The Role of (E)-2-octenyl Acetate as a Pheromone of Bagrada hilaris (Burmeister): Laboratory and Field Evaluation
Abstract
1. Introduction
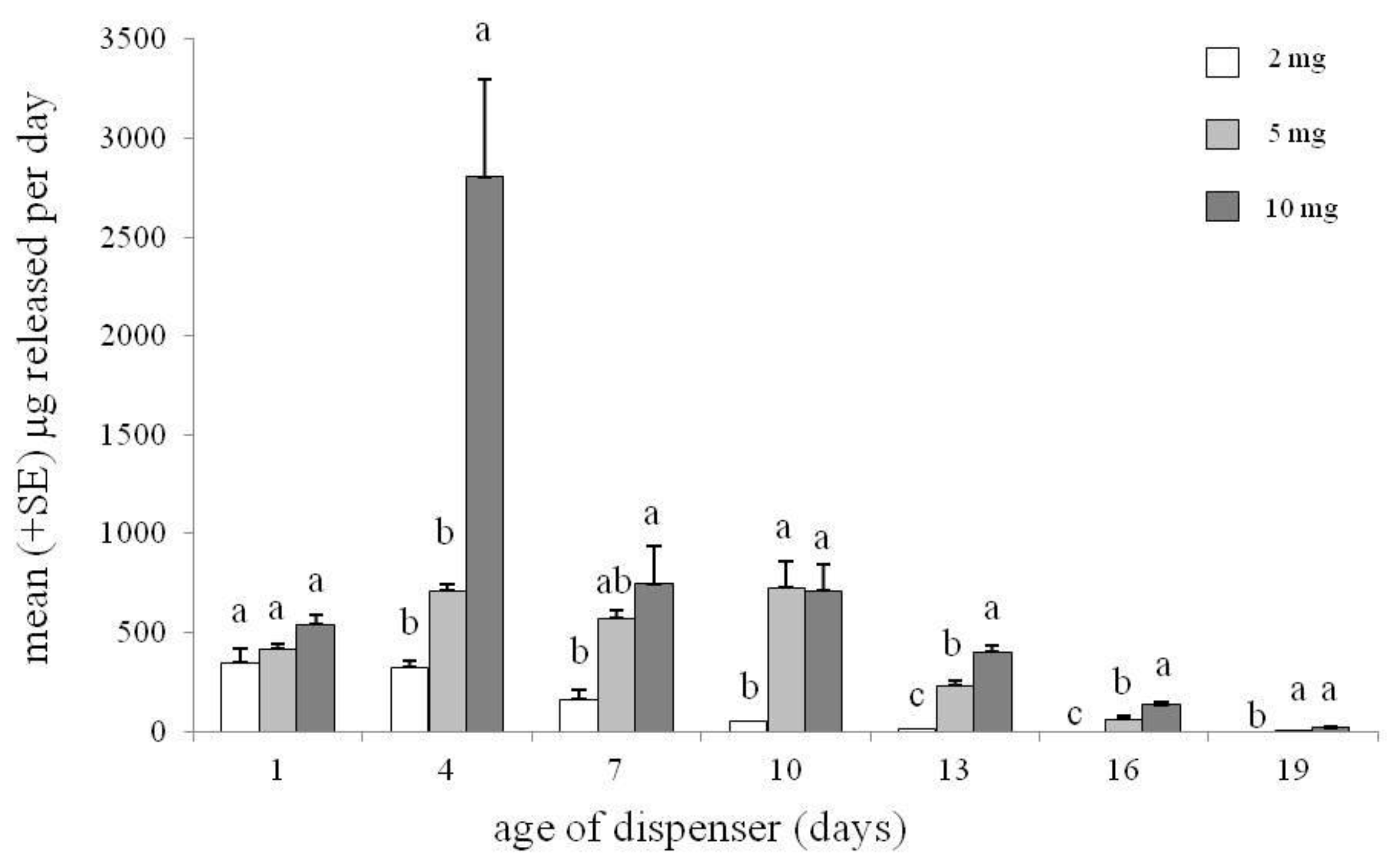
2. Materials and Methods
2.1. Insects and Plants
2.2. Electroantennography (EAG)
2.3. Open Vertical Y-Shaped Olfactometer
2.4. Field Bioassay
2.5. Statistics and Data Analyses
3. Results
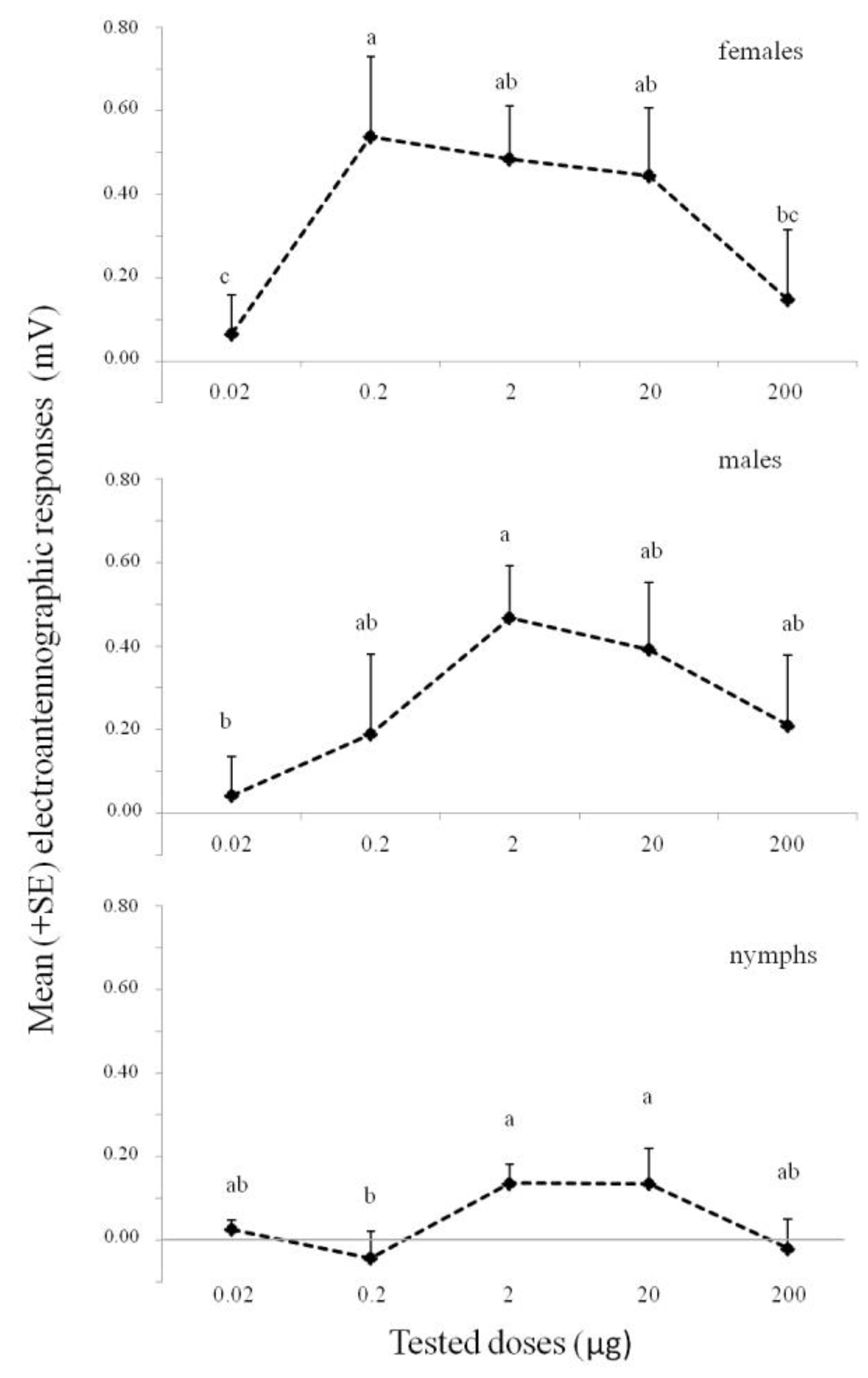
3.1. Electroantennography (EAG)
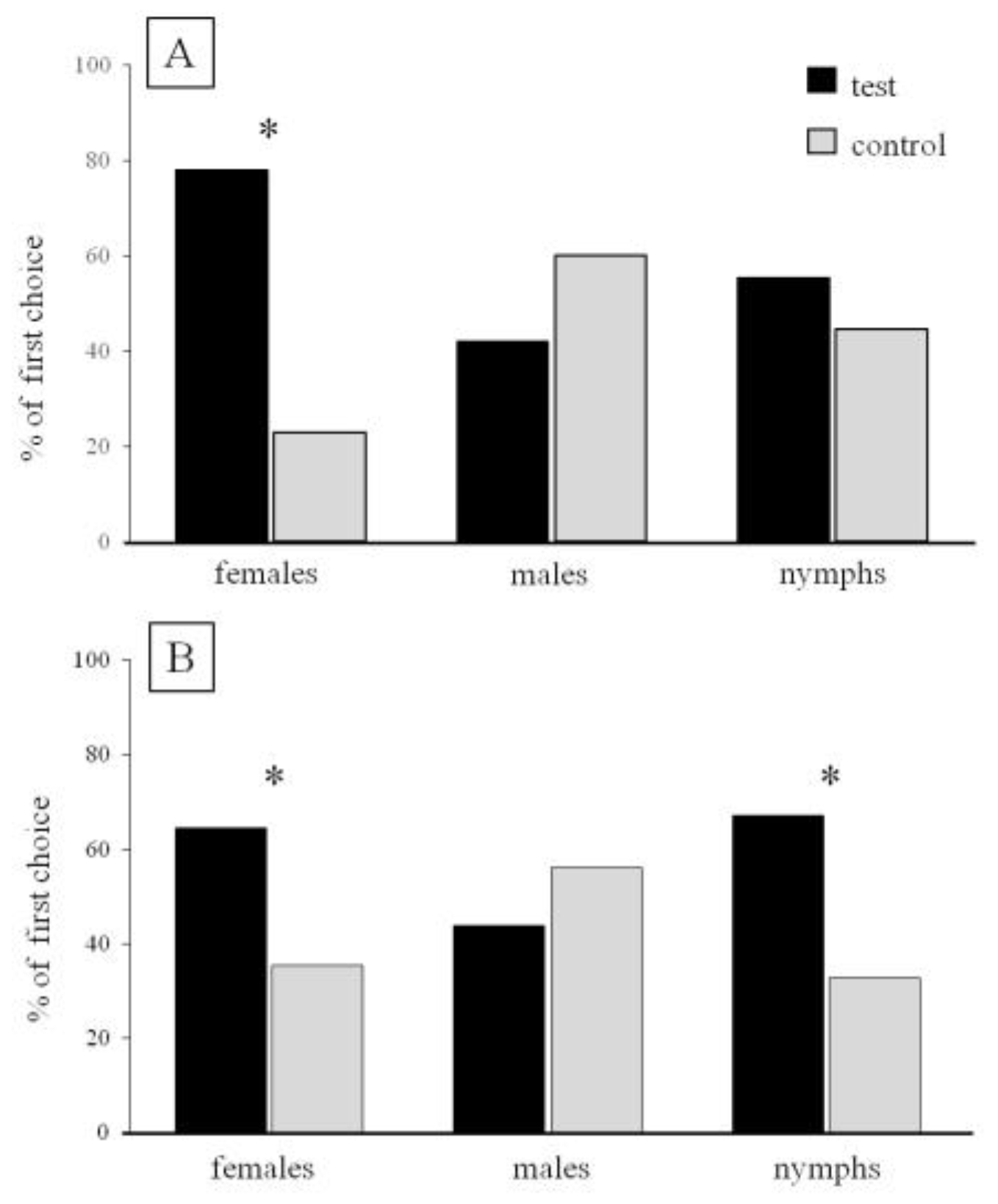
3.2. Open Vertical Y-Shaped Olfactometer
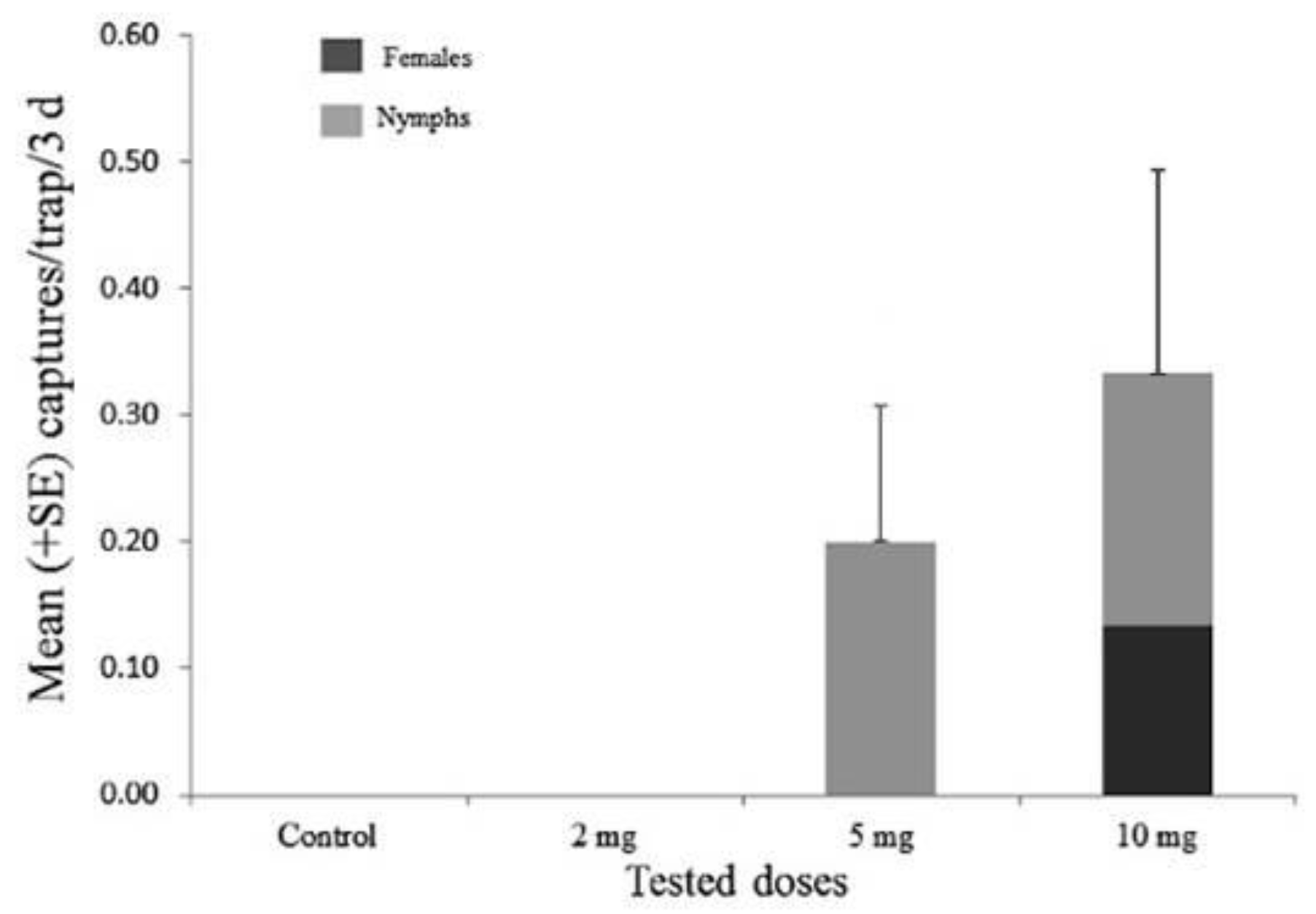
3.3. Field Bioassay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, T.I.; Reed, D.A.; Perring, T.M.; Palumbo, J.C. Feeding damage by Bagrada hilaris (Hemiptera: Pentatomidae) and impact on growth and chlorophyll content of Brassicaceous plant species. Arthropod Plant Interact. 2014, 8, 89–100. [Google Scholar] [CrossRef]
- Reed, D.A.; Palumbo, J.C.; Perring, T.M.; May, C. Bagrada hilaris (Hemiptera: Pentatomidae), an invasive stink bug attacking cole crops in the southwestern United States. J. Integr. Pest Manag. 2013, 4, C1–C7. [Google Scholar] [CrossRef]
- Bundy, C.S.; Perring, T.M.; Reed, D.A.; Palumbo, J.C.; Grasswitz, T.R.; Jones, W.A. Bagrada hilaris. In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 205–242. [Google Scholar]
- Palumbo, J.C.; Natwick, E.T. The bagrada bug (Hemiptera: Pentatomidae): A new invasive pest of cole crops in Arizona and California. Plant Health Prog. 2010, 11, 50. [Google Scholar] [CrossRef]
- Bundy, C.S.; Grasswitz, T.R.; Sutherland, C. First report of the invasive stink bug Bagrada hilaris (Burmeister) (Heteroptera: Pentatomidae) from New Mexico, with notes on its biology. Southwest Entomol. 2012, 37, 411–415. [Google Scholar] [CrossRef]
