Biology of Pseudacteon Decapitating Flies (Diptera: Phoridae) That Parasitize Ants of the Solenopsis saevissima Complex (Hymenoptera: Formicidae) in South America
Abstract
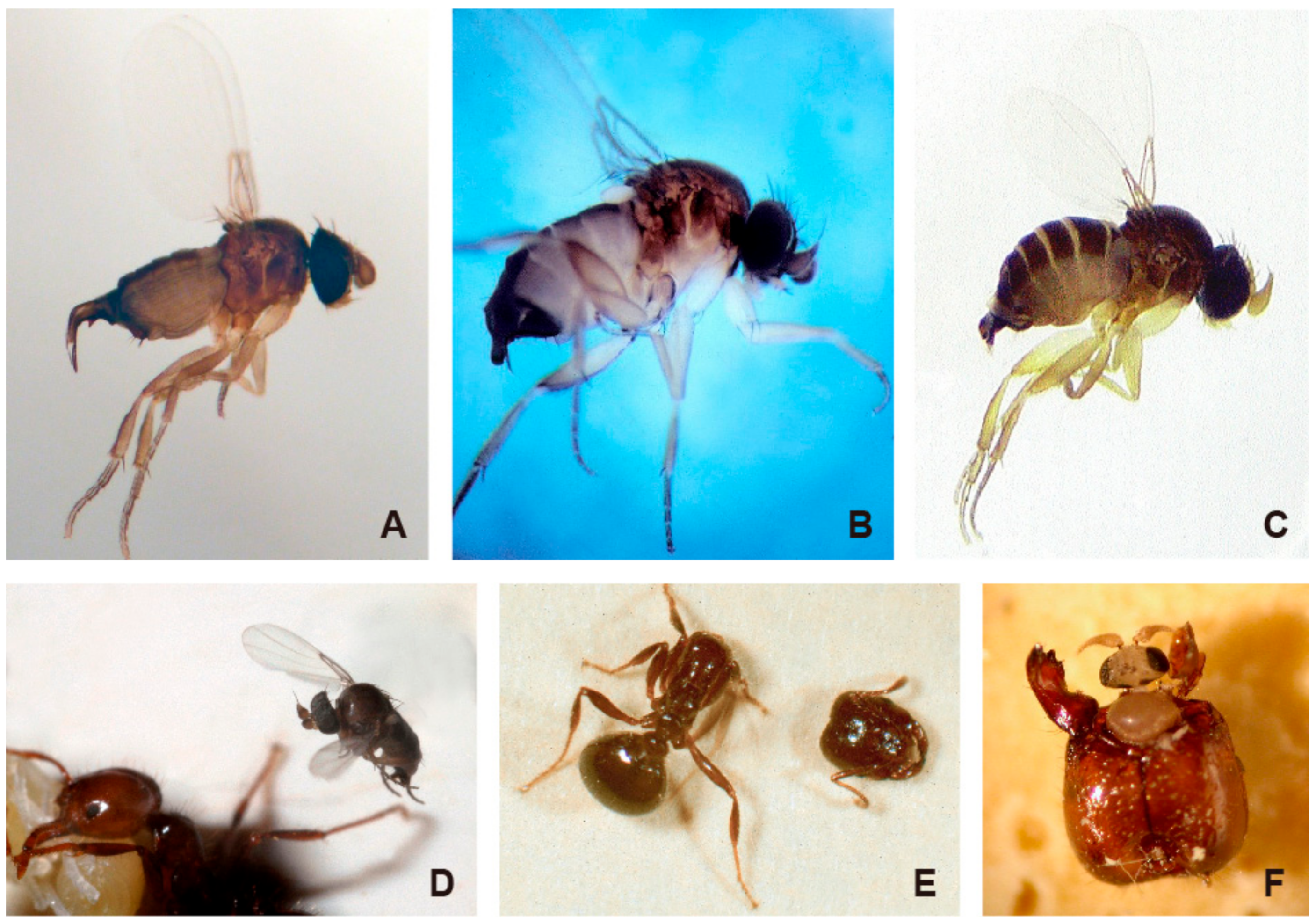
:1. Introduction
2. Parasitic Life of Pseudacteon Phorid Flies
3. Distribution
4. Phenology and Activity Pattern
5. Phorid Fly–Fire Ant Association
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Disney, R.H.L. Scuttle Flies: The Phoridae; Chapman & Hall: London, UK, 1994; p. 467. [Google Scholar]
- Chen, L.; Fadamiro, H.Y. Pseudacteon phorid flies: Host specificity and impacts on Solenopsis fire ants. Annu. Rev. Entomol. 2018, 63, 47–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrock, R.J.W.; Porter, S.D.; Gilbert, L.E.; Folgarait, P.J. Distributional patterns of Pseudacteon associated with the Solenopsis saevissima complex in South America. J. Insect Sci. 2009, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, S.D.; Pesquero, M.A. Illustrated key to Pseudacteon decapitating flies (Diptera: Phoridae) that attack Solenopsis saevissima complex fire ants in South America. Fla. Entomol. 2001, 84, 691–699. [Google Scholar] [CrossRef]
- Estrada, C.; Patrock, R.W.; Folgarait, P.J.; Gilbert, L.E. Host specificity of four Pseudacteon spp. (Diptera: Phoridae), parasitoids of fire ants in Argentina (Hymenoptera: Formicidae). Fla. Entomol. 2006, 89, 462–468. [Google Scholar] [CrossRef]
- Morrison, L.W. Biology of Pseudacteon (Diptera: Phoridae) ant parasitoids and their potential to control imported Solenopsis fire ants (Hymenoptera: Formicidae). Recent Res. Dev. Entomol. 2000, 3, 1–13. [Google Scholar]
- Porter, S.D. Biology and behavior of Pseudacteon decapitating flies (Diptera: Phoridae) that parasitize Solenopsis fire ants (Hymenoptera: Formicidae). Fla. Entomol. 1998, 81, 292–309. [Google Scholar] [CrossRef]
- Porter, S.D.; Alonso, L.E. Host specificity of fire ant decapitating flies (Diptera: Phoridae) in laboratory oviposition tests. J. Econ. Entomol. 1999, 92, 110–114. [Google Scholar] [CrossRef]
- Porter, S.D. Host-specific attraction of Pseudacteon flies (Diptera: Phoridae) to fire ant colonies in Brazil. Fla. Entomol. 1998, 81, 423–429. [Google Scholar] [CrossRef]
- Porter, S.D.; Gilbert, L.E. Assessing host specificity and field release potential of fire ant decapitating flies (Phoridae: Pseudacteon). In Assessing Host Ranges for Parasitoids and Predators Used for Classical Biological Control: A Guide to Best Practice; van Driesche, R.G., Reardon, R., Eds.; Forest Health Technology Enterprise Team-2004-03; USDA Forest Service: Morgantown, WV, USA, 2004; pp. 152–176. [Google Scholar]
- Porter, S.D. Host specificity and risk assessment of releasing the decapitating fly Pseudacteon curvatus as a classical biocontrol agent for imported fire ants. Biol. Control 2000, 19, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Porter, S.D.; Fowler, H.G.; Campiolo, S.; Pesquero, M.A. Host specificity of several Pseudacteon (Diptera: Phoridae) parasites of fire ants (Hymenoptera: Formicidae) in South America. Fla. Entomol. 1995, 78, 70–75. [Google Scholar] [CrossRef]
