Testing Seven Hypotheses to Determine What Explains the Current Planthopper (Fulgoridae) Geographical and Species Richness Patterns in China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
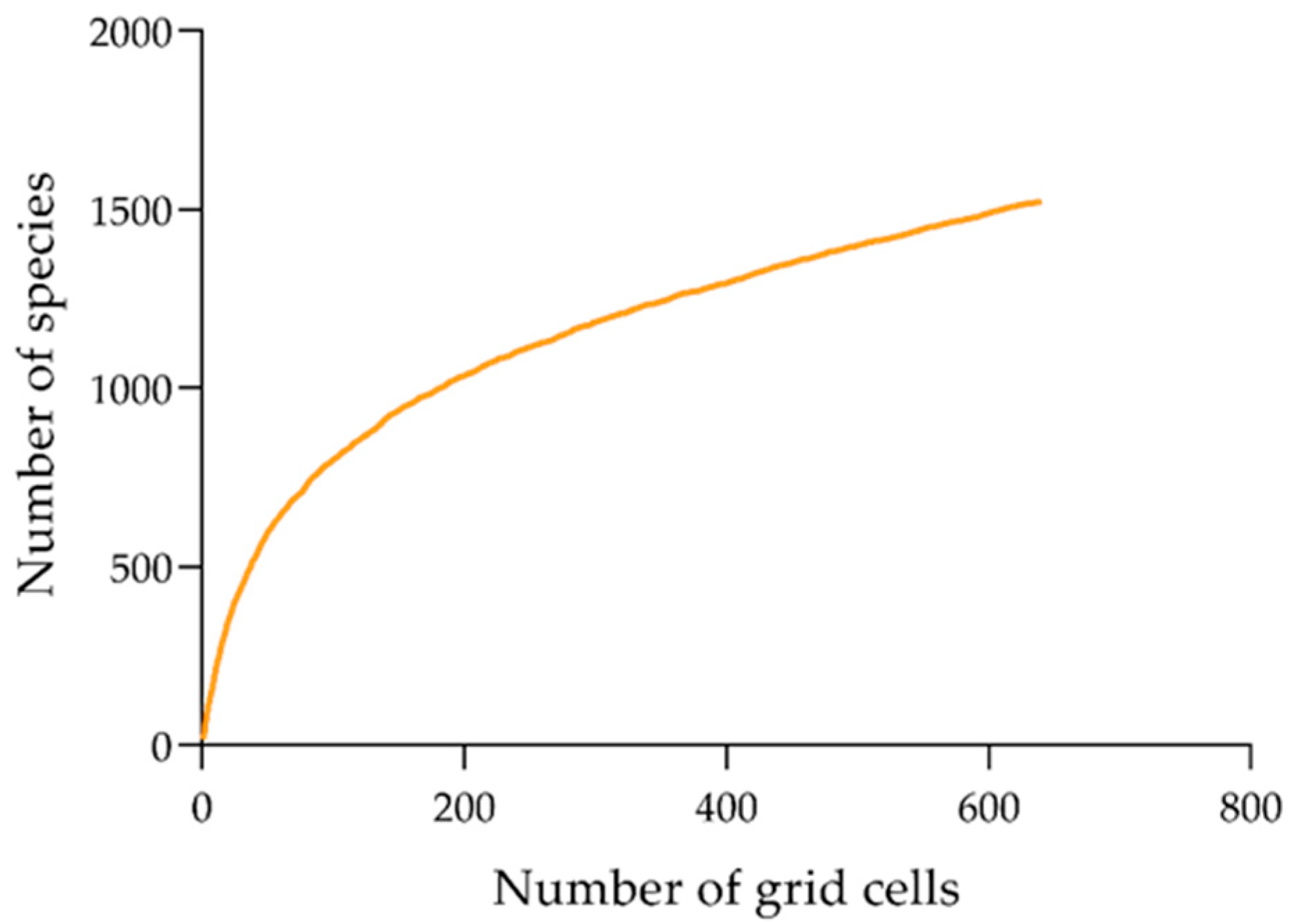
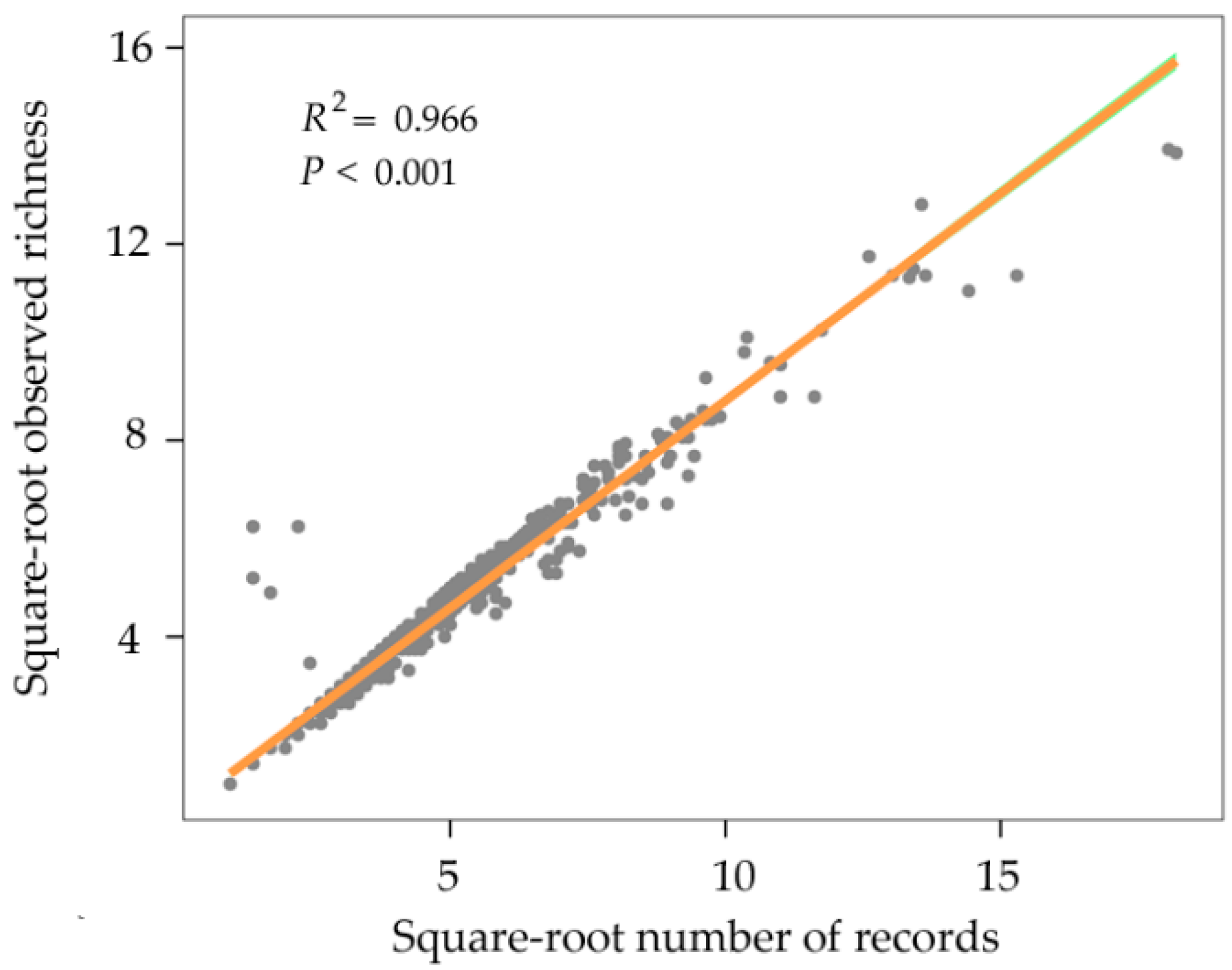
2.1. Species Distributional Data
2.2. Environmental Data
2.3. Statistical Analyses
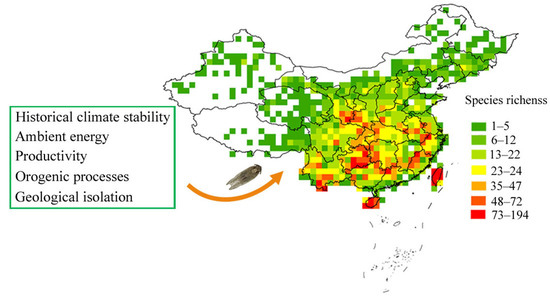
3. Results
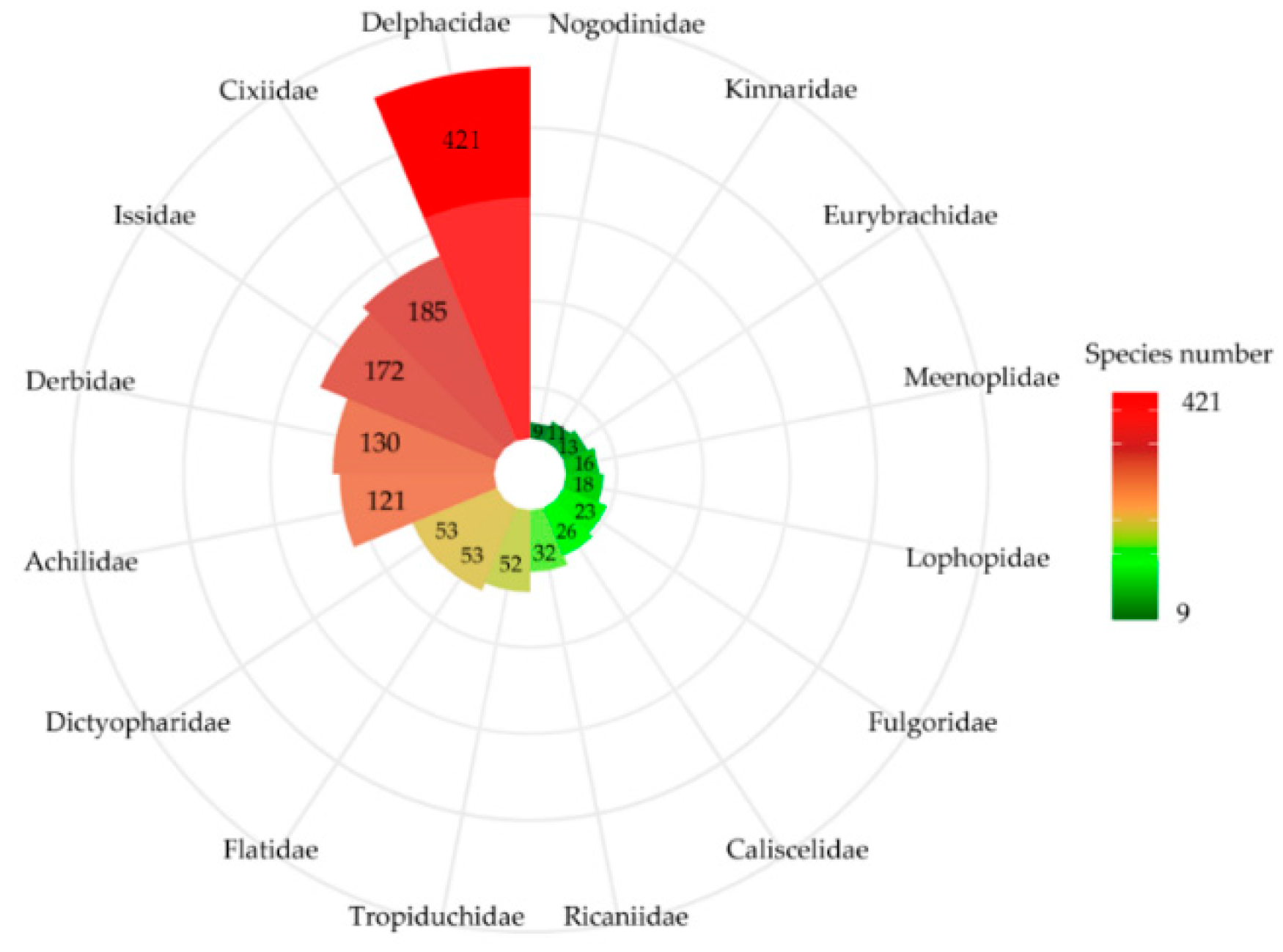
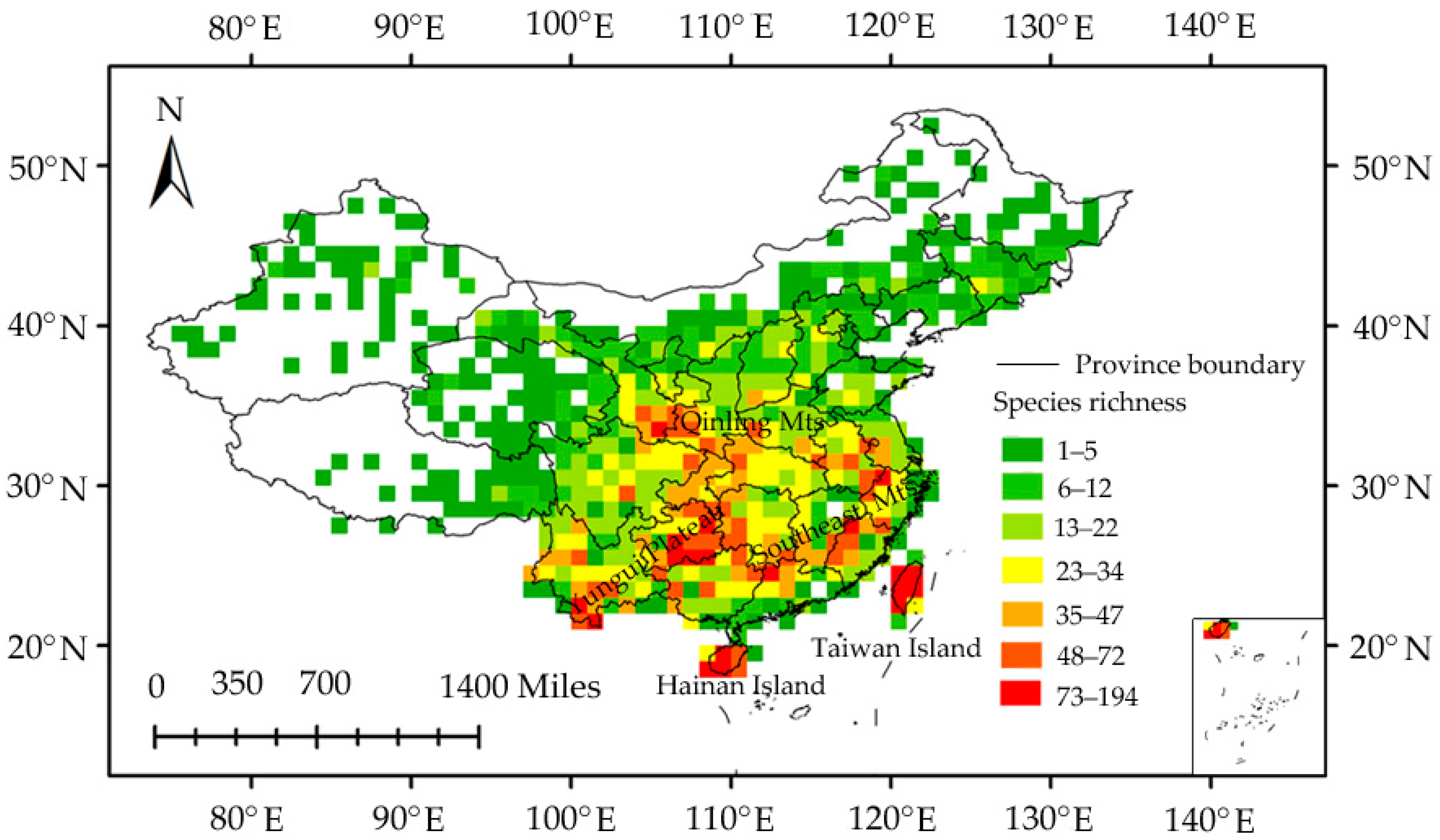
3.1. Planthopper Taxonomic Diversity
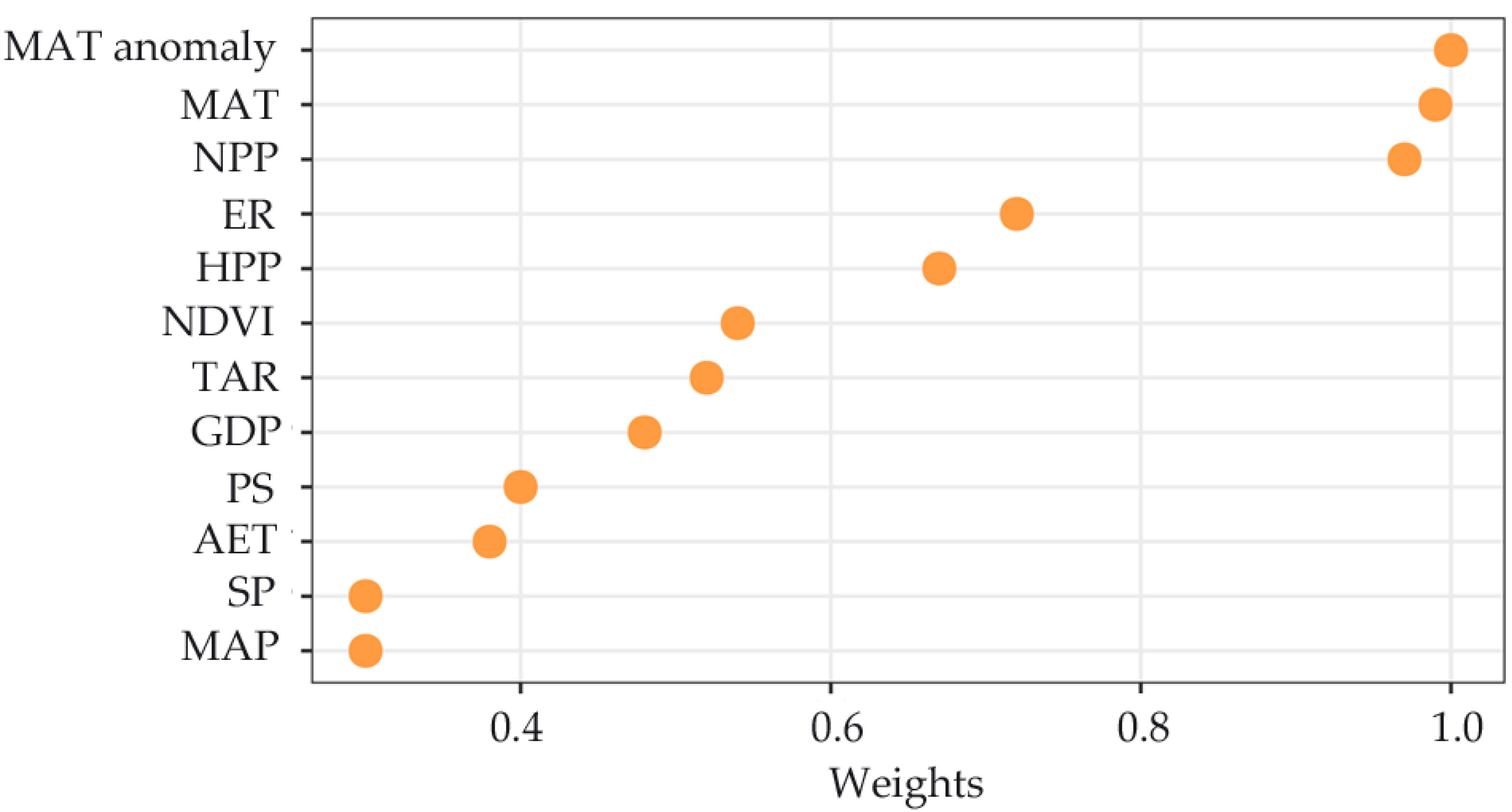
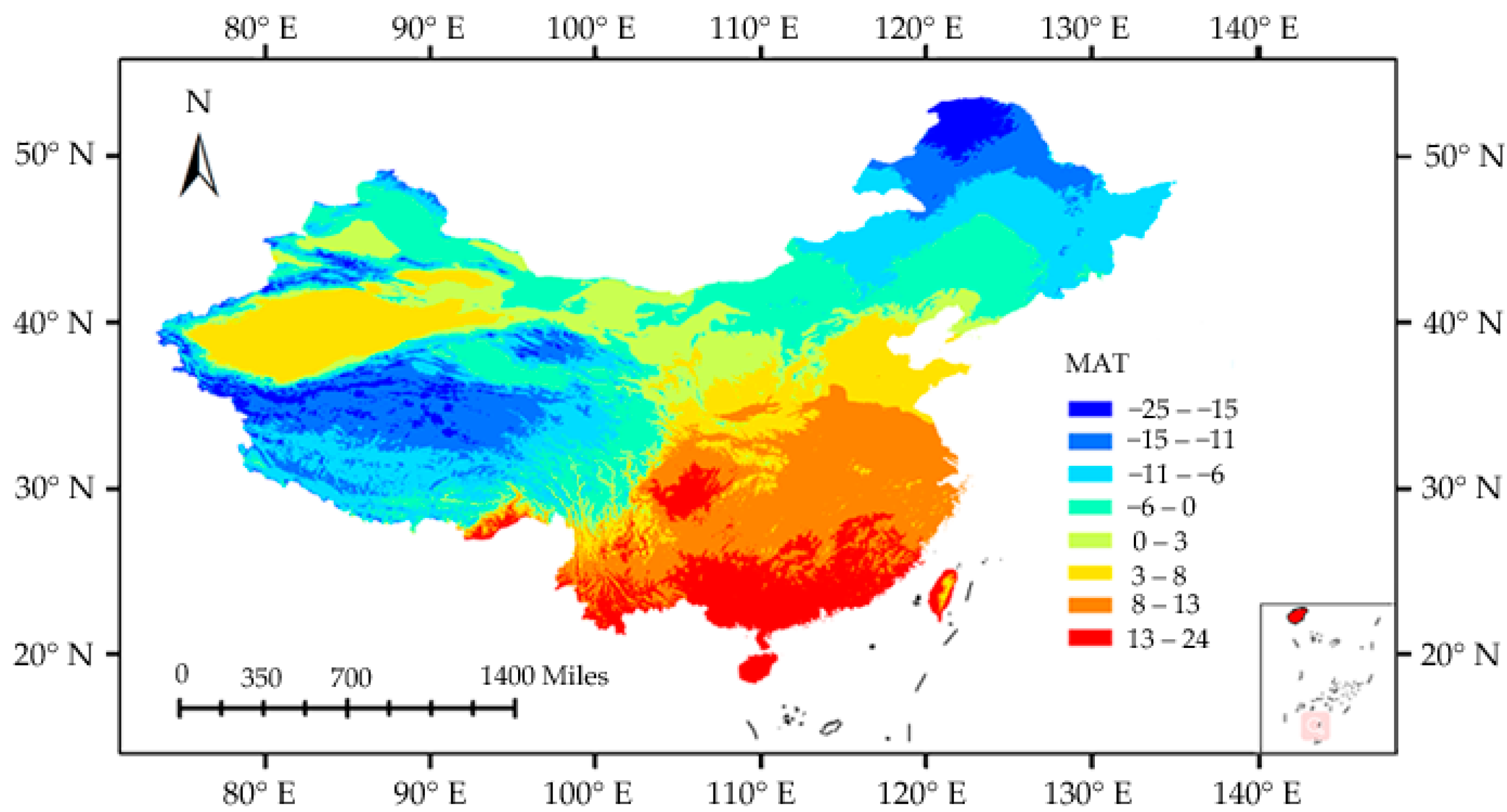
3.2. Species Richness–Environment Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Currie, D.J. Energy and large–scale patterns of animal–and plant–species richness. Am. Nat. 1991, 137, 27–49. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H. On the generality of the latitudinal gradient. Am. Nat. 2004, 163, 192–211. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Qian, H. Global tests of regional effect on species richness of vascular plants and terrestrial vertebrates. Ecography 2009, 32, 553–560. [Google Scholar] [CrossRef]
- Field, R.; Hawkins, B.A.; Cornell, H.V.; Currie, D.J.; Diniz–Filho, J.A.F.; Guégan, J.F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; et al. Spatial species–richness gradients across scales: A meta–analysis. J. Biogeogr. 2009, 36, 132–147. [Google Scholar] [CrossRef]
- Araújo, M.B.; David, N.B.; Diniz–Filho, J.A.F.; Haywood, A.M.; Valdes, P.J.; Rahbek, C. Quaternary climate changes explain diversity among reptiles and amphibians. Ecography 2008, 31, 8–15. [Google Scholar] [CrossRef]