- Sánchez-Peña, S.R. First record in Mexico of the invasive stink bug Bagrada hilaris, on cultivated crucifers in Saltillo. Southwest Entomol. 2014, 39, 375–378. [Google Scholar] [CrossRef]
- Palumbo, J.C.; Perring, T.M.; Millar, J.G.; Reed, D.A. Biology, ecology, and management of an invasive stink bug, Bagrada hilaris, in North America. Ann. Rev. Entomol. 2016, 61, 453–473. [Google Scholar] [CrossRef]
- Torres-Acosta, R.I.; Sánchez-Peña, S.R.; Torres-Castillo, J.A. Feeding by Bagrada Bug, Bagrada hilaris, on Moringa oleifera (Brassicales: Moringaceae) in Mexico. Southwest Entomol. 2017, 42, 919–923. [Google Scholar] [CrossRef]
- Guarino, S.; Peri, E.; Colazza, S.; Luchi, N.; Michelozzi, M.; Loreto, F. Impact of the invasive painted bug Bagrada hilaris on physiological traits of its host Brassica oleracea var botrytis. Arthropod Plant Interact. 2017, 11, 649–658. [Google Scholar] [CrossRef]
- Guarino, S.; Arif, M.A.; Millar, J.G.; Colazza, S.; Peri, E. Volatile unsaturated hydrocarbons emitted by seedlings of Brassica species provide host location cues to Bagrada hilaris. PLoS ONE 2018, 13, e0209870. [Google Scholar] [CrossRef]
- Baker, R.; Borges, M.; Cooke, N.G.; Herbert, R.H. Identification and synthesis of (Z)-(1ʹ S, 3ʹ R, 4ʹ S)(–)-2-(3ʹ, 4ʹ-epoxy-4ʹ-methylcyclohexyl)-6-methylhepta-2, 5-diene, the sex pheromone of the southern green stinkbug, Nezara viridula (L.). J. Chem. Soc. Chem. Commun. 1987, 6, 414–416. [Google Scholar] [CrossRef]
- Aldrich, J.R. Chemical ecology of the Heteroptera. Ann. Rev. Entomol. 1988, 33, 211–238. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Numata, H.; Borges, M.; Bin, F.; Waite, G.K.; Lusby, W.R. Artifacts and pheromone blends from Nezara spp. and other stink bugs (Heteroptera: Pentatomidae). Z. Naturforschung C. 1993, 48, 73–79. [Google Scholar] [CrossRef]
