Interspecific Mating Effects on Locomotor Activity Rhythms and Refractoriness of Aedes albopictus (Diptera: Culicidae) Females
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Rearing and Dissections of Male Accessory Glands
2.2. Injections of Ae. albopictus Females and Analysis of Locomotor Activity
2.3. Statistical Analysis
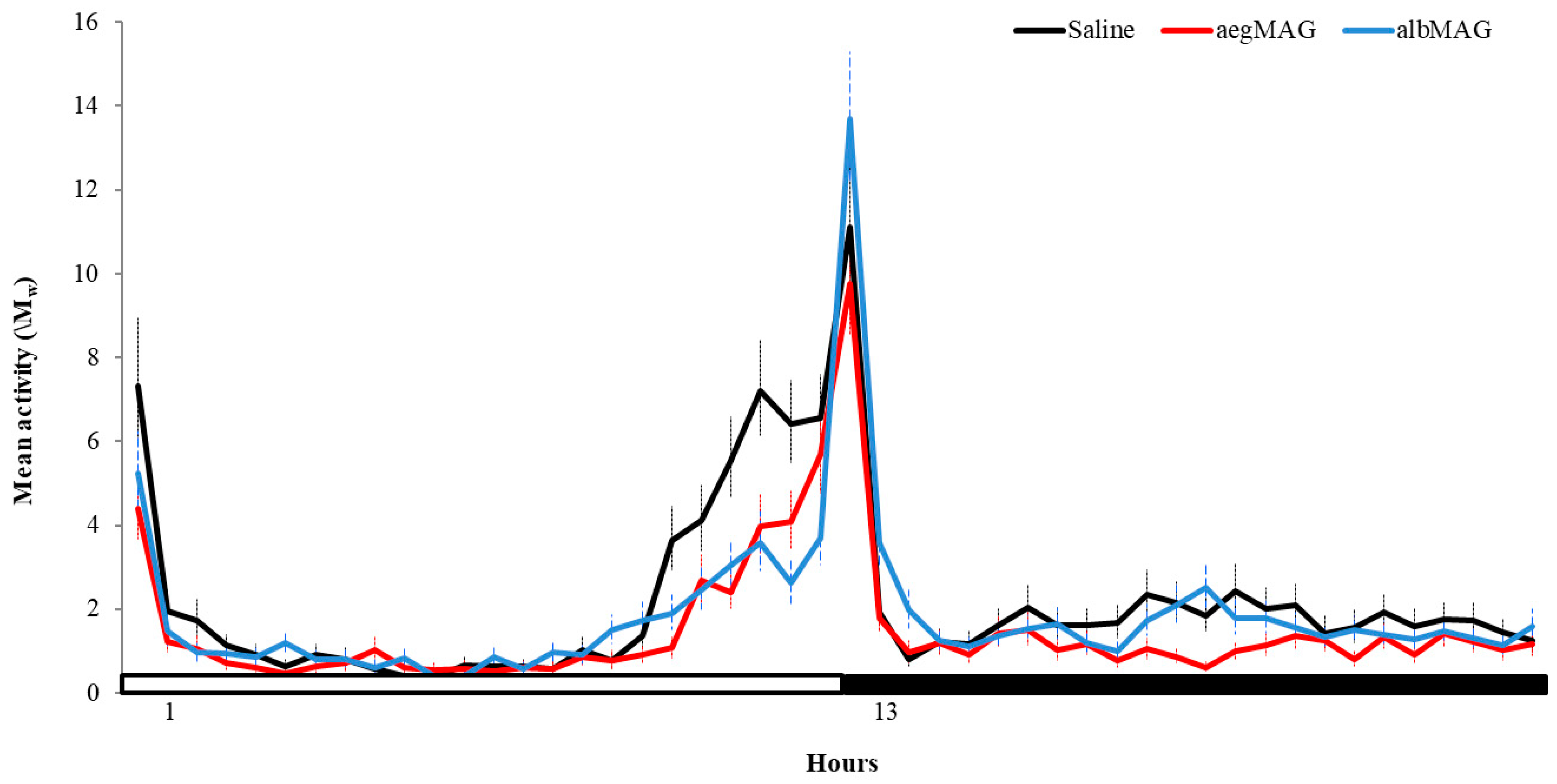
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lounibos, L.P.; Juliano, S.A. Where Vectors Collide: The Importance of Mechanisms Shaping the Realized Niche for Modeling Ranges of Invasive Aedes Mosquitoes. Biol. Invasions 2018, 20, 1913–1929. [Google Scholar] [CrossRef] [PubMed]
- Christophers, S.R. Aedes aegypti (L.), the Yellow Fever Mosquito. Its Life History, Bionomics, and Structure; Cambridge University Press: New York, NY, USA, 1960; ISBN 978-0-521-11302-1. [Google Scholar]
- Soghigian, J.; Gloria-Soria, A.; Robert, V.; Goff, G.L.; Failloux, A.-B.; Powell, J.R. Genetic evidence for the origin of Aedes aegypti, the yellow fever mosquito, in the southwestern Indian Ocean. Mol. Ecol. 2020, 29, 3593–3606. [Google Scholar] [CrossRef]
- Lounibos, L.P. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002, 47, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.E.G. The history of dengue in tropical Asia and its probable relationship to the mosquito Aedes aegypti. J. Trop. Med. Hyg. 1956, 59, 243–251. [Google Scholar] [PubMed]
- Huang, Y.-M. Medical entomology studies–XI. The subgenus Stegomyia of Aedes in the Oriental region with keys to the species (Diptera: Culicidae). Contrib. Am. Entomol. Inst. 1979, 15, 1–79. [Google Scholar]
- Urbanski, J.; Mogi, M.; O’Donnell, D.; DeCotiis, M.; Toma, T.; Armbruster, P. Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. Am. Nat. 2012, 179, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, L.; Kendell, D.; Robertson, D.; Livdahl, T.; Khatchikian, C. Aedes aegypti and Aedes albopictus in Bermuda: Extinction, invasion, invasion and extinction. Biol. Invasions 2010, 12, 3277–3288. [Google Scholar] [CrossRef]
- Lounibos, L.P.; Bargielowski, I.; Carrasquilla, M.C.; Nishimura, N. Coexistence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Peninsular Florida Two Decades After Competitive Displacements. J. Med. Entomol. 2016, 53, 1385–1390. [Google Scholar] [CrossRef]