- Sandel, B.; Arge, L.; Dalsgaard, B.; Davies, R.G.; Gaston, K.J.; Sutherland, J.; Svenning, J.C. The influence of Late Quaternary climate–change velocity on species endemism. Science 2011, 334, 660–664. [Google Scholar] [CrossRef]
- Liu, Y.P.; Shen, Z.H.; Wang, Q.G.; Su, X.Y.; Zhang, W.J.; Shrestha, N.; Xu, X.Y.; Wang, Z.H. Determinants of richness patterns differ between rare and common species: Implications for Gesneriaceae conservation in China. Divers. Distrib. 2017, 23, 235–246. [Google Scholar] [CrossRef]
- Wright, D.H. Species–energy theory: An extension of species–area theory. Oikos 1983, 41, 496–506. [Google Scholar] [CrossRef]
- O’Brien, E.M. Climatic gradients in woody plant species richness: Towards an explanation based on an analysis of southern Africa’s woody flora. J. Biogeogr. 1993, 20, 181–198. [Google Scholar] [CrossRef]
- Kerr, J.T.; Packer, L. Habitat heterogeneity as a determinant of mammal species richness in high–energy regions. Nature 1997, 385, 252–254. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Field, R.; Cornell, H.V.; Currie, D.J.; Guégan, J.F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; O’Brien, E.M.; et al. Energy, water, and broad–scale geographic patterns of species richness. Ecology 2003, 84, 3105–3177. [Google Scholar] [CrossRef]
- Feng, G.; Yan, H.; Yang, X.T. Climate and food diversity as drivers of mammal diversity in Inner Mongolia. Ecol. Evol. 2019, 9, 2142–2148. [Google Scholar] [CrossRef]
- Xu, X.T.; Dimitrov, D.; Shrestha, N.; Rahbek, C.; Wang, Z.H. A consistent species richness–climate relationship for oaks across the northern Hemisphere. Glob. Ecol. Biogeogr. 2019, 28, 1051–1066. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, Z.G.; Ying, L.X.; Zang, R.G.; Jiang, Y.X. Effects of current climate, paleo–climate, and habitat heterogeneity in determining biogeographical patterns of evergreen broad–leaved woody plants in China. J. Geogr. Sci. 2019, 29, 1142–1158. [Google Scholar] [CrossRef]