- Borges, M.; Aldrich, J.R. Attractant pheromone for Nearctic stink bug, Euschistus obscurus (Heteroptera: Pentatomidae): Insight into a Neotropical relative. J. Chem. Ecol. 1994, 20, 1095–1102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borges, M. Attractant compounds of the southern green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae). An. Soc. Entomol. Bras. 1995, 24, 215–225. [Google Scholar]
- Borges, M.; Birkett, M.; Aldrich, J.R.; Oliver, J.E.; Chiba, M.; Murata, Y.; Laumann, R.A.; Barrigossi, J.A.; Pickett, J.A.; Moraes, M.C. Sex attractant pheromone from the rice stalk stink bug, Tibraca limbativentris Stal. J. Chem. Ecol. 2006, 32, 2749. [Google Scholar] [CrossRef]
- Weber, D.C.; Khrimian, A.; Blassioli-Moraes, M.C.; Millar, J.G. Semiochemistry of Pentatomoidea. In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 677–725. [Google Scholar]
- Millar, J.G. Pheromones of true bugs. In The Chemistry of Pheromones and Other Semiochemicals II; Schulz, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 37–84. [Google Scholar]
- Fucarino, A.; Millar, J.G.; McElfresh, J.S.; Colazza, S. Chemical and physical signals mediating conspecific and heterospecific aggregation behavior of first instar stink bugs. J. Chem. Ecol. 2004, 30, 1257–1269. [Google Scholar] [CrossRef]
- Guarino, S.; De Pasquale, C.; Peri, E.; Alonzo, G.; Colazza, S. Role of volatile and contact pheromones in the mating behaviour of Bagrada hilaris (Heteroptera: Pentatomidae). Eur. J. Entomol. 2008, 105, 613. [Google Scholar] [CrossRef]
- Brézot, P.; Malosse, C.; Mori, K.; Renou, M. Bisabolene epoxides in sex pheromone in Nezara viridula (L.) (Heteroptera: Pentatomidae): Role of cis isomer and relation to specificity of pheromone. J. Chem. Ecol. 1994, 20, 3133–3147. [Google Scholar] [CrossRef]
