Flea Communities on Small Rodents in Eastern Poland
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
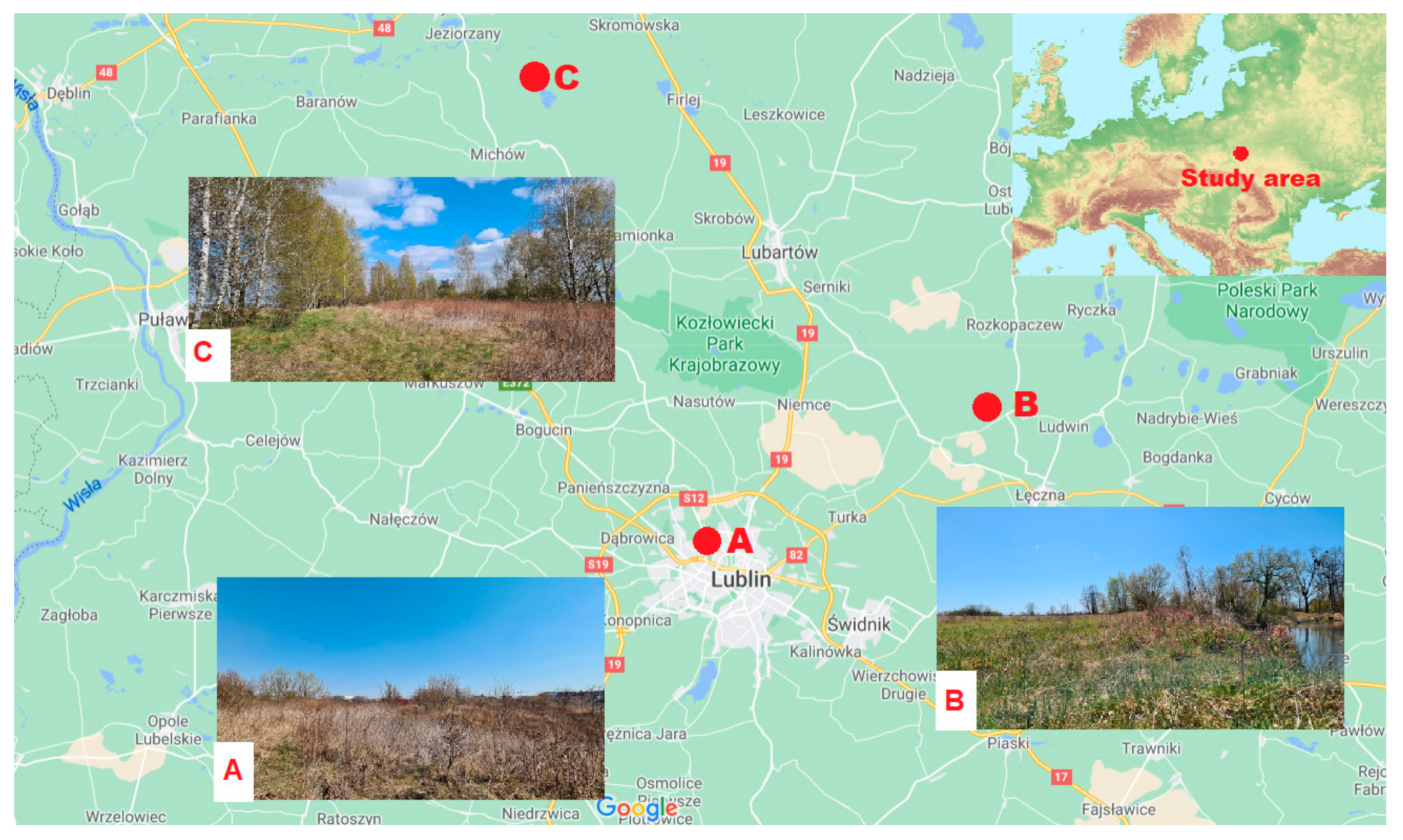
2.1. Study Area
2.2. Rodent Trapping Procedure
2.3. Identification of Flea Species
2.4. Analysis of the Quantitative Structure of Flea Populations and Their Host Preferences
2.5. Analysis of Weather Conditions
2.6. Statistical Analysis
3. Results
3.1. Occurrence and Seasonal Activity of Fleas
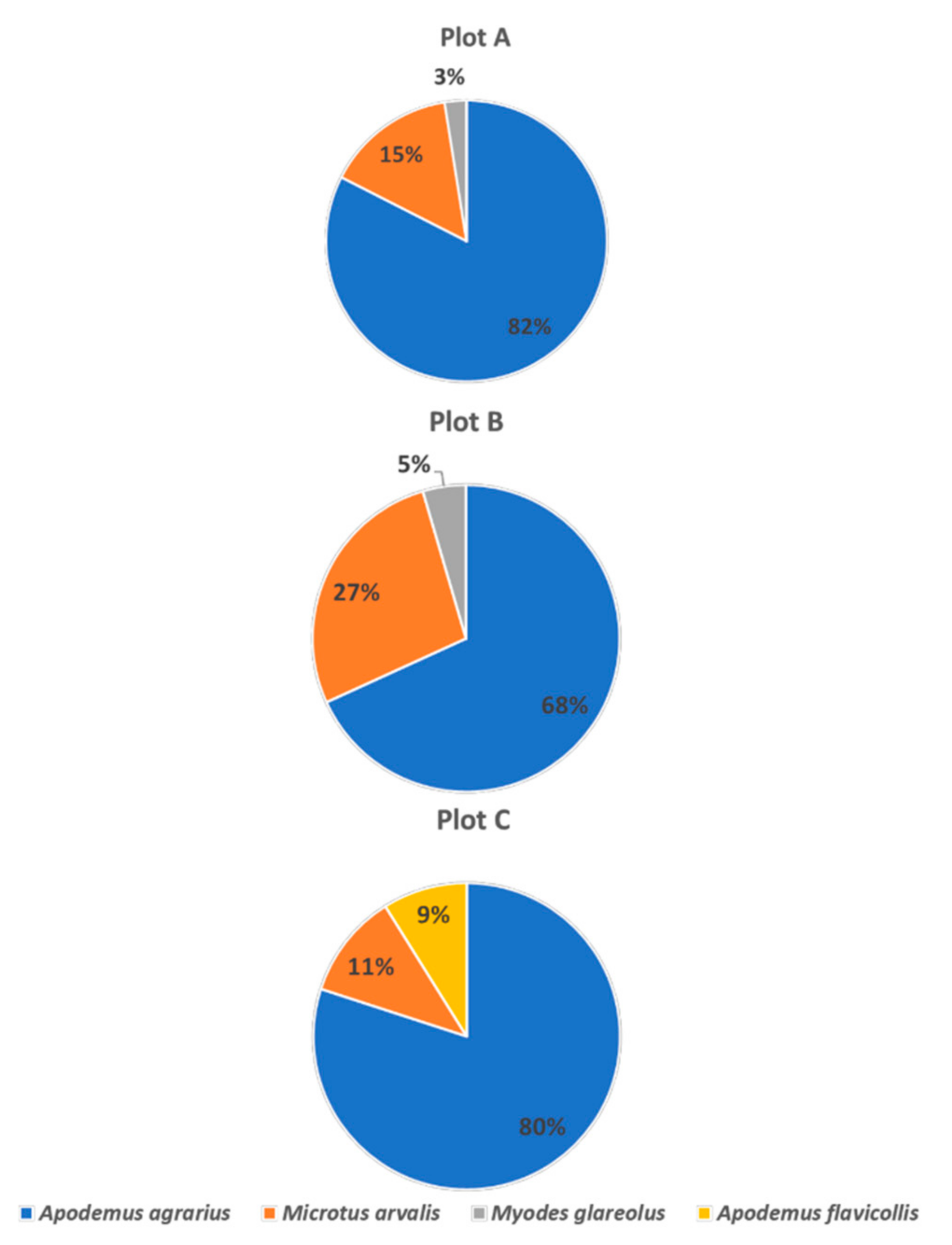
3.2. Flea Host Range
3.3. Weather Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maleki-Ravasan, N.; Solhjouy-Fard, S.; Beaucournu, J.C.; Laudisoit, A.; Mostafavi, E. The fleas (Siphonaptera) in Iran: Diversity, host range, and medical importance. PLoS Negl. Trop. Dis. 2017, 11, e0005260. [Google Scholar] [CrossRef] [PubMed]
- Bitam, I.; Dittmar, K.; Parola, P.; Whiting, M.F.; Raoult, D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Aiguo, Z. The characteristics of geographic distribution of Xenopsylla Cheopis and the current of epidemic situation of the plague. Chin. J. Pest. Cont. 2003, 10, 577–579. [Google Scholar]
- Zurita, A.; Callejón, R.; García-sánchez, Á.M.; Urdapilleta, M.; Lareschi, M.; Cutillas, C. Origin, evolution, phylogeny and taxonomy of Pulex irritans. Med. Vet. Entomol. 2019, 33, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Linardi, P.M.; Calheiros, C.M.L.; Campelo-Junior, E.B.; Duarte, E.M.; Heukelbach, J.; Feldmeier, H. Occurrence of the off-host life stages of Tunga penetrans (Siphonaptera) in various environments in Brazil. Ann. Trop. Med. Parasitol. 2010, 104, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Deka, M.A. Mapping the geographic distribution of tungiasis in Sub-Saharan Africa. Trop. Med. Infect. Dis. 2020, 5, 122. [Google Scholar] [CrossRef]
