Perriere, G.; Laudet, V.; Robinson-Rechavi, M. Nurebase: Database of nuclear hormone receptors. Nucleic Acids Res. 2002, 30, 364–368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Germain, P.; Staels, B.; Dacquet, C.; Spedding, M.; Laudet, V. Overview of nomenclature of nuclear receptors. Pharmacol. Rev. 2006, 58, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Rechavi, M.; Carpentier, A.S.; Duffraisse, M.; Laudet, V. How many nuclear hormone receptors are there in the human genome? Trends Genet. 2001, 17, 554–556. [Google Scholar] [CrossRef]
- Sluder, A.E.; Maina, C.V. Nuclear receptors in nematodes: Themes and variations. Trends Genet. 2001, 17, 206–213. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Burch, P.E.; Cooney, A.J.; Lanz, R.B.; Pereira, F.A.; Wu, J.Q.; Gibbs, R.A.; Weinstock, G.; Wheeler, D.A. Genomic analysis of the nuclear receptor family: New insights into structure, regulation, and evolution from the rat genome. Genome Res. 2004, 14, 580–590. [Google Scholar] [CrossRef]
- King-Jones, K.; Thummel, C.S. Nuclear receptors-A perspective from Drosophila. Nat. Rev. Genet. 2005, 6, 311–323. [Google Scholar] [CrossRef]
- Cheng, D.; Xia, Q.; Duan, J.; Wei, L.; Huang, C.; Li, Z.; Wang, G.; Xiang, Z. Nuclear receptors in Bombyx mori: Insights into genomic structure and developmental expression. Insect Biochem. Mol. Biol. 2008, 38, 1130–1137. [Google Scholar] [CrossRef]
- Velarde, R.A.; Robinson, G.E.; Fahrbach, S.E. Nuclear receptors of the honey bee: Annotation and expression in the adult brain. Insect Mol. Biol. 2006, 15, 583–595. [Google Scholar] [CrossRef]
- Bonneton, F.; Chaumot, A.; Laudet, V. Annotation of Tribolium nuclear receptors reveals an increase in evolutionary rate of a network controlling the ecdysone cascade. Insect Biochem. Mol. Biol. 2008, 38, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Jarvela, A.M.C.; Pick, L. The Function and Evolution of Nuclear Receptors in Insect Embryonic Development. In Nuclear Receptors in Development and Disease; Forrest, D., Tsai, S., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2017; Volume 125, pp. 39–70. [Google Scholar]
- Bernardo, T.J.; Dubrovskaya, V.A.; Jannat, H.; Maughan, B.; Dubrovsky, E.B. Hormonal Regulation of the E75 Gene in Drosophila: Identifying Functional Regulatory Elements through Computational and Biological Analysis. J. Mol. Biology 2009, 387, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Mazina, M.Y.; Kocheryzhkina, E.V.; Nikolenko, J.V.; Krasnov, A.N.; Georgieva, S.G.; Vorobyeva, N.E. Nuclear receptors EcR, Usp, E75, DHR3, and ERR regulate transcription of ecdysone cascade genes. Dokl. Biochem. Biophys. 2017, 473, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Afschar, S.; Toivonen, J.M.; Hoffmann, J.M.; Tain, L.S.; Wieser, D.; Finlayson, A.J.; Driege, Y.; Alic, N.; Emran, S.; Stinn, J.; et al. Nuclear hormone receptor DHR96 mediates the resistance to xenobiotics but not the increased lifespan of insulin-mutant Drosophila. Proc. Natl. Acad. Sci. USA 2016, 113, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Marxreiter, S.; Thummel, C.S. Adult functions for the Drosophila DHR78 nuclear receptor. Dev. Dyn. 2018, 247, 315–322. [Google Scholar] [CrossRef]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef]
- Wang, J.J.; Wei, D.; Dou, W.; Hu, F.; Liu, W.F.; Wang, J.J. Toxicities and synergistic effects of several insecticides against the oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2013, 106, 970–978. [Google Scholar] [CrossRef]
- Rothe, M.; Nauber, U.; Jackle, H. Three hormone receptor-like Drosophila genes encode an identical DNA-binding finger. Embo J. 1989, 8, 3087–3094. [Google Scholar] [CrossRef]
- Lundell, M.J.; Hirsh, J. Eagle is required for the specification of serotonin neurons and other neuroblast 7-3 progeny in the Drosophila CNS. Development 1998, 125, 463–472. [Google Scholar]