- Morrison, L.W. Biological control of Solenopsis fire ants by Pseudacteon parasitoids: Theory and practice. Psyche 2012, 2012, 424817. [Google Scholar]
- Feener, D.H., Jr.; Brown, B.V. Diptera as parasitoids. Annu. Rev. Entomol. 1997, 42, 73–97. [Google Scholar] [CrossRef]
- Consoli, F.L.; Wuellner, C.T.; Vinson, S.B.; Gilbert, L.E. Immature development of Pseudacteon tricuspis (Diptera: Phoridae), an endoparasitoid of the red imported fire ant (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 2001, 94, 97–109. [Google Scholar] [CrossRef]
- Porter, S.D.; Pesquero, M.A.; Campiolo, S.; Fowler, H.G. Growth and development of Pseudacteon phorid fly maggots (Diptera: Phoridae) in the heads of Solenopsis fire ant workers (Hymenoptera: Formicidae). Environ. Entomol. 1995, 24, 475–479. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Patrock, R.J.W.; Gilbert, L.E. Development of Pseudacteon nocens (Diptera: Phoridae) on Solenopsis invicta and Solenopsis richteri fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2006, 99, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Folgarait, P.J.; Chirino, M.G.; Patrock, R.J.W.; Gilbert, L.E. Development of Pseudacteon obtusus (Diptera: Phoridae) on Solenopsis invicta and Solenopsis richteri fire ants (Hymenoptera: Formicidae). Environ. Entomol. 2005, 34, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Porter, S.D.; Williams, D.F.; Patterson, R.S. Rearing the decapitating fly Pseudacteon tricuspis (Diptera: Phoridae) in imported fire ants (Hymenoptera: Formicidae) from the United States. J. Econ. Entomol. 1997, 90, 135–138. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Bruzzone, O.A.; Gilbert, L.E. Development of Pseudacteon cultellatus (Diptera: Phoridae) on Solenopsis invicta and Solenopsis richteri fire ants (Hymenoptera: Formicidae). Environ. Entomol. 2002, 31, 403–410. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Bruzzone, O.A.; Patrock, R.J.W.; Gilbert, L.E. Developmental rates and host specificity for Pseudacteon parasitoids (Diptera: Phoridae) of fire ants (Hymenoptera: Formicidae) in Argentina. J. Econ. Entomol. 2002, 95, 1151–1158. [Google Scholar] [CrossRef]
- Porter, S.D.; Briano, J.A. Parasitoid-host matching between the little decapitating fly Pseudacteon curvatus from Las Flores, Argentina and the black fire ant Solenopsis richteri. Fla. Entomol. 2000, 83, 422–427. [Google Scholar] [CrossRef]
- Morrison, L.W.; Dall’Aglio-Holvorcem, C.G.; Gilbert, L.E. Oviposition behavior and development of Pseudacteon flies (Diptera: Phoridae), parasitoids of Solenopsis fire ants (Hymenoptera: Formicidae). Environ. Entomol. 1997, 26, 716–724. [Google Scholar] [CrossRef]
- Fadamiro, H.Y.; Chen, L.; Onagbola, E.O.; Graham, L. Lifespan and patterns of accumulation and mobilization of nutrients in the sugar-fed phorid fly, Pseudacteon tricuspis. Physiol. Entomol. 2005, 30, 212–224. [Google Scholar] [CrossRef]
- Ajayi, O.S.; Fadamiro, H.Y. Comparing longevity of Pseudacteon species of different sizes: Effect of sugar feeding. Physiol. Entomol. 2016, 41, 260–266. [Google Scholar] [CrossRef]