- Urdapilleta, M.; Linardi, P.M.; Lareschi, M. Fleas associated with sigmodontine rodents and marsupials from the Paranaense Forest in Northeastern Argentina. Acta Trop. 2019, 193, 71–77. [Google Scholar] [CrossRef]
- Slifer, E.H. Chemoreceptors and Other Sense Organs on the Antennal Flagellum of the flea, Ctenocephalides canis (Siphonaptera: Pulicidae). Ann. Entomol. Soc. Am. 1980, 73, 418–419. [Google Scholar] [CrossRef]
- Durden, L.A.; Hinkle, N.C. Fleas (Siphonaptera). In Medical and Veterinary Entomology, 3rd ed.; Mullen, G.R., Durden, L.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 145–169. ISBN 978-0-12-814043-7. [Google Scholar]
- Fielden, L.; Krasnov, B.; Khokhlova, I.; Arakelyan, M. Respiratory gas exchange in the desert flea Xenopsylla ramesis (Siphonaptera: Pulicidae): Response to temperature and blood-feeding. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 557–565. [Google Scholar] [CrossRef]
- Humphries, D. Behavioural aspects of the ecology of the sand martin flea Ceratophyllus styx jordani Smit (Siphonaptera). Parasitology 1969, 59, 311–334. [Google Scholar] [CrossRef]
- Durden, L.A.; Wills, W.; Clark, K.L. The fleas (Siphonaptera) of South Carolina with an assessment of their vectorial importance. J. Vector Ecol 1999, 24, 171–181. [Google Scholar] [PubMed]
- Krasnov, B.R. Functional and Evolutionary Ecology of Fleas: A Model for Ecological Parasitology; Cambridge University Press: Cambridge, UK, 2008; ISBN 978-0521882774. [Google Scholar]