- Dong, Y.J.; Wu, N.Q.; Li, F.J.; Chen, X.Y.; Zhang, D.; Zhang, Y.T.; Huang, L.P.; Wu, B.; Lu, H.Y. Influence of monsoonal water–energy dynamics on terrestrial mollusk species–diversity gradients in northern China. Sci. Total Environ. 2019, 676, 206–214. [Google Scholar] [CrossRef]
- Currie, D.J.; Mittelbach, G.G.; Cornell, H.V.; Field, R.; Guégan, J.F.; Hawkins, B.A.; Kaufman, D.M.; Kerr, J.T.; Oberdorff, T.; O’Brien, M.; et al. Predictions and tests of climate–based hypotheses of broad–scale variation in taxonomic richness. Ecol. Lett. 2004, 7, 1121–1134. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Porter, E.P.; Diniz–Filho, J.A.F. Productivity and history as predictors of the latitudinal diversity gradient of terrestrial birds. Ecology 2003, 84, 1608–1623. [Google Scholar] [CrossRef]
- Francis, A.P.; Currie, D.J. A globaly consistent richness–climate relationship for angiosperms. Am. Nat. 2003, 161, 523–536. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Porter, E.E. Relative influences of current and historical factors on mammal and bird diversity patterns in deglaciated North America. Glob. Ecol. Biogeogr. 2003, 12, 475–481. [Google Scholar] [CrossRef]
- Feng, G.; Mao, L.F.; Sandel, B.; Swenson, N.G. High plant endemism in China is partially linked to reduced glacial-interglacial climate change. J. Biogeogr. 2016, 43, 145–154. [Google Scholar] [CrossRef]