- Millar, J.G. Methyl (2E, 4Z, 6Z)-deca-2, 4, 6-trienoate, a thermally unstable, sex-specific compound from the stink bug Thyanta pallidovirens. Tetrahedron Lett. 1997, 38, 7971–7972. [Google Scholar] [CrossRef]
- Blassioli-Moraes, M.C.; Laumann, R.A.; Oliveira, M.W.M.; Woodcock, C.M.; Mayon, P.; Hooper, A.; Pickett, J.A.; Birkett, M.A.; Borges, M. Sex pheromone communication in two sympatric neotropical stink bug species Chinavia ubica and Chinavia impicticornis. J. Chem. Ecol. 2012, 38, 836–845. [Google Scholar] [CrossRef]
- Krupke, C.H.; Brunner, J.F.; Doerr, M.D.; Kahn, A.D. Field attraction of the stink bug Euschistus conspersus (Hemiptera: Pentatomidae) to synthetic pheromone-baited host plants. J. Econ. Entomol. 2001, 94, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R., III; Allen, M.; Leskey, T.C. Behavioural response of the invasive Halyomorpha halys (Hemiptera: Pentatomidae) to host plant stimuli augmented with semiochemicals in the field. Agric. For. Entomol. 2018, 20, 62–72. [Google Scholar] [CrossRef]
- Ho, H.Y.; Millar, J.G. Identification and synthesis of a male-produced sex pheromone from the stink bug Chlorochroa sayi. J. Chem. Ecol. 2001, 27, 1177–1201. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.R.; Oliver, J.E.; Lusby, W.R.; Kochansky, J.P.; Lockwood, J.A. Pheromone strains of the cosmopolitan pest, Nezara viridula (Heteroptera: Pentatomidae). J. Exp. Zool. 1987, 244, 171–175. [Google Scholar] [CrossRef]
- Panizzi, A.R. Wild hosts of pentatomids: Ecological significance and role in their pest status on crops. Ann. Rev. Entomol. 1997, 42, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.G.; McBrien, H.L.; Ho, H.Y.; Rice, R.E.; Cullen, E.; Zalom, F.G.; Uokl, A. Pentatomid bug pheromones in IPM: Possible applications and limitations. IOBC Wprs Bull. 2002, 25, 241–250. [Google Scholar]