- Chen, L.; Onagbola, E.O.; Fadamiro, H.Y. Effects of temperature, sugar availability, gender, mating, and size on the longevity of phorid fly Pseudacteon tricuspis (Diptera: Phoridae). Environ. Entomol. 2005, 34, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Fadamiro, H.Y. Comparing the effects of five naturally occurring monosaccharide and oligosaccharide sugars on longevity and carbohydrate nutrient levels of a parasitic phorid fly, Pseudacteon tricuspis. Physiol. Entomol. 2006, 31, 46–56. [Google Scholar] [CrossRef]
- Henneberry, T.J.; Jech, L.F.; de la Torre, T.; Hendrix, D.L. Cotton aphid (Homoptera: Aphididae) biology, honeydew production, sugar quality and quantity, and relationships to sticky cotton. Southwest. Entomol. 2000, 25, 161–174. [Google Scholar]
- Zacaro, A.A.; Porter, S.D. Female reproductive system of the decapitating fly Pseudacteon wasmanni Schmitz (Diptera: Phoridae). Arthropod Struct. Dev. 2003, 31, 329–337. [Google Scholar] [CrossRef]
- Calcaterra, L.A.; Delgado, A.; Tsutsui, N.D. Activity patterns and parasitism rates of fire ant-decapitating flies (Diptera: Phoridae: Pseudacteon spp.) in their native Argentina. Ann. Entomol. Soc. Am. 2008, 101, 539–550. [Google Scholar] [CrossRef] [Green Version]
- Campiolo, S.; Pesquero, M.A.; Fowler, H.G. Size-selective oviposition by phorid (Diptera: Phoridae) parasitoids on workers of the fire ant, Solenopsis saevissima (Hymenoptera: Formicidae). Etologia 1994, 4, 85–86. [Google Scholar]
- Fowler, H.G. Morphological prediction of worker size discrimination and relative abundance of sympatric species of Pseudacteon (Dipt., Phoridae) parasitoids of the fire ant, Solenopsis saevissima (Hym., Formicidae) in Brazil. J. Appl. Entomol. 1997, 121, 37–40. [Google Scholar] [CrossRef]
- Morrison, L.W.; Porter, S.D.; Gilbert, L.E. Sex ratio variation as a function of host size in Pseudacteon flies (Diptera: Phoridae), parasitoids of Solenopsis fire ants (Hymenoptera: Formicidae). Biol. J. Linn. Soc. 1999, 66, 257–267. [Google Scholar] [CrossRef]
- Chirino, M.G.; Gilbert, L.E.; Folgarait, P.J. Behavior and development of Pseudacteon curvatus (Diptera: Phoridae) varies according to the social form of its host Solenopsis invicta (Hymenoptera: Formicidae) in its native range. Environ. Entomol. 2009, 38, 198–206. [Google Scholar] [CrossRef]
- Chirino, M.G.; Folgarait, P.J.; Gilbert, L.E. Pseudacteon tricuspis: Its behavior and development according to the social form of its host and the role of interference competition among females. J. Econ. Entomol. 2012, 105, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, L.; Fletcher, D.J.C.; Vinson, S.B. Differences in worker size and mound distribution in monogynous and polygynous colonies of the fire ant Solenopsis invicta Buren. J. Kans. Entomol. Soc. 1985, 58, 9–18. [Google Scholar]
- Morrison, L.W.; Gilbert, L.E. Parasitoid-host relationships when host size varies: The case of Pseudacteon flies and Solenopsis fire ants. Ecol. Entomol. 1998, 23, 409–416. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Bruzzone, O.; Porter, S.D.; Pesquero, M.A.; Gilbert, L.E. Biogeography and macroecology of phorid flies that attack fire ants in south-eastern Brazil and Argentina. J. Biogeogr. 2005, 32, 353–367. [Google Scholar] [CrossRef]