- Feng, G.; Ma, Z.Y.; Sandel, B.; Mao, L.F.; Normand, N.; Ordonez, A.; Svenning, J.C. Species and phylogenetic endemism in angiosperm trees across the Northern Hemisphere are jointly shaped by modern climate and glacial–interglacial climate change. Glob. Ecol. Biogeogr. 2019, 28, 1393–1402. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, H.H.; Wu, Y.X.; Zeng, L.D.; Huang, X.L. Spatial patterns and determinants of the diversity of Hemipteran insects in the Qinghai-Tibetan Plateau. Front. Ecol. Evol. 2019, 7, 165. [Google Scholar] [CrossRef]
- Sosa, V.; Loera, I. Influence of current climate, historical climate stability and topography on species richness and endemism in Mesoamerican geophyte plants. PeerJ 2017, 5, e3932. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Jin, B.C.; Zhou, Z.X.; Yan, L.; Long, J.K.; Chen, X.S. Determinants of Delphacidae richness and endemism in China. Ecol. Entomol. 2020, 45, 1396–1407. [Google Scholar] [CrossRef]
- Burrows, M.; Sutton, G.P. Interacting gears synchronize propulsive leg movements in a jumping insect. Science 2013, 341, 1254–1256. [Google Scholar] [CrossRef]
- Burrows, M. Jumping mechanisms in Flatid planthoppers (Hemiptera, Flatidae). J. Exp. Biol. 2014, 217, 2590–2600. [Google Scholar] [CrossRef]
- Wilson, S.W.; Mitter, C.; Denno, R.F.; Wilson, M.R. Evolutionary patterns of host plant use by delphacid planthoppers and their relatives. In Planthoppers: Their Ecology and Management; Denno, R.F., Perfect, T.J., Eds.; Chapman and Hall: New York, NY, USA, 1994; pp. 7–45. [Google Scholar]
- Wheeler, A.G., Jr. Bryophagy in the Auchenorrhyncha: Seasonal history and habits of a moss specialist, Javesella Opaca (Beamer) (Fulgoroidea: Delphacidae). Proc. Entomol. Soc. Wash. 2003, 105, 599–610. [Google Scholar]
- O’Brien, L.B.; Wilson, S.W. Planthopper systematics and externalmorphology. In The Leafhoppers and Planthoppers; Nault, L.R., Rodriguez, J.G., Eds.; Wiley: New York, NY, USA, 1985; pp. 61–102. [Google Scholar]