- Aldrich, J.R.; Hoffmann, M.P.; Kochansky, J.P.; Lusby, W.R.; Eger, J.E.; Payne, J.A. Identification and attractiveness of a major pheromone component for Nearctic Euschistus spp. stink bugs (Heteroptera: Pentatomidae). Environ. Entomol. 1991, 20, 477–483. [Google Scholar] [CrossRef]
- Sugie, H.; Yoshida, M.; Kawasaki, K.; Noguchi, H.; Moriya, S.; Takagi, K.; Fukuda, H.; Fujiie, A.; Yamanaka, M.; Ohira, Y.; et al. Identification of the aggregation pheromone of the brown-winged green bug, Plautia stali Scott (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 1996, 31, 427–431. [Google Scholar] [CrossRef]
- Borges, M.; Millar, J.G.; Laumann, R.A.; Moraes, M.C. A male-produced sex pheromone from the neotropical redbanded stink bug, Piezodorus guildinii (W.). J. Chem. Ecol. 2007, 33, 1235–1248. [Google Scholar] [CrossRef]
- McBrien, H.L.; Millar, J.G.; Rice, R.E.; McElfresh, J.S.; Cullen, E.; Zalom, F.G. Sex attractant pheromone of the red-shouldered stink bug Thyanta pallidovirens: A pheromone blend with multiple redundant components. J. Chem. Ecol. 2002, 28, 1797–1818. [Google Scholar] [CrossRef]
- De Oliveira, M.W.M.; Borges, M.; Andrade, C.K.Z.; Laumann, R.A.; Barrigossi, J.A.F.; Blassioli-Moraes, M.C. Zingiberenol,(1 S, 4 R, 1ʹ S)-4-(1ʹ, 5ʹ-dimethylhex-4ʹ-enyl)-1-methylcyclohex-2-en-1-ol, identified as the sex pheromone produced by males of the rice stink bug Oebalus poecilus (Heteroptera: Pentatomidae). J. Agric. Food Chem. 2013, 61, 7777–7785. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, B.H.; Kuwahara, S.; Leal, W.S.; Men, H.C. Synthesis of the racemate of (Z)-exo-α-bergamotenal, a pheromone component of the white-spotted spined bug, Eysarcoris parvus Uhler. Biosci. Biotech. Biochem. 