- Sherman, D.M. Tending Animals in the Global Village: A Guide to International Veterinary Medicine; John Wiley & Sons: Baltimore, MD, USA, 2007; ISBN 0-683-18051-7. [Google Scholar]
- Ramana, K.V.; Rao, S.D.; Rao, R.; Mohanty, S.K.; Wilson, C.G. Human dipylidiasis: A case report of Dipylidium caninum infection from Karimnagar. Online J. Health Allied Sci. 2011, 10, 28. [Google Scholar]
- Kandi, V.; Koka, S.S.; Bhoomigari, M.R. Hymenolepiasis in a Pregnant Woman: A Case Report of Hymenolepis nana Infection. Cureus 2019, 11, e3810. [Google Scholar] [CrossRef] [PubMed]
- Youssefi, M.R.; Ebrahimpour, S.; Rezaei, M.; Ahmadpour, E.; Rakhshanpour, A.; Rahimi, M.T. Dermatitis caused by Ctenocephalides felis (cat flea) in human. Casp. J. Intern. Med. 2014, 5, 248–250. [Google Scholar]
- Juckett, G. Arthropod bites. Am. Fam. Physician 2013, 88, 841–847. [Google Scholar] [PubMed]
- Elsheikha, H.M. Flea allergy dermatitis: The continued challenge. Vet. Nurs. J. 2012, 3, 350–356. [Google Scholar] [CrossRef]
- Cucchi-Stefanoni, K.; Juan-Sallés, C.; Parás, A.; Garner, M.M. Fatal anemia and dermatitis in captive agoutis (Dasyprocta mexicana) infested with Echidnophaga fleas. Vet. Parasitol. 2008, 155, 336–339. [Google Scholar] [CrossRef]
- Kowalski, K.; Eichert, U.; Bogdziewicz, M.; Rychlik, L. Differentiation of flea communities infesting small mammals across selected habitats of the Baltic coast, central lowlands, and southern mountains of Poland. Parasitol. Res. 2014, 113, 1725–1734. [Google Scholar] [CrossRef]
- Haitlinger, R. Drobne ssaki bezleśnych wydm nadmorskich i ich fauna pcheł. Przegląd Zool. 1972, 16, 231–237. [Google Scholar]