- Chen, C.K.; Kuhnlein, R.P.; Eulenberg, K.G.; Vincent, S.; Affolter, M.; Schuh, R. The transcription factors Knirps and Knirps related control cell migration and branch morphogenesis during Drosophila tracheal development. Development 1998, 125, 4959–4968. [Google Scholar]
- Higashijima, S.; Shishido, E.; Matsuzaki, M.; Saigo, K. Eagle, a member of the steroid receptor gene superfamily, is expressed in a subset of neuroblasts and regulates the fate of their putative progeny in the Drosophila CNS. Development 1996, 122, 527–536. [Google Scholar] [PubMed]
- Marvin, K.A.; Reinking, J.L.; Lee, A.J.; Pardee, K.; Krause, H.M.; Burstyn, J.N. Nuclear receptors Homo sapiens Rev-erb beta and Drosophila melanogaster E75 are thiolate-ligated heme proteins which undergo redox-mediated ligand switching and bind CO and NO. Biochemistry 2009, 48, 7056–7071. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zheng, W.W.; Tariq, K.; Yu, S.N.; Zhang, H.Y. MicroRNA Let-7 targets the ecdysone signaling pathway E75 gene to control larval-pupal development in Bactrocera dorsalis. Insect Sci. 2019, 26, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Ruaud, A.-F.; Lam, G.; Thummel, C.S. The Drosophila nuclear receptors DHR3 and beta FTZ-F1 control overlapping developmental responses in late embryos. Development 2010, 137, 123–131. [Google Scholar] [CrossRef]
- Ables, E.T.; Bois, K.E.; Garcia, C.A.; Drummond-Barbosa, D. Ecdysone response gene E78 controls ovarian germline stem cell niche formation and follicle survival in Drosophila. Dev. Biology 2015, 400, 33–42. [Google Scholar] [CrossRef]
- Cong, L.; Yang, W.J.; Shen, G.M.; Dou, W.; Wang, J.J. Molecular characterization of the cDNA encoding ecdysone receptor isoform B1 and its expression in the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol. 2012, 95, 650–658. [Google Scholar] [CrossRef]
- Barry, W.E.; Thummel, C.S. The Drosophila HNF4 nuclear receptor promotes glucose-stimulated insulin secretion and mitochondrial function in adults. Elife 2016, 5, e11183. [Google Scholar] [CrossRef]
- Hall, B.L.; Thummel, C.S. The RXR homolog Ultraspiracle is an essential component of the Drosophila ecdysone receptor. Development 1998, 125, 4709–4717. [Google Scholar]
- Janssens, H.; Crombach, A.; Wotton, K.R.; Cicin-Sain, D.; Surkova, S.; Lim, C.L.; Samsonova, M.; Akam, M.; Jaeger, J. Lack of tailless leads to an increase in expression variability in Drosophila embryos. Dev. Biol. 2013, 377, 305–317. [Google Scholar] [CrossRef]
- Sung, C.; Wong, L.E.; Sen, L.Q.C.; Nguyen, E.; Lazaga, N.; Ganzer, G.; McNabb, S.L.; Robinow, S. The unfulfilled/DHR51 gene of Drosophila melanogaster modulates wing expansion and fertility. Dev. Dyn. 2009, 238, 171–182. [Google Scholar] [CrossRef]
- Kanai, M.I.; Okabe, M.; Hiromi, Y. Seven-up controls switching of transcription factors that specify temporal identities of Drosophila neuroblasts. Dev. Cell 2005, 8, 203–213. [Google Scholar] [CrossRef]
- Maurange, C.; Cheng, L.; Gould, A.P. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell 2008, 133, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.J.E.; Tang, J.C.Y.; Ridyard, M.S.; Lian, T.S.; Keatings, K.; Allan, D.W. Gene regulatory mechanisms underlying the spatial and temporal regulation of target-dependent gene expression in Drosophila neurons. PLoS Genet. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, E.V.; Mazina, M.Y.; Krasnov, A.N.; Vorobyeva, N.E. The Drosophila nuclear receptors ECR and ERR jointly regulate the expression of genes involved in carbohydrate metabolism. Insect Biochem. Mol. Biol. 2019, 112, 103184. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.D.; Shewchuk, L.M.; Kozlova, T.; Makishima, M.; Hassell, A.; Wisely, B.; Caravella, J.A.; Lambert, M.H.; Reinking, J.L.; Krause, H.; et al. The Drosophila orphan nuclear receptor DHR38 mediates an atypical ecdysteroid signaling pathway. Cell 2003, 113, 731–742. [Google Scholar] [CrossRef]