- Honório, N.A.; Carrasquilla, M.C.; Bargielowski, I.E.; Nishimura, N.; Swan, T.; Lounibos, L.P. Male origin determines satyrization potential of Aedes aegypti by invasive Aedes albopictus. Biol. Invasions 2018, 20, 653–664. [Google Scholar] [CrossRef]
- Braks, M.A.H.; Honório, N.A.; Lourenço-De-Oliveira, R.; Juliano, S.A.; Lounibos, L.P. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida. J. Med. Entomol. 2003, 40, 785–794. [Google Scholar] [CrossRef]
- Juliano, S.A. Species Introduction and Replacement Among Mosquitoes: Interspecific Resource Competition or Apparent Competition? Ecology 1998, 79, 255–268. [Google Scholar] [CrossRef]
- Ribeiro, J.M. Can satyrs control pests and vectors? J. Med. Entomol. 1988, 25, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Tripet, F.; Lounibos, L.P.; Robbins, D.; Moran, J.; Nishimura, N.; Blosser, E.M. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am. J. Trop. Med. Hyg. 2011, 85, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Bargielowski, I.E.; Lounibos, L.P.; Shin, D.; Smartt, C.T.; Carrasquilla, M.C.; Henry, A.; Navarro, J.C.; Paupy, C.; Dennett, J.A. Widespread evidence for interspecific mating between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in nature. Infect. Genet. Evol. 2015, 36, 456–461. [Google Scholar] [CrossRef]
- Bargielowski, I.E.; Lounibos, L.P. Satyrization and satyrization-resistance in competitive displacements of invasive mosquito species. Insect Sci. 2016, 23, 162–174. [Google Scholar] [CrossRef]
- Bargielowski, I.; Lounibos, L.P. Rapid evolution of reduced receptivity to interspecific mating in the dengue vector Aedes aegypti in response to satyrization by invasive Aedes albopictus. Evol. Ecol. 2014, 28, 193–203. [Google Scholar] [CrossRef]
- Craig, G.B., Jr. Mosquitoes: Female monogamy induced by male accessory gland substance. Science 1967, 156, 1499–1501. [Google Scholar] [CrossRef]
- Jones, M.D.R.; Gubbins, S.J. Modification of circadian flight activity in the mosquito Anopheles gambiae after insemination. Nature 1977, 268, 731–732. [Google Scholar] [CrossRef]
- Jones, M.D.R. The programming of circadian flight-activity in relation to mating and the gonotrophic cycle in the mosquito, Aedes aegypti. Physiol. Entomol. 1981, 6, 307–313. [Google Scholar] [CrossRef]