- Kaczmarek, S.; Mohr, A. Pchły z gniazd nurogesia [Mergus merganser L.]. Wiadomości. Parazytol. 1995, 41, 221–224. [Google Scholar]
- Karbowiak, G.; Solarz, K.; Asman, M.; Wróblewski, Z.; Slivinska, K.; Werszko, J. Phoresy of astigmatic mites on ticks and fleas in Poland. Biol. Lett. 2013, 50, 89–96. [Google Scholar] [CrossRef]
- Haitlinger, R. Arthropods (Acari, Anoplura, Siphonaptera) of small mammals from Kujawsko-Pomorskie Province. Zesz. Nauk. Uniwersyeteu Przyr. Wrocławiu 2011, 63, 59–78. [Google Scholar]
- Haitlinger, R. Arthropod communities occurring on small mammals from non-wooded areas of urban agglomeration of Wroclaw. Acta Parasitol. Pol. 1989, 34, 45–66. [Google Scholar]
- Haitlinger, R. Arthropods (Acari, Anoplura, Siphonaptera) of small mammals of Lubelskie Province. In Zeszyty Naukowe Uniwersytetu Przyrodniczego We Wrocławiu—Biologia i Hodowla Zwierząt; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2010. [Google Scholar]
- Skuratowicz, W. Pchły-Siphonaptera (Aphaniptera). In Klucze do Oznaczania Owadów Polski; Opracowanie Zbiorowe, Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1967. [Google Scholar]
- Kowalski, K. Klucz do Oznaczania Ssaków Polski; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1984; pp. 149–240. [Google Scholar]
- Available online: https://otop.org.pl/wp-content/uploads/2016/04/Rozporz%C4%85dzenie-ministra-srodowiska-w-sprawie-ochrony-gatunkowej-zwierzat-16_12_2016.pdf (accessed on 15 October 2020).
- Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32010L0063&from=EN (accessed on 15 October 2020).