2002, 66, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Fávaro, C.F.; Rodrigues, M.A.D.M.; Aldrich, J.R.; Zarbin, P.H. Identification of semiochemicals in adults and nymphs of the stink bug Pallantia macunaima Grazia (Hemiptera: Pentatomidae). J. Braz. Chem. Soc. 2011, 22, 58–64. [Google Scholar] [CrossRef]
- Weber, D.C.; Walsh, G.C.; Di Meglio, A.S.; Athanas, M.M.; Leskey, T.C.; Khrimian, A. Attractiveness of harlequin bug, Murgantia histrionica, aggregation pheromone: Field response to isomers, ratios, and dose. J. Chem. Ecol. 2014, 40, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Khrimian, A.; Shirali, S.; Vermillion, K.E.; Siegler, M.A.; Guzman, F.; Chauhan, K.; Aldrich, J.A.; Weber, D.C. Determination of the stereochemistry of the aggregation pheromone of harlequin bug, Murgantia histrionica. J. Chem. Ecol. 2014, 40, 1260–1268. [Google Scholar] [CrossRef]
- Takita, M.; Sugie, H.; Tabata, J.; Ishii, S.; Hiradate, S. Isolation and estimation of the aggregation pheromone from Eysarcoris lewisi (Distant) (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 2008, 43, 11–17. [Google Scholar] [CrossRef]
- Khrimian, A.; Zhang, A.; Weber, D.C.; Ho, H.Y.; Aldrich, J.R.; Vermillion, K.E.; Siegler, M.A.; Shirali, S.; Guzman, F.; Leskey, T.C. Discovery of the aggregation pheromone of the brown marmorated stink bug (Halyomorpha halys) through the creation of stereoisomeric libraries of 1-bisabolen-3-ols. J. Nat. Prod. 2014, 77, 1708–1717. [Google Scholar] [CrossRef]
- Aldrich, J.R.; Kochansky, J.P.; Lusby, W.R.; Sexton, J.D. Semiochemicals from a predaceous stink bug, Podisus maculiventris (Hemiptera: Pentatomidae). J. Wash. Acad. Sci. 1984, 74, 39–46. [Google Scholar]