- Nault, L.R.; Ammar, E.D. Leafhopper and planthopper transmission of plant viruses. Annu. Rev. Entomol. 1989, 34, 503–529. [Google Scholar] [CrossRef]
- Gatineau, F.; Larrue, J.; Clair, D.; Lorton, F.; Richardmolard, M.; Boudonpadieu, E. A new natural planthopper vector of stolbur phytoplasma in the genus pentastiridius (Hemiptera: Cixiidae). Eur. J. Plant Pathol. 2001, 107, 263–271. [Google Scholar] [CrossRef]
- Arocha, Y.; López, M.; Fernández, M.; Piñol, B.; Horta, D.; Peralta, E.L.; Almeida, R.; Carvajal, O.; Picornell, S.; Wilson, M.R.; et al. Transmission of a sugarcane yellow leaf phytoplasma by the delphacid planthopper Saccharosydne saccharivora, a new vector of sugarcane yellow leaf syndrome. Plant Pathol. 2005, 54, 634–642. [Google Scholar] [CrossRef]
- Wilson, S.W. Keys to the families of Fulgoromorpha with emphasis on planthoppers of potential economic importance in the southeastern United States (Hemiptera: Auchenorrhyncha). Fla. Entomol. 2005, 88, 464–481. [Google Scholar] [CrossRef]
- Liang, A.P. Cladistic biogeography of Cercopoidea and Fulgoroidea (Insecta: Homoptera) in China and adjacent regions. Acta Zootaxonomica Sin. 1998, 23, 132–167. [Google Scholar]
- Qian, H.; Wang, S.L.; Li, Y.L.; Wang, X.H. Breeding bird diversity in relation to environmental gradients in China. Acta Oecolo. 2009, 35, 819–823. [Google Scholar] [CrossRef]
- Luo, Z.H.; Tang, S.H.; Li, C.W.; Fang, H.X.; Hu, H.J.; Yang, J.; Ding, J.J.; Jiang, Z.G. Environmental effects on vertebrate species richness: Testing the energy, environmental stability and habitat heterogeneity hypotheses. PLoS ONE 2012, 7, e35514. [Google Scholar]