- Baláž, I.; Zigová, M. Flea Communities on Small Mammals in Lowland Environment. Ekológia (Bratisl.) 2020, 39, 260–269. [Google Scholar] [CrossRef]
- Schwerdtfeger, F. Ökologie der Tiere. Band III: Synökologie; Verlag Paul Parey: Hamburg, Germany, 1975. [Google Scholar]
- Margolis, L.; Esch, G.W.; Holmes, J.C.; Kuris, A.M.; Schad, G.A. The use of ecological terms in parasitology (report of an ad hoc committee of the American Society of Parasitologists). J. Parasitol. 1982, 68, 131–133. [Google Scholar] [CrossRef]
- Dudich, A. Synecological Study of Small Mammals’ Ectoparasites of Spruce Forests in Stredné Beskydy Mts. (West Carpathians). Ph.D. Thesis, Technická Univerzita, Zvolen, Slovakia, 1995. [Google Scholar]
- Stanko, M. Striped Field Mouse (Apodemus Agrarius, Rodentia) in Slovakia; Institute of Parasitology, SAS: Košice, Slovakia, 2014. [Google Scholar]
- Zając, Z.; Kulisz, J.; Woźniak, A. The striped field mouse (Apodemus agrarius) as a host of fleas (Siphonaptera) and tapeworms (Cestoda) in suburban environment of Lublin (eastern Poland). Folia Biol. Oecologica 2019, 15, 7–15. [Google Scholar] [CrossRef]
- Vukićević-Radić, O.; Matić, R.; Kataranovski, D.; Stamenković, S. Spatial organization and home range of Apodemus flavicollis and A. agrarius on Mt. Avala, Serbia. Acta Zool. Acad. Sci. Hung. 2006, 52, 81–96. [Google Scholar]
- Kowalski, K.; Bogdziewicz, M.; Eichert, U.; Rychlik, L. Sex differences in flea infections among rodent hosts: Is there a male bias? Parasitol. Res. 2015, 114, 337–341. [Google Scholar] [CrossRef]
- Jacob, J.; Manson, P.; Barfknecht, R.; Fredricks, T. Common vole (Microtus arvalis) ecology and management: Implications for risk assessment of plant protection products. Pest. Manag. Sci. 2014, 70, 869–878. [Google Scholar] [CrossRef]
- Canova, L. Distribution and habitat preference of small mammals in a biotope of the north Italian plain. Ital. J. Zool. 1992, 59, 417–420. [Google Scholar] [CrossRef]
- Devevey, G.; Christe, P. Flea infestation reduces the life span of the common vole. Parasitology 2009, 136, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Heglasová, I.; Víchová, B.; Stanko, M. Detection of Rickettsia spp. in Fleas Collected from Small Mammals in Slovakia, Central Europe. Vec. Borne Zoonotic Dis. 2020, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, B.R.; Stanko, M.; Miklisova, D.; Morand, S. Habitat variation in species composition of flea assemblages on small mammals in central Europe. Ecol. Res. 2006, 21, 460. [Google Scholar] [CrossRef]
- Klimpel, S.; Förster, M.; Schmahl, G. Parasites of two abundant sympatric rodent species in relation to host phylogeny and ecology. Parasitol. Res. 2007, 100, 867. [Google Scholar] [CrossRef]
- Vashchenok, V.S. Species composition, host association and niche differentiation in fleas of small mammals in the Ilmen-Volkhov lowland. Parazitologiia 2006, 40, 425–437. [Google Scholar]
- Fielden, L.J.; Krasnov, B.R.; Still, K.M.; Khokhlova, I.S. Water balance in two species of desert fleas, Xenopsylla ramesis and X. conformis (Siphonaptera: Pulicidae). J. Med. Entomol. 2002, 39, 875–881. [Google Scholar] [CrossRef]