- Fávaro, C.F.; Soldi, R.A.; Ando, T.; Aldrich, J.R.; Zarbin, P.H. (6 R, 10 S)-Pallantione: The first ketone identified as sex pheromone in stink bugs. Org. Lett. 2013, 15, 1822–1825. [Google Scholar] [CrossRef]
- Marques, F.D.A.; McElfresh, J.S.; Millar, J.G. Female-produced sex pheromone of the predatory bug Geocoris punctipes. J. Chem. Ecol. 2000, 26, 2843–2855. [Google Scholar] [CrossRef]
- Leal, W.S.; Ueda, Y.; Ono, M. Attractant pheromone for male rice bug, Leptocorisa chinensis: Semiochemicals produced by both male and female. J. Chem. Ecol. 1996, 22, 1429. [Google Scholar] [CrossRef] [PubMed]
- Pareja, M.; Borges, M.; Laumann, R.A.; Moraes, M.C. Inter-and intraspecific variation in defensive compounds produced by five neotropical stink bug species (Hemiptera: Pentatomidae). J. Insect Physiol. 2007, 53, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Noge, K.; Prudic, K.L.; Becerra, J.X. Defensive roles of (E)-2-alkenals and related compounds in Heteroptera. J. Chem. Ecol. 2012, 38, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, J.A.; Story, R.N. Defensive secretion of the southern green stink bug (Hemiptera: Pentatomidae) as an alarm pheromone. Ann. Entomol. Soc. Am. 1987, 80, 686–691. [Google Scholar] [CrossRef]
- Ruther, J.; Reinecke, A.; Tolasch, T.; Hilker, M. Make love not war: A common arthropod defence compound as sex pheromone in the forest cockchafer Melolontha ippocastani. Oecologia 2001, 128, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Geiselhardt, S.; Ockenfels, P.; Peschke, K. 1-Tridecene—Male-produced sex pheromone of the tenebrionid beetle Parastizopus transgariepinus. Naturwissenschaften 2008, 95, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.; Rössler, T.; Hofferberth, J.; Brummer, M.; Ruther, J.; Stökl, J. A nonspecific defensive compound evolves into a competition avoidance cue and a female sex pheromone. Nat. Commun. 2013, 4, 2767. [Google Scholar] [CrossRef]