- Trager, J.C. A revision of the fire ants, Solenopsis geminata group (Hymenoptera: Formicidae: Myrmicinae). J. N. Y. Entomol. Soc. 1991, 99, 141–198. [Google Scholar]
- Calcaterra, L.A.; Vander Meer, R.K.; Pitts, J.P.; Livore, J.P.; Tsutsui, N.D. Survey of Solenopsis fire ants and their parasitoid flies (Diptera: Phoridae: Pseudacteon) in central Chile and central western Argentina. Ann. Entomol. Soc. Am. 2007, 100, 512–521. [Google Scholar] [CrossRef]
- Allen, G.E.; Buren, W.F.; Williams, R.N.; Menezes, M.D.; Whitcomb, W.H. The red imported fire ant, Solenopsis invicta; Distribution and habitat in Mato Grosso, Brazil. Ann. Entomol. Soc. Am. 1974, 67, 43–46. [Google Scholar] [CrossRef]
- Buren, W.F.; Allen, G.E.; Whitcomb, W.H.; Lennartz, F.E.; Williams, R.N. Zoogeography of the imported fire ants. J. N. Y. Entomol. Soc. 1974, 82, 113–124. [Google Scholar]
- Tschinkel, W.R. The Fire Ants; Harvard University Press: Cambridge, UK, 2006; p. 723. [Google Scholar]
- Ross, K.G.; Trager, J.C. Systematics and population genetics of fire ants (Solenopsis saevissima complex) from Argentina. Evolution 1990, 44, 2113–2134. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.G.; Shoemaker, D.D. Species delimitation in native South American fire ants. Mol. Ecol. 2005, 14, 3419–3438. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L.W.; Porter, S.D.; Daniels, E.; Korzukhin, M.D. Potential global range expansion of the invasive fire ant, Solenopsis invicta. Biol. Invasions 2004, 6, 183–191. [Google Scholar] [CrossRef]
- Calcaterra, L.A.; Porter, S.D.; Briano, J.A. Distribution and abundance of fire ant decapitating flies (Diptera: Phoridae: Pseudacteon) in three regions of southern South America. Ann. Entomol. Soc. Am. 2005, 98, 85–95. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Patrock, R.J.W.; Gilbert, L.E. The influence of ambient conditions and space on the phenological patterns of a Solenopsis phorid guild in an arid environment. Biol. Control 2007, 42, 262–273. [Google Scholar] [CrossRef]
- Brown, B.V.; Folgarait, P.; Gilbert, L. A new species of Pseudacteon attacking Solenopsis fire ants (Hymenoptera: Formicidae) in Argentina. Sociobiology 2003, 41, 685–688. [Google Scholar]
- Folgarait, P.J.; Bruzzone, O.A.; Gilbert, L.E. Seasonal patterns of activity among species of black fire ant parasitoid flies (Pseudacteon: Phoridae) in Argentina explained by analysis of climatic variables. Biol. Control 2003, 28, 368–378. [Google Scholar] [CrossRef]
- Pesquero, M.A.; Dias, A. Geographical transition zone of Solenopsis fire ants (Hymenoptera: Formicidae) and Pseudacteon fly parasitoids (Diptera: Phoridae) in the state of São Paulo, Brazil. Neotrop. Entomol. 2011, 40, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Fowler, H.G.; Pesquero, M.A.; Campiolo, S.; Porter, S.D. Seasonal activity of species of Pseudacteon (Diptera: Phoridae) parasitoids of fire ant (Solenopsis saevissima) (Hymenoptera: Formicidae) in Brazil. Cientifica 1995, 23, 367–371. [Google Scholar]