- Jones, M.D.R.; Gubbins, S.J. Modification of female circadian flight-activity by a male accessory gland pheromone in the mosquito, Culex pipiens quinquefasciatus. Physiol. Entomol. 1979, 4, 345–351. [Google Scholar] [CrossRef]
- Lima-Camara, T.N.; Lima, J.B.P.; Bruno, R.V.; Peixoto, A.A. Effects of insemination and blood-feeding on locomotor activity of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) females under laboratory conditions. Parasit. Vectors 2014, 7, 304. [Google Scholar] [CrossRef] [PubMed]
- Lima-Camara, T.N.; Codeco, C.T.; Honorio, N.A.; Bruno, R.V.; Peixoto, A.A.; Lounibos, L.P. Male accessory gland substances from Aedes albopictus affect the locomotor activity of Aedes aegypti females. Mem. Inst. Oswaldo Cruz 2013, 108, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. The check is in the male: Male mosquitoes affect female physiology and behavior. J. Am. Mosq. Control Assoc. 1999, 15, 213–220. [Google Scholar] [PubMed]
- Consoli, R.A.G.B.; Lourenço-de-Oliveira, R. Principais Mosquitos de Importância Sanitária no Brasil; Editora Fiocruz: Rio de Janeiro, Brazil, 1994; ISBN 85-85676-03-5. [Google Scholar]
- Bargielowski, I.E.; Lounibos, L.P.; Carrasquilla, M.C. Evolution of resistance to satyrization through reproductive character displacement in populations of invasive dengue vectors. Proc. Natl. Acad. Sci. USA 2013, 110, 2888–2892. [Google Scholar] [CrossRef]
- Padilha, K.P.; Resck, M.E.B.; da Cunha, O.A.T.; Teles-de-Freitas, R.; Campos, S.S.; Sorgine, M.H.F.; Lourenço-de-Oliveira, R.; Farnesi, L.C.; Bruno, R.V. Zika infection decreases Aedes aegypti locomotor activity but does not influence egg production or viability. Mem. Inst. Oswaldo Cruz 2018, 113. [Google Scholar] [CrossRef]
- Gentile, C.; Rivas, G.B.S.; Meireles-Filho, A.C.A.; Lima, J.B.P.; Peixoto, A.A. Circadian expression of clock genes in two mosquito disease vectors: Cry2 is different. J. Biol. Rhythms 2009, 24, 444–451. [Google Scholar] [CrossRef]
- Lima-Camara, T.N.; Bruno, R.V.; Luz, P.M.; Castro, M.G.; Lourenço-de-Oliveira, R.; Sorgine, M.H.F.; Peixoto, A.A. Dengue Infection Increases the Locomotor Activity of Aedes aegypti Females. PLoS ONE 2011, 6, e17690. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 14 July 2020).