- Knülle, W. Physiological properties and biological implications of the water vapour sorption mechanism in larvae of the oriental rat flea, Xenopsylla cheopis (Roths.). J. Insect Physiol. 1967, 13, 333–357. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Matthee, S.; Lareschi, M.; Korallo-Vinarskaya, N.P.; Vinarski, M.V. Co-occurrence of ectoparasites on rodent hosts: Null model analyses of data from three continents. Oikos 2010, 119, 120–128. [Google Scholar] [CrossRef]
- Krasnov, B.R.; Shenbrot, G.I.; Khokhlova, I.S.; Poulin, R. Is abundance a species attribute? An example with haematophagous ectoparasites. Oecologia 2006, 150, 132–140. [Google Scholar] [CrossRef]
| Plot | Species | Number of Fleas | Statistics | ||
|---|---|---|---|---|---|
| F | M | F + M | |||
| A | C. agyrtes | 48 | 60 | 108 | t = −1.086, p = 0.280 |
| C. assimilis | 0 | 0 | 0 | - | |
| H. talpae | 4 | 2 | 6 | t = 0.837, p = 0.462 | |
| N. fasciatus | 3 | 6 | 9 | t = −1.044, p = 0.299 | |
| B | C. agyrtes | 80 | 91 | 171 | t = −0.767, p = 0.444 |
| C. assimilis | 9 | 11 | 20 | t = −0.447, p = 0.655 | |
| H. talpae | 5 | 7 | 12 | t = −0.562, p = 0.575 | |
| N. fasciatus | 1 | 2 | 3 | t = −0.581, p = 0.562 | |
| C | C. agyrtes | 48 | 66 | 114 | t = −1.740, p = 0.846 |
| C. assimilis | 6 | 3 | 9 | t = −1.038, p = 0.313 | |
| H. talpae | 3 | 5 | 8 | t = −0.646, p = 0.259 | |
| N. fasciatus | 5 | 9 | 14 | t = −1.104, p = 0.272 | |
| Plot | Month | Rodent Species | N | Mean Level of Infestation of Rodents (Number of Fleas/Animal) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. agyrtes | C. assimilis | H. talpae | N. fasciatus | Total F + M | ||||||||||||
| F | M | F + M | F | M | F + M | F | M | F + M | F | M | F + M | |||||
| A | May | A. agrarius | 6 | 0.3 | 1.3 | 1.6 | - | - | - | 0.2 | - | 0.2 | - | - | - | 1.8 |
| M. arvalis | 1 | 1.0 | - | 1.0 | - | - | - | - | - | - | - | - | - | 1.0 | ||
| June | A. agrarius | 9 | 1.0 | 1.2 | 2.2 | - | - | - | 0.3 | 0.1 | 0.4 | 0.1 | 0.1 | 0.3 | 2.9 | |
| M. arvalis | 2 | 1.5 | 3.0 | 4.5 | - | - | - | - | - | - | - | - | - | 4.5 | ||
| July | A. agrarius | 9 | 1.5 | 1.5 | 3.0 | - | - | - | - | - | - | 0.1 | 0.1 | 0.2 | 3.2 | |
| M. arvalis | 1 | 2.0 | 2.0 | 4.0 | - | - | - | - | - | - | - | 1.0 | 1.0 | 5.0 | ||
| August | A. agrarius | 1 | 2.0 | 1.0 | 3.0 | - | - | - | - | - | - | - | - | - | 3.0 | |
| M. arvalis | 1 | - | 3.0 | 3.0 | - | - | - | - | - | - | - | - | - | 3.0 | ||
| M. glareolus | 1 | 2.0 | 1.0 | 3.0 | - | - | - | - | - | - | - | - | - | 3.0 | ||
| September | A. agrarius | 5 | 1.2 | 0.3 | 1.5 | - | - | - | - | - | - | - | - | - | 1.5 | |
| M. arvalis | 1 | - | - | - | - | - | - | - | - | - | 1.0 | 1.0 | 2.0 | 2.0 | ||
| October | A. agrarius | 3 | - | 1.3 | 1.3 | - | - | - | - | - | - | - | 0.3 | 0.3 | 1.6 | |
| B | May | A. agrarius | 4 | 1.7 | 1.5 | - | - | - | - | - | - | - | - | 0.2 | 0.2 | 1.7 |
| M. arvalis | 2 | 1.5 | 0.5 | 2.0 | - | - | - | - | - | - | - | - | - | 2.0 | ||
| June | A. agrarius | 6 | 1.2 | 1.7 | 2.9 | 0.2 | 0.3 | 0.5 | 0.2 | 0.3 | 0.5 | - | - | - | 3.9 | |
| M. arvalis | 4 | 1.5 | - | 1.5 | 0.2 | 0.5 | 0.7 | 0.2 | 0.2 | 0.4 | - | - | - | 2.6 | ||