- Pesquero, M.A.; Campiolo, S.; Fowler, H.G.; Porter, S.D. Diurnal patterns of ovipositional activity in two Pseudacteon fly parasitoids (Diptera: Phoridae) of Solenopsis fire ants (Hymenoptera: Formicidae). Fla. Entomol. 1996, 79, 455–457. [Google Scholar] [CrossRef]
- Pesquero, M.A.; Vaz, A.P.A.; Arruda, F.V. Laboratory rearing and niche resources of Pseudacteon spp. Coquillett (Diptera: Phoridae) parasitoids of Solenopsis saevissima (Smith) (Hymenoptera: Formicidae). Sociobiology 2013, 60, 484–486. [Google Scholar] [CrossRef] [Green Version]
- Folgarait, P.J.; Patrock, R.J.W.; Gilbert, L.E. Associations of fire ant phorids and microhabitats. Environ. Entomol. 2007, 36, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Feener, D.H., Jr.; Brown, B.V. Reduced foraging of Solenopsis geminata (Hymenoptera: Formicidae) in the presence of parasitic Pseudacteon spp. (Diptera: Phoridae). Ann. Entomol. Soc. Am. 1992, 85, 80–84. [Google Scholar] [CrossRef]
- Orr, M.R.; Seike, S.H.; Gilbert, L.E. Foraging ecology and patterns of diversification in dipteran parasitoids of fire ants in south Brazil. Ecol. Entomol. 1997, 22, 305–314. [Google Scholar] [CrossRef]
- Porter, S.D.; Vander Meer, R.K.; Pesquero, M.A.; Campiolo, S.; Fowler, H.G. Solenopsis (Hymenoptera: Formicidae) fire ant reactions to attacks of Pseudacteon flies (Diptera: Phoridae) in southeastern Brazil. Ann. Entomol. Soc. Am. 1995, 88, 570–575. [Google Scholar] [CrossRef]
- Calcaterra, L.A.; Livore, J.P.; Delgado, A.; Briano, J.A. Ecological dominance of the red imported fire ant, Solenopsis invicta, in its native range. Oecologia 2008, 156, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Mathis, K.A.; Philpott, S.M. Current understanding and future prospects of host selection, acceptance, discrimination, and regulation of phorid fly parasitoids that attack ants. Psyche 2012, 2012, 895424. [Google Scholar] [CrossRef] [Green Version]
- Vander Meer, R.K.; Slowik, T.J.; Thorvilson, H.G. Semiochemicals released by electrically stimulated red imported fire ants, Solenopsis invicta. J. Chem. Ecol. 2002, 28, 2585–2600. [Google Scholar] [CrossRef]
- Vander Meer, R.K.; Porter, S.D. Fire ant, Solenopsis invicta, worker alarm pheromones attract Pseudacteon phorid flies. In Proceedings of the 2002 Imported Fire Ant Conference, Athens, GA, USA, 24–26 March 2002; pp. 77–80. [Google Scholar]
- Barr, C.L.; Calixto, A.A. Electrical stimulation of Solenopsis invicta to enhance phorid fly, Pseudacteon tricuspis, detection. Southwest. Entomol. 2005, 30, 165–168. [Google Scholar]
- Chen, L.; Sharma, K.R.; Fadamiro, H.Y. Fire ant venom alkaloids act as key attractants for the parasitic phorid fly, Pseudacteon tricuspis (Diptera: Phoridae). Naturwissenschaften 2009, 96, 1421–1429. [Google Scholar] [CrossRef]
- Chen, L.; Fadamiro, H.Y. Re-investigation of venom chemistry in Solenopsis fire ants. I. Identification of novel alkaloids in S. richteri. Toxicon 2009, 53, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fadamiro, H.Y. Re-investigation of venom chemistry in Solenopsis fire ants. II. Identification of novel alkaloids in S. invicta. Toxicon 2009, 53, 479–486. [Google Scholar] [CrossRef]