- RStudio Team RStudio: Integrated Development for R. RStudio. Available online: http://www.rstudio.com/ (accessed on 14 July 2020).
- Sirot, L.K.; Hardstone, M.C.; Helinski, M.E.H.; Ribeiro, J.M.C.; Kimura, M.; Deewatthanawong, P.; Wolfner, M.F.; Harrington, L.C. Towards a semen proteome of the dengue vector mosquito: Protein identification and potential functions. PLoS Negl. Trop. Dis. 2011, 5, e989. [Google Scholar] [CrossRef] [PubMed]
- Boes, K.E.; Ribeiro, J.M.C.; Wong, A.; Harrington, L.C.; Wolfner, M.F.; Sirot, L.K. Identification and characterization of seminal fluid proteins in the Asian tiger mosquito, Aedes albopictus. PLoS Negl. Trop. Dis. 2014, 8, e2946. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. Endogenous Factors Regulating Mosquito Host-Seeking Behaviour. In Ciba Foundation Symposium 200-Olfaction in Mosquito-Host Interactions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 212–232. ISBN 978-0-470-51494-8. [Google Scholar]
- Gillott, C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003, 48, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Nasci, R.S.; Hare, S.G.; Willis, F.S. Interspecific mating between Louisiana strains of Aedes albopictus and Aedes aegypti in the field and laboratory. J. Am. Mosq. Control Assoc. 1989, 5, 416–421. [Google Scholar] [PubMed]
- Nazni, W.A.; Lee, H.L.; Dayang, H.A.B.; Azahari, A.H. Cross-mating between Malaysian strains of Aedes aegypti and Aedes albopictus in the laboratory. Southeast Asian J. Trop. Med. Public Health 2009, 40, 40–46. [Google Scholar]
- Carrasquilla, M.C.; Lounibos, L.P. Satyrization without evidence of successful insemination from interspecific mating between invasive mosquitoes. Biol. Lett. 2015, 11, 20150527. [Google Scholar] [CrossRef]

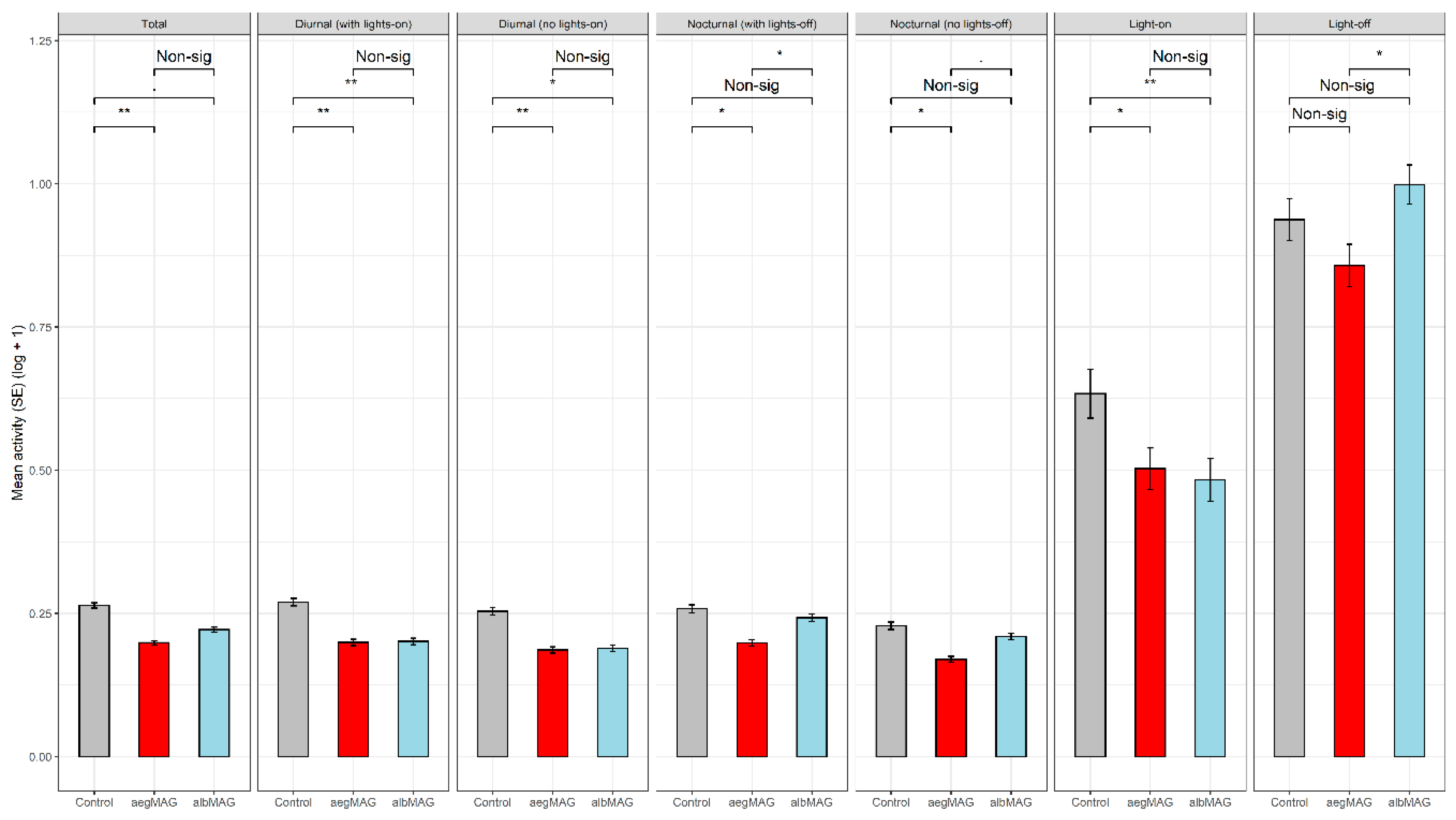
| Saline (n = 63) | Ae. aegypti MAG (aegMAG) (n = 70) | Ae. albopictus MAG (albMAG) (n = 74) | F Test b | |
|---|---|---|---|---|
| Total Activity | 0.264 (0.005) | 0.199 (0.004) | 0.222 (0.004) | F2, 201 = 4.3296, p < 0.05 |