- Ricklefs, R.E. Community diversity: Relative roles of local and regional processes. Science 1987, 235, 167–171. [Google Scholar] [CrossRef]
- Hortal, J.; Lobo, J.M.; Jimenez–Valverde, A. Limitations of biodiversity databases: Case study on seed–plant diversity in Tenerife, Canary Islands. Conserv. Biol. 2007, 21, 853–863. [Google Scholar] [CrossRef]
- Yang, W.J.; Ma, K.P.; Kreft, H. Geographical sampling bias in a large distributional database and its effects on species richness–environment models. J. Biogeogr. 2013, 40, 1415–1426. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. In User’s Guide and Application; EstimateS: Storrs, CT, USA, 2013. [Google Scholar]
- Dutilleul, P.; Clifford, P.; Richardson, S.; Hemon, D. Modifying the t test for assessing the correlation between two spatial processes. Biometrics 1993, 49, 305–314. [Google Scholar] [CrossRef]
- Rangel, T.F.; Diniz–Filho, J.A.F.; Bini, L.M. SAM: A comprehensive application for spatial analysis in macroecology. Ecography 2010, 33, 46–50. [Google Scholar] [CrossRef]
- Kissling, W.D.; Carl, G. Spatial autocorrelation and the selection of simultaneous autoregressive models. Glob. Ecol. Biogeogr. 2008, 17, 59–71. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bartoń, K. MuMIn: Multi–Model Inference, R package version 1.43.17; 2018. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 15 September 2020).
- Bivand, R.; Altman, M.; Anselin, L.; Assunção, R.; Berke, O.; Bernat, A.; Blanchet., G.; Blankmeyer, E.; Carvalho, M.; Christensen, B.; et al. spdep: Spatial Dependence: Weighting Schemes, Statistics and Models, R package version 1.1–2; 2019. Available online: https://CRAN.R-project.org/package=spdep (accessed on 20 May 2020).