- Blum, M.S. Semiochemical parsimony in the Arthropoda. Ann. Rev. Entomol. 1996, 41, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Ota, D.; Čokl, A. Mate location in the southern green stink bug, Nezara viridula (Heteroptera: Pentatomidae), mediated through substrate-borne signals on ivy. J. Insect Behav. 1991, 4, 441–447. [Google Scholar] [CrossRef]
- Ryan, M.A.; Walter, G.H. Sound transmission in Nezara viridula (L.) (Heteroptera: Pentatomidae): Further evidence that signal transmission is substrate-borne. Experientia 1992, 48, 1112–1115. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, M.A.; Guarino, S.; Colazza, S.; Peri, E. The Role of (E)-2-octenyl Acetate as a Pheromone of Bagrada hilaris (Burmeister): Laboratory and Field Evaluation. Insects 2020, 11, 109. https://doi.org/10.3390/insects11020109
Arif MA, Guarino S, Colazza S, Peri E. The Role of (E)-2-octenyl Acetate as a Pheromone of Bagrada hilaris (Burmeister): Laboratory and Field Evaluation. Insects. 2020; 11(2):109. https://doi.org/10.3390/insects11020109
Chicago/Turabian StyleArif, Mokhtar Abdulsattar, Salvatore Guarino, Stefano Colazza, and Ezio Peri. 2020. "The Role of (E)-2-octenyl Acetate as a Pheromone of Bagrada hilaris (Burmeister): Laboratory and Field Evaluation" Insects 11, no. 2: 109. https://doi.org/10.3390/insects11020109
APA StyleArif, M. A., Guarino, S., Colazza, S., & Peri, E. (2020). The Role of (E)-2-octenyl Acetate as a Pheromone of Bagrada hilaris (Burmeister): Laboratory and Field Evaluation. Insects, 11(2), 109. https://doi.org/10.3390/insects11020109