| Diurnal Activity | 0.270 (0.007) | 0.199 (0.006) | 0.201 (0.006) | F2, 201 = 5.1346, p < 0.01 |
| Diurnal Activity Without Lights-on | 0.254 (0.007) | 0.186 (0.006) | 0.189 (0.006) | F2, 201 = 4.5596, p < 0.05 |
| Lights-on Activity | 0.634 (0.043) | 0.503 (0.036) | 0.483 (0.037) | F2, 201 = 4.6340, p < 0.05 |
| Nocturnal Activity | 0.258 (0.007) | 0.198 (0.006) | 0.242 (0.006) | F2, 201 = 2.9092, p = 0.05682 |
| Nocturnal Activity Without Lights-off | 0.228 (0.006) | 0.170 (0.005) | 0.209 (0.006) | F2, 201 = 2.7769, p = 0.06462 |
| Lights-off Activity | 0.937 (0.036) | 0.857 (0.037) | 0.999 (0.034) | F2, 201 = 2.5070, p =0.08406 |
| Fixed Effects | Diurnal Activity | ||||
| Estimate | SE | DF | T Value | p-Value | |
| Intercept | 0.27893 | 0.0171 | 201 | 16.314 | <0.001 |
| Block: Experiment 2 | −0.0342 | 0.03291 | 201 | −1.039 | 0.30003 |
| Status: Ae. aegypti MAG | −0.07935 | 0.02507 | 201 | −3.165 | <0.01 |
| Status: Ae. albopictus MAG | −0.07572 | 0.0278 | 201 | −2.724 | <0.01 |
| Block: Experiment 2 x Status: Ae. aegypti MAG | 0.03362 | 0.04322 | 201 | 0.778 | 0.43748 |
| Block: Experiment 2 x Status: Ae. albopictus MAG | 0.03069 | 0.04308 | 201 | 0.712 | 0.47712 |
| Fixed effects | Diurnal Activity Without Lights-on | ||||
| Estimate | SE | DF | T Value | p-Value | |
| Intercept | 0.26529 | 0.01656 | 201 | 16.022 | <0.001 |
| Block: Experiment 2 | −0.0423 | 0.03188 | 201 | −1.327 | 0.18603 |
| Status: Ae. aegypti MAG | −0.07771 | 0.02428 | 201 | −3.201 | <0.01 |
| Status: Ae. albopictus MAG | −0.0678 | 0.02692 | 201 | −2.519 | <0.05 |
| Block: Experiment 2 x Status: Ae. aegypti MAG | 0.03894 | 0.04185 | 201 | 0.93 | 0.35326 |
| Block: Experiment 2 x Status: Ae. albopictus MAG | 0.02827 | 0.04172 | 201 | 0.678 | 0.49886 |
| Fixed effects | Lights-on Activity | ||||
| Estimate | SE | DF | T Value | p-Value | |
| Intercept | 0.59269 | 0.05714 | 201 | 10.373 | <0.001 |
| Block: Experiment 2 | 0.15206 | 0.11 | 201 | 1.382 | 0.16837 |
| Status: Ae. aegypti MAG | −0.11711 | 0.08378 | 201 | −1.398 | 0.16373 |