- Dynesius, M.; Jansson, R. Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc. Natl. Acad. Sci. USA 2000, 97, 9115–9120. [Google Scholar] [CrossRef] [PubMed]
- Jansson, R.; Dynesius, M. The fate of clades in a world of re-current climatic change: Milankovitch oscillations and evolution. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 741–777. [Google Scholar] [CrossRef]
- Jansson, R. Global patterns in endemism explained by past climatic change. Proc. R. Soc. B Biol. Sci. 2003, 270, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Tribsch, A. Areas of endemism of vascular plants in the Eastern Alps in relation to Pleistocene glaciation. J. Biogeogr. 2004, 31, 747–760. [Google Scholar] [CrossRef]
- López–Pujol, J.; Zhang, F.M.; Sun, H.Q.; Ying, T.S.; Ge, S. Centres of plant endemism in China: Places for survival or for speciation? J. Biogeogr. 2011, 38, 1267–1280. [Google Scholar] [CrossRef]
- Svenning, J.C.; Skov, F. Limited filling of the potential range in European tree species. Ecol. Lett. 2004, 7, 565–573. [Google Scholar] [CrossRef]
- Svenning, J.C.; Skov, F. Ice age legacies in the geographical distribution of tree species richness in Europe. Glob. Ecol. Biogeogr. 2007, 16, 234–245. [Google Scholar] [CrossRef]
- Yi, H.J.; Sun, X.M.; Zhang, G.F. The effect of short-term temperature drop on the development of brown planthopper nymphs. China Plant Prot. 2008, 28, 15–16. [Google Scholar]
- Zhang, H.J.; Yang, H.Z.; Li, B.L. Effects of low temperature stress on occurrence of brown planthopper population. J. Anhui Agric. Sci. 2018, 46, 146–148. [Google Scholar]
- Feng, B.C.; Huang, C.W.; Wang, H.D.; Yao, J.; Song, L.J. Influence of temperature on the population dynamics of the whitebacked planthopper Sogatella furcifera (Horvath). Acta Entomol. Sin. 1985, 28, 390–397. [Google Scholar]
- von Humboldt, A. Ansichten der Natur: Mit Wissenschaftlichen Erläuterungen: Beide Teile in Enem Bande; JG Cotta’scher Verlag: Tübingen, Germany, 1808. [Google Scholar]
- Fine, P.V.A. Ecological and evolutionary drivers of geographic variation in species diversity. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 369–392. [Google Scholar] [CrossRef]
- Srivastava, D.S.; Lawton, J.H. Why more productive sites have more species: An experimental test of theory using tree–hole communities. Am. Nat. 1998, 152, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Hurlbert, A.H. Species–energy relationships and habitat complexity in bird communities. Ecol. Lett. 2004, 7, 714–720. [Google Scholar] [CrossRef]
- Evans, K.L.; James, N.A.; Gaston, K.J. Abundance, species richness and energy availability in the north American avifauna. Glob. Ecol. Biogeogr. 2006, 15, 372–385. [Google Scholar] [CrossRef]
- Turner, J.R.G.; Gatehouse, C.M.; Corey, C.A. Does solar energy control organic diversity? Butterflies, moths and the British climate. Oikos 1987, 48, 195–205. [Google Scholar] [CrossRef]
- Turner, J.R.G.; Lennon, J.J.; Lawrenson, J.A. British bird species distributions and the energy theory. Nature 1988, 335, 539–541. [Google Scholar] [CrossRef]
- Turner, J.R.G. Explaining the global biodiversity gradient: Energy, area, history and natural selection. Basic Appl. Ecol. 2004, 5, 435–448. [Google Scholar] [CrossRef]
- Li, R.D. Population growth of the brown planthopper, Nilaparvata lugens (Stål), as influenced by temperature. Acta Phytophylacica Sin. 1984, 11, 101–107. [Google Scholar]
- Ye, Z.X.; Qin, H.G.; Li, H. Effects of temperature and nutrition condition on the population in crease of white–backed planthopper. Acta Phytophylacica Sin. 1994, 21, 209–213. [Google Scholar]
- Shi, B.K.; Hu, C.X.; Huang, J.L.; Hou, M.L. Modeling the influence of temperature on development, survival and oviposition of the brown planthopper, Nilaparvata lugens. Acta Ecol. Sin. 2014, 34, 5868–5874. [Google Scholar]
- Qian, H.; Wang, X.H.; Wang, S.L.; Li, Y.L. Environmental determinants of amphibian and reptile species richness in China. Ecography 2007, 30, 471–482. [Google Scholar] [CrossRef]
- Fang, J.Y.; Wang, X.P.; Liu, Y.N.; Tang, Z.Y.; White, P.S.; Sanders, N.J. Multi–scale patterns of forest structure and species composition in relation to climate in northeast China. Ecography 2012, 35, 1072–1082. [Google Scholar] [CrossRef]
- Wang, Q.G.; Su, X.Y.; Shrestha, N.; Liu, Y.P.; Wang, S.Y.; Xu, X.Y.; Wang, Z.H. Historical factors shaped species diversity and composition of Salix in eastern Asia. Sci. Rep. 2017, 7, 42038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Quan, Q.; Wu, Y.J.; Chen, Y.H.; He, P.; Qu, Y.H.; Lei, F.M. Topographic heterogeneity and temperature amplitude explain species richness patterns of birds in the Qinghai–Tibetan Plateau. Curr. Zool. 2017, 63, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Beck, J.; Meyer, C.; Waldmann, E.; Weigelt, P.; Kreft, H. Differential effects of environmental heterogeneity on global mammal species richness. Glob. Ecol. Biogeogr. 2015, 24, 1072–1083. [Google Scholar] [CrossRef]
- Shen, M.W.; Chen, S.B.; Bi, M.J.; Chen, W.D.; Zhou, K.X. Relationships between geographic patterns of ant species richness and environmental factors in China. Acta Ecol. Sin. 2016, 36, 7732–7739. [Google Scholar]
- Wei, J.F.; Niu, M.M.; Feng, J.N. Diversity and distribution patterns of scale insects in China. Ann. Entomol. Soc. Am. 2016, 109, 405–414. [Google Scholar] [CrossRef]
- Lyu, Y.M.; Wang, X.P.; Luo, J.C. Geographic patterns of insect diversity across China’s nature reserves: The roles of niche conservatism and range overlapping. Ecol. Evol. 2020, 10, 3305–3317. [Google Scholar] [CrossRef]