- Ruaud, A.-F.; Lam, G.; Thummel, C.S. The Drosophila NR4A nuclear receptor DHR38 regulates carbohydrate metabolism and glycogen storage. Mol. Endocrinol. 2011, 25, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Borsos, B.N.; Pankotai, T.; Kovacs, D.; Popescu, C.; Pahi, Z.; Boros, I.M. Acetylations of FTZ-F1 and histone H4K5 are required for the fine-tuning of ecdysone biosynthesis during Drosophila metamorphosis. Dev. Biol. 2015, 404, 80–87. [Google Scholar] [CrossRef]
- Sullivan, A.A.; Thummel, C.S. Temporal profiles of nuclear receptor gene expression reveal coordinate transcriptional responses during Drosophila development. Mol. Endocrinol. 2003, 17, 2125–2137. [Google Scholar] [CrossRef]
- Ou, Q.; Magico, A.; King-Jones, K. Nuclear receptor DHR4 controls the timing of steroid hormone pulses during Drosophila development. PLoS Biol. 2011, 9, e1001160. [Google Scholar] [CrossRef]
- Thomson, S.A.; Baldwin, W.S.; Wang, Y.H.; Kwon, G.; LeBlanc, G.A. Annotation, phylogenetics, and expression of the nuclear receptors in Daphnia pulex. BMC Genom. 2009, 10, 500. [Google Scholar] [CrossRef]


| Group | B. dorsalis | Genbank accession no. | Protein length/aa | M.W. /kDa | pI | D. melanogaster |
|---|---|---|---|---|---|---|
| 0A | BdKNRL | XP_019844421 | 750 | 79.4 | 6.4 | KNRL |
| 0A | BdEGON | XP_011213892 | 477 | 50.2 | 9.2 | EGON |
| 0A | BdKNI | XP_029404449 | 519 | 54.5 | 6.2 | KNI |
| 1D | BdE75 | XP_019846264 | 1302 | 144.7 | 8.2 | E75 |
| 1E | BdE78 | XP_019846486 | 363 | 38.8 | 5.2 | E78 |
| 1F | BdHR3 | XP_011208441 | 485 | 54.8 | 6.1 | HR3 |
| 1H | BdECR | XP_011203444 | 566 | 62.8 | 6.9 | ECR |
| 1J | BdHR96 | XP_019847193 | 847 | 94.8 | 5.7 | HR96 |
| 2A | BdHNF4 | XP_029404261 | 698 | 76.8 | 6.4 | HNF4 |
| 2B | BdUSP | XP_019845796 | 453 | 51.3 | 8.5 | USP |
| 2D | BdHR78 | XP_011201915 | 630 | 69.2 | 5.5 | HR78 |
| 2E | BdTLL | XP_011214380 | 516 | 57.2 | 5.6 | TLL |
| 2E | BdDSF | XP_011214839 | 603 | 64.8 | 7.1 | DSF |
| 2E | BdHR83 | XP_029407834 | 318 | 35.7 | 9.0 | HR83 |
| 2E | BdHR51 | XP_011200189 | 339 | 37.4 | 5.0 | HR51 |
| 2F | BdSVP | XP_029407513 | 574 | 60.8 | 8.6 | SVP |
| 3B | BdERR | XP_019846473 | 524 | 57.4 | 7.0 | ERR |
| 4A | BdHR38 | XP_011212592 | 822 | 93.1 | 9.0 | HR38 |
| 5A | BdFTZ-F1 | XP_029405285 | 751 | 80.9 | 6.8 | FTZ-F1 |
| 5B | BdHR39 | XP_019844854 | 856 | 89.4 | 7.3 | HR39 |
| 6A | BdHR4 | XP_019846713 | 1573 | 169.1 | 9.1 | HR4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.-J.; Chen, E.-H.; Song, Z.-H.; He, W.; Liu, S.-H.; Dou, W.; Wang, J.-J. Molecular Characterization and Expression Profiling of Nuclear Receptor Gene Families in Oriental Fruit Fly, Bactrocera Dorsalis (Hendel). Insects 2020, 11, 126. https://doi.org/10.3390/insects11020126
Yang P-J, Chen E-H, Song Z-H, He W, Liu S-H, Dou W, Wang J-J. Molecular Characterization and Expression Profiling of Nuclear Receptor Gene Families in Oriental Fruit Fly, Bactrocera Dorsalis (Hendel). Insects. 2020; 11(2):126. https://doi.org/10.3390/insects11020126
Chicago/Turabian StyleYang, Pei-Jin, Er-Hu Chen, Zhong-Hao Song, Wang He, Shi-Huo Liu, Wei Dou, and Jin-Jun Wang. 2020. "Molecular Characterization and Expression Profiling of Nuclear Receptor Gene Families in Oriental Fruit Fly, Bactrocera Dorsalis (Hendel)" Insects 11, no. 2: 126. https://doi.org/10.3390/insects11020126
APA StyleYang, P.-J., Chen, E.-H., Song, Z.-H., He, W., Liu, S.-H., Dou, W., & Wang, J.-J. (2020). Molecular Characterization and Expression Profiling of Nuclear Receptor Gene Families in Oriental Fruit Fly, Bactrocera Dorsalis (Hendel). Insects, 11(2), 126. https://doi.org/10.3390/insects11020126