| Status: Ae. albopictus MAG | −0.25776 | 0.09289 | 201 | −2.775 | <0.01 |
| Block: Experiment 2 x Status: Ae. aegypti MAG | −0.08873 | 0.14443 | 201 | −0.614 | 0.53967 |
| Block: Experiment 2 x Status: Ae. albopictus MAG | 0.08631 | 0.14397 | 201 | 0.599 | 0.54954 |
| Fixed effects | Nocturnal Activity | ||||
| Estimate | SE | DF | T Value | p-Value | |
| Intercept | 0.27335 | 0.01913 | 201 | 14.293 | <0.001 |
| Block: Experiment 2 | −0.05689 | 0.03682 | 201 | −1.545 | 0.1239 |
| Status: Ae. aegypti MAG | −0.06134 | 0.02804 | 201 | −2.187 | <0.05 |
| Status: Ae. albopictus MAG | −0.03111 | 0.03109 | 201 | −1.001 | 0.3182 |
| Block: Experiment 2 x Status: Ae. aegypti MAG | 0.02493 | 0.04834 | 201 | 0.516 | 0.6067 |
| Block: Experiment 2 x Status: Ae. albopictus MAG | 0.05709 | 0.04819 | 201 | 1.185 | 0.2376 |
| Fixed effects | Nocturnal Activity Without Lights-off | ||||
| Estimate | SE | DF | T Value | p-Value | |
| Intercept | 0.24321 | 0.0184 | 201 | 13.215 | <0.001 |
| Block: Experiment 2 | −0.05467 | 0.03543 | 201 | −1.543 | 0.1244 |
| Status: Ae. aegypti MAG | −0.06043 | 0.02699 | 201 | −2.239 | <0.05 |
| Status: Ae. albopictus MAG | −0.03788 | 0.02992 | 201 | −1.266 | 0.2069 |
| Block: Experiment 2 x Status: Ae. aegypti MAG | 0.02406 | 0.04652 | 201 | 0.517 | 0.6055 |
| Block: Experiment 2 x Status: Ae. albopictus MAG | 0.06136 | 0.04637 | 201 | 1.323 | 0.1873 |
| Fixed effects | Lights-off Activity | ||||
| Estimate | SE | DF | T Value | p-Value | |
| Intercept | 0.96659 | 0.06406 | 201 | 15.09 | <0.001 |
| Block: Experiment 2 | −0.10789 | 0.12331 | 201 | −0.875 | 0.383 |
| Status: Ae. aegypti MAG | −0.08222 | 0.09392 | 201 | −0.875 | 0.382 |
| Status: Ae. albopictus MAG | 0.1246 | 0.10414 | 201 | 1.197 | 0.233 |
| Block: Experiment 2 x Status: Ae. aegypti MAG | 0.04482 | 0.16191 | 201 | 0.277 | 0.782 |
| Block: Experiment 2 x Status: Ae. albopictus MAG | −0.04112 | 0.1614 | 201 | −0.255 | 0.799 |
| Fixed effects | Total Activity | ||||
| Estimate | SE | DF | T Value | p-Value | |
| Intercept | 0.27614 | 0.01498 | 201 | 18.432 | <0.001 |