- Balmford, A.; Moore, J.L.; Brooks, T.; Burgess, N.D.; Hansen, L.A.; Williams, P.; Rahbek, C. Conservation conflicts across Africa. Science 2001, 291, 2616–2619. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.L.; Gaston, K.J. People, energy and avian species richness. Glob. Ecol. Biogeogr. 2005, 14, 187–196. [Google Scholar] [CrossRef]
- Luck, G.W. The relationships between net primary productivity, human population density and species conservation. J. Biogeogr. 2007, 34, 201–212. [Google Scholar] [CrossRef]
- Wang, Z.H.; Rahbek, C.; Fang, J.Y. Effects of geographical extent on the determinants of woody plant diversity. Ecography 2013, 35, 1160–1167. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, L.Y.; Chen, J.; Qiao, G.X. DNA barcoding reveals a mysterious high species diversity of conifer–feeding aphids in the mountains of southwest China. Sci. Rep. 2016, 6, 20123. [Google Scholar] [CrossRef]
- Cheng, R.; Jiang, N.; Xue, D.Y.; Li, X.X.; Ban, X.S.; Han, H.X. The evolutionary history of biston suppressaria (guenée) (Lepidoptera: Geometridae) related to complex topography and geological history. Syst. Entomol. 2016, 41, 732–743. [Google Scholar] [CrossRef]
- Che, Y.L.; Bai, Q.K.; Deng, W.B.; Liao, S.R.; He, J.J.; Simon, Y.W.H.; Wang, Z.Q. Uplift–driven diversification revealed by the historical biogeography of the cockroach Cryptocercus Scudder (Blattodea: Cryptocercidae) in eastern Asia. Syst. Entomol. 2020, 45, 60–72. [Google Scholar] [CrossRef]
- Yuan, S.; Huang, M.; Wang, X.S.; Ji, L.Q.; Zhang, Y.L. Centers of endemism and diversity patterns for Typhlocybine leafhoppers (Hemiptera: Cicadellidae: Typhlocybinae) in China. Insect Sci. 2013, 21, 523–526. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, C.Z.; Liu, J.Z.; Wei, J.F. Areas of endemism for scale insects in China. J. Asia Pac. Entomol. 2017, 20, 1170–1174. [Google Scholar] [CrossRef]
- Gao, C.; Chen, J.; Li, Y.; Jiang, L.Y.; Qiao, G.X. Congruent patterns between species richness and areas of endemism of the Greenideinae aphids (Hemiptera: Aphididae) revealed by global–scale data. Zool. J. Linn. Soc. 2018, 83, 791–807. [Google Scholar] [CrossRef]
- Huang, X.L.; Qiao, G.X.; Lei, F.M. Use of parsimony analysis to identify areas of endemism of Chinese birds: Implications for conservation and biogeography. Int. J. Mol. Sci. 2010, 11, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
| Hypotheses | Environmental Variables |
|---|---|
| Ambient energy | Mean annual temperature (MAT) |
| Annual potential evapotranspiration (PET) | |
| Water–energy dynamics | Mean annual precipitation (MAP) |
| Annual actual evapotranspiration (AET) | |
| Productivity | Normalized difference vegetation index (NDVI) |
| Net primary productivity (NPP) | |
| Environmental stability | Temperature annual range (TAR) |
| Precipitation seasonality (PS) | |
| Habitat heterogeneity | Elevation range (ER) |
| Topographical roughness (TR) | |
| Slope (SP) | |
| Human activities | Human population (HPP) |
| Gross domestic product (GDP) | |
| Historical climate stability | Mean annual temperature change since the LGM (MAT anomaly) |
| Mean annual precipitation change since the LGM (MAP anomaly) |
| Species Richness | ||||
|---|---|---|---|---|
| CoefOLS | R2OLS | CoefSAR | Pseudo R2SAR | |
| MAT | 0.56 | 31.9 *** | 0.45 | 52.6 *** |
| PET | −0.03 | 0.1 ns | −0.04 | 46 ns |
| MAP | 0.57 | 33.4 *** | 0.51 | 54 *** |
| AET | 0.59 | 35.4 *** | 0.55 | 54.9 *** |
| NDVI | 0.46 | 21.3 *** | 0.39 | 51.5 *** |
| NPP | 0.55 | 30.3 *** | 0.48 | 54.2 *** |
| TAR | −0.54 | 29.6 *** | −0.46 | 52.4 *** |
| PS | −0.37 | 13.8 *** | −0.28 | 48.8 *** |
| ER | 0.12 | 1.6 *** | 0.17 | 47.6 *** |
| TR | 0.05 | 0.2 ns | 0.15 | 46.1 ns |
| SP | 0.18 | 3.5 *** | 0.18 | 47.5 *** |
| HPP | 0.44 | 19.4 *** | 0.36 | 50.7 *** |
| GDP | 0.38 | 14.6 *** | 0.31 | 49.5 *** |
| MAT anomaly | −0.55 | 30.5 *** | −0.5 | 53.9 *** |
| MAP anomaly | 0.06 | 0.4 ns | −0.01 | 45.9 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.-X.; Yang, L.; Long, J.-K.; Chang, Z.-M.; Zhou, Z.-X.; Zhi, Y.; Yang, L.-J.; Li, H.-X.; Sui, Y.-J.; Gong, N.; et al. Testing Seven Hypotheses to Determine What Explains the Current Planthopper (Fulgoridae) Geographical and Species Richness Patterns in China. Insects 2020, 11, 892. https://doi.org/10.3390/insects11120892
Zhao Z-X, Yang L, Long J-K, Chang Z-M, Zhou Z-X, Zhi Y, Yang L-J, Li H-X, Sui Y-J, Gong N, et al. Testing Seven Hypotheses to Determine What Explains the Current Planthopper (Fulgoridae) Geographical and Species Richness Patterns in China. Insects. 2020; 11(12):892. https://doi.org/10.3390/insects11120892
Chicago/Turabian StyleZhao, Zheng-Xue, Lin Yang, Jian-Kun Long, Zhi-Min Chang, Zheng-Xiang Zhou, Yan Zhi, Liang-Jing Yang, Hong-Xing Li, Yong-Jin Sui, Nian Gong, and et al. 2020. "Testing Seven Hypotheses to Determine What Explains the Current Planthopper (Fulgoridae) Geographical and Species Richness Patterns in China" Insects 11, no. 12: 892. https://doi.org/10.3390/insects11120892
APA StyleZhao, Z.-X., Yang, L., Long, J.-K., Chang, Z.-M., Zhou, Z.-X., Zhi, Y., Yang, L.-J., Li, H.-X., Sui, Y.-J., Gong, N., Wang, X.-Y., & Chen, X.-S. (2020). Testing Seven Hypotheses to Determine What Explains the Current Planthopper (Fulgoridae) Geographical and Species Richness Patterns in China. Insects, 11(12), 892. https://doi.org/10.3390/insects11120892