| July | A. agrarius | 7 | 1.6 | 3.3 | 4.9 | 0.3 | 0.4 | 0.7 | 0.2 | 0.2 | 0.4 | - | 0.2 | 0.2 | 6.2 | |
| M. arvalis | 2 | 2.5 | 3.0 | 5.2 | - | - | - | - | - | - | - | - | - | 5.2 | ||
| M. glareolus | 1 | 2 | 2 | 4.0 | - | - | - | - | - | - | - | - | - | 4.0 | ||
| August | A. agrarius | 8 | 2.8 | 3.1 | 5.9 | 0.1 | 0.1 | 0.2 | - | - | - | - | - | - | 6.1 | |
| M. arvalis | 1 | 4.0 | 5.0 | 9.0 | - | - | - | 1.0 | - | 1.0 | - | - | - | 10.0 | ||
| September | A. agrarius | 4 | 1.8 | 1.5 | 3.3 | 0.2 | - | 0.2 | - | - | - | - | - | - | 3.5 | |
| M. glareolus | 1 | 2.0 | - | 2.0 | - | - | - | - | - | - | - | - | - | 2.0 | ||
| October | A. agrarius | 1 | 2.0 | 2.0 | 4.0 | 1.0 | - | 1.0 | - | - | - | - | - | - | 5.0 | |
| M. arvalis | 3 | 1.3 | 2.0 | 3.3 | - | - | - | - | - | - | - | - | - | 3.3 | ||
| C | May | A. agrarius | 7 | 0.6 | 07 | 1.1 | 0.3 | 0.3 | 0.6 | - | - | - | - | - | - | 1.7 |
| June | A. agrarius | 1 | 1.2 | 1.4 | 2.6 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.3 | - | - | - | 3.1 | |
| A. flavicollis | 1 | - | - | - | - | - | - | - | - | - | 1.0 | - | 1.0 | 1.0 | ||
| July | A. agrarius | 7 | 1.8 | 2.1 | 3.9 | - | - | - | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.3 | 4.4 | |
| A. flavicollis | 1 | 2.0 | 3.0 | 5.0 | - | - | - | - | - | - | - | - | - | 5.0 | ||
| M. arvalis | 1 | 2.0 | - | 2.0 | - | - | - | - | - | - | - | - | - | 2.0 | ||
| August | A. agrarius | 7 | 1.0 | 1.8 | 2.8 | - | - | - | - | 0.1 | 0.1 | 0.1 | - | 0.1 | 3.0 | |
| M. arvalis | 2 | 1.5 | 2.0 | 3.5 | - | - | - | - | - | - | - | - | - | 3.5 | ||
| September | A. agrarius | 8 | 1.1 | 1.2 | 2.3 | 0.1 | 0 | 0.1 | - | - | - | - | 0.5 | 0.5 | 2.9 | |
| A. flavicollis | 2 | - | 1.5 | 1.5 | - | - | - | - | - | - | - | - | - | 1.5 | ||
| October | A. agrarius | 6 | 0.7 | 1.0 | 1.7 | - | - | - | - | - | - | 0.2 | 0.3 | 0.5 | 2.2 | |
| A. flavicollis | 1 | 3.0 | 4.0 | 7.0 | - | - | - | - | - | - | - | - | - | 7.0 | ||
| M. arvalis | 2 | - | - | - | - | - | - | 0.5 | 0.5 | 1.0 | 0.5 | 0.5 | 1.0 | 2.0 | ||
| Flea Species | D (%) | P (%) |
|---|---|---|
| C. agyrtes | 82.91 | 84 |
| C. assimilis | 6.11 | 12 |
| H. talpae | 5.49 | 11 |
| N. fasciatus | 5.49 | 15 |
| Flea Species | Rodent Species | |||
|---|---|---|---|---|
| A. agrarius | A. flavicolis | M. arvalis | M. glareolus | |
| C. agyrtes | 2.47 | 3.00 | 3.25 | 2.25 |
| C. assimilis | 0.21 | 0.20 | 0.13 | - |
| H. talpae | 0.17 | - | 0.25 | - |
| N. fasciatus | 0.16 | 0.20 | 0.25 | - |
Publisher’sNote: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, Z.; Kulisz, J.; Woźniak, A. Flea Communities on Small Rodents in Eastern Poland. Insects 2020, 11, 894. https://doi.org/10.3390/insects11120894
Zając Z, Kulisz J, Woźniak A. Flea Communities on Small Rodents in Eastern Poland. Insects. 2020; 11(12):894. https://doi.org/10.3390/insects11120894
Chicago/Turabian StyleZając, Zbigniew, Joanna Kulisz, and Aneta Woźniak. 2020. "Flea Communities on Small Rodents in Eastern Poland" Insects 11, no. 12: 894. https://doi.org/10.3390/insects11120894
APA StyleZając, Z., Kulisz, J., & Woźniak, A. (2020). Flea Communities on Small Rodents in Eastern Poland. Insects, 11(12), 894. https://doi.org/10.3390/insects11120894