| Block: Experiment 2 | −0.04554 | 0.02884 | 201 | −1.579 | 0.11587 |
| Status: Ae. aegypti MAG | −0.07035 | 0.02197 | 201 | −3.202 | <0.01 |
| Status: Ae. albopictus MAG | −0.05341 | 0.02436 | 201 | −2.193 | <0.05 |
| Block: Experiment 2 x Status: Ae. aegypti MAG | 0.02928 | 0.03787 | 201 | 0.773 | 0.44038 |
| Block: Experiment 2 x Status: Ae. albopictus MAG | 0.04389 | 0.03775 | 201 | 1.163 | 0.24638 |
| Injection | Positive Spermathecae n (%) | Negative Spermathecae n (%) |
|---|---|---|
| Saline (Control) | 33 (86.84%) | 5 (13.16%) |
| Ae. aegypti MAG (aegMAG) | 25 (83.33%) | 5 (16.67%) |
| Ae. albopictus MAG (albMAG) | 2 (10.00%) | 18 (90.00%) |
| Total | 60 (68.18%) | 28 (31.82%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feitoza, T.d.S.; Ferreira-de-Lima, V.H.; Câmara, D.C.P.; Honório, N.A.; Lounibos, L.P.; Lima-Camara, T.N. Interspecific Mating Effects on Locomotor Activity Rhythms and Refractoriness of Aedes albopictus (Diptera: Culicidae) Females. Insects 2020, 11, 874. https://doi.org/10.3390/insects11120874
Feitoza TdS, Ferreira-de-Lima VH, Câmara DCP, Honório NA, Lounibos LP, Lima-Camara TN. Interspecific Mating Effects on Locomotor Activity Rhythms and Refractoriness of Aedes albopictus (Diptera: Culicidae) Females. Insects. 2020; 11(12):874. https://doi.org/10.3390/insects11120874
Chicago/Turabian StyleFeitoza, Thais de Souza, Victor Henrique Ferreira-de-Lima, Daniel Cardoso Portela Câmara, Nildimar Alves Honório, L. Philip Lounibos, and Tamara Nunes Lima-Camara. 2020. "Interspecific Mating Effects on Locomotor Activity Rhythms and Refractoriness of Aedes albopictus (Diptera: Culicidae) Females" Insects 11, no. 12: 874. https://doi.org/10.3390/insects11120874
APA StyleFeitoza, T. d. S., Ferreira-de-Lima, V. H., Câmara, D. C. P., Honório, N. A., Lounibos, L. P., & Lima-Camara, T. N. (2020). Interspecific Mating Effects on Locomotor Activity Rhythms and Refractoriness of Aedes albopictus (Diptera: Culicidae) Females. Insects, 11(12), 874. https://doi.org/10.3390/insects11120874