- Williams, D.F.; Banks, W.A. Pseudacteon obtusus (Diptera: Phoridae) attacking Solenopsis invicta (Hymenoptera: Formicidae) in Brazil. Psyche 1987, 94, 9–13. [Google Scholar] [CrossRef]
- Smith, C.R.; Gilbert, L.E. Differential attraction of a parasitoid to dead host ants. Fla. Entomol. 2003, 86, 479–480. [Google Scholar] [CrossRef]
- Pesquero, M.A.; Campiolo, S.; Fowler, H.G. Phorids (Diptera: Phoridae) associated with mating swarms of Solenopsis saevissima (Hymenoptera: Formicidae). Fla. Entomol. 1993, 76, 179–181. [Google Scholar] [CrossRef] [Green Version]
- Alonso, L.E.; Vander Meer, R.K. Source of alate excitant pheromones in the red imported fire ant Solenopsis invicta (Hymenoptera: Formicidae). J. Insect Behav. 1997, 10, 541–555. [Google Scholar] [CrossRef]
- Obin, M.S.; Vander Meer, R.K. Alate semiochemicals release worker behavior during fire ant nuptial flights. J. Entomol. Sci. 1994, 29, 143–151. [Google Scholar] [CrossRef]
| Species | Temp. (°C) | Host Species | Development Time (d) | Reference | ||
|---|---|---|---|---|---|---|
| Larval | Pupal | Total | ||||
| P. borgmeieri | 22–27 | S. richteri | 26.0 | 30.0 | 56.0 | [21] |
| S. invicta | 34.0 | 29.0 | 62.0 | [21] | ||
| P. cultellatus | 22–27 | S. richteri | 20.5 | 27.0 | 47.0 | [21] |
| S. invicta | 19.0 | 23.0 | 42.0 | [21] | ||
| P. curvatus | 22–27 | S. richteri | 13.0 | 18.0 | 31.0 | [21] |
| P. litoralis | 23 | S. invicta | 22.0 | 24.0 | 47.0 | [16] |
| 30 | S. invicta | 18.4 | 18.7 | 37.1 | [23] | |
| P. nocens | 22–27 | S. richteri | 32.0 | 32.5 | 65.0 | [21] |
| S. invicta | 25.0 | 26.5 | 51.5 | [21] | ||
| P. nudicornis | 22–27 | S. richteri | 20.0 | 19.0 | 42.0 | [21] |
| S. invicta | 16.0 | 19.0 | 35.0 | [21] | ||
| P. tricuspis | 22–27 | S. richteri | 19.0 | 17.0 | 38.0 | [21] |
| 24 | S. invicta, hybrid | 20.0 | 19.0 | 39.0 | [19] | |
| 30 | S. invicta | 15.9 | 17.2 | 33.1 | [23] | |
| P. obtusus | 22–27 | S. richteri | 15.0 | 23.0 | 38.0 | [21] |
| S. invicta | 22.0 | 27.0 | 49.0 | [21] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Porter, S.D. Biology of Pseudacteon Decapitating Flies (Diptera: Phoridae) That Parasitize Ants of the Solenopsis saevissima Complex (Hymenoptera: Formicidae) in South America. Insects 2020, 11, 107. https://doi.org/10.3390/insects11020107
Chen L, Porter SD. Biology of Pseudacteon Decapitating Flies (Diptera: Phoridae) That Parasitize Ants of the Solenopsis saevissima Complex (Hymenoptera: Formicidae) in South America. Insects. 2020; 11(2):107. https://doi.org/10.3390/insects11020107
Chicago/Turabian StyleChen, Li, and Sanford D. Porter. 2020. "Biology of Pseudacteon Decapitating Flies (Diptera: Phoridae) That Parasitize Ants of the Solenopsis saevissima Complex (Hymenoptera: Formicidae) in South America" Insects 11, no. 2: 107. https://doi.org/10.3390/insects11020107
APA StyleChen, L., & Porter, S. D. (2020). Biology of Pseudacteon Decapitating Flies (Diptera: Phoridae) That Parasitize Ants of the Solenopsis saevissima Complex (Hymenoptera: Formicidae) in South America. Insects, 11(2), 107. https://doi.org/10.3390/insects11020107





