A Pesticide Residues Insight on Honeybees, Bumblebees and Olive Oil after Pesticidal Applications against the Olive Fruit Fly Bactrocera oleae (Diptera: Tephritidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
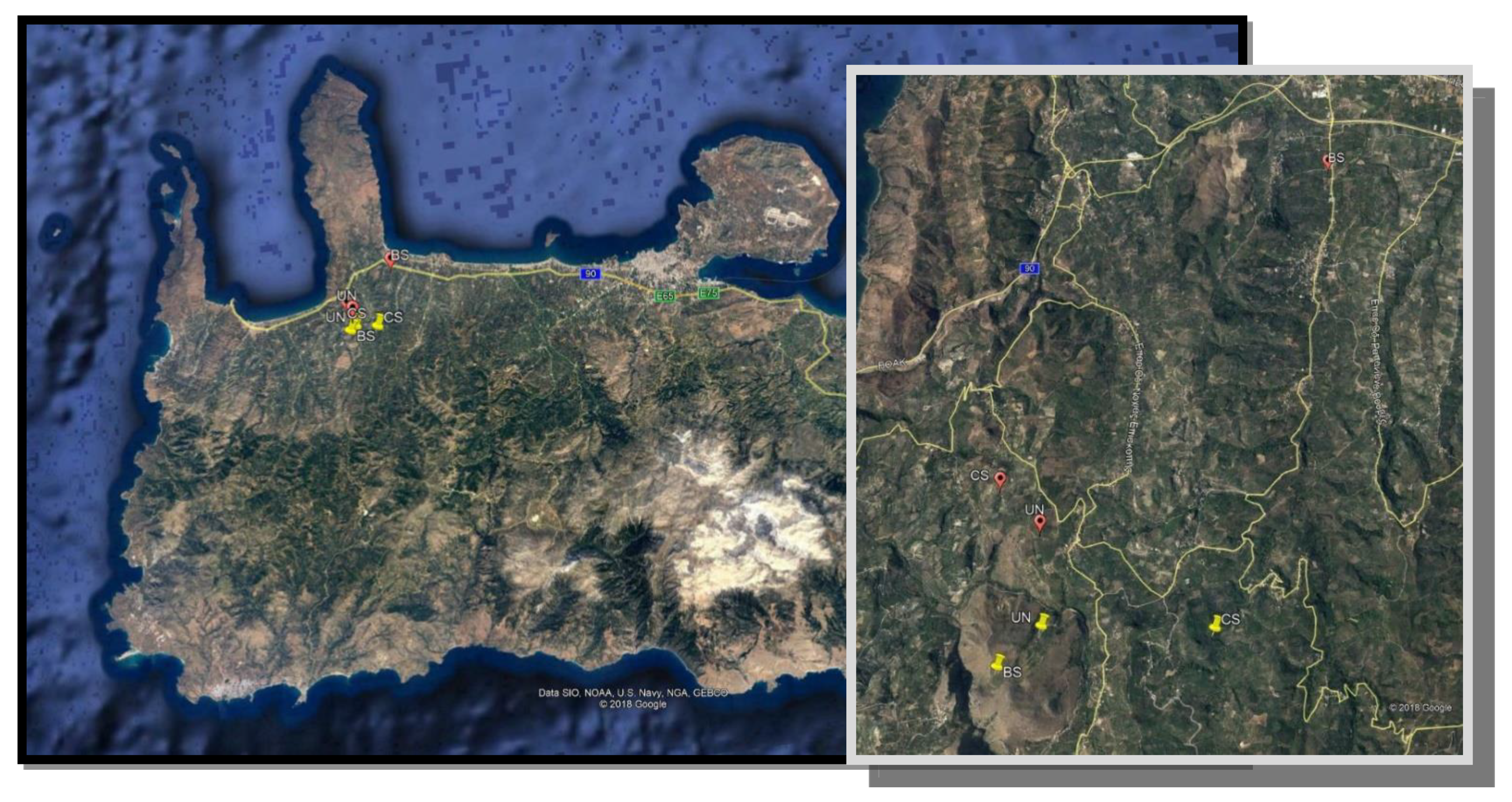
2.1. Experimental Areas
2.2. The Insecticide Applications
2.3. Timing of the Bait Spray and Cover Spray Applications
2.4. Sampling of Honey, Honeybees, Bumblebees and Olive Fruits
2.4.1. Sampling of Honeybees, Bumblebees and Honey
2.4.2. Sampling of Olive Fruits for Olive Oil Analysis
2.5. Analysis of Samples
2.5.1. Reagents and Standards
2.5.2. Preparation of Standard Solutions
2.5.3. Extraction Procedure
Olive Oil
Bees and Honey
2.6. Analytical Method Validation
2.7. Determination of Compounds—Instrumentation
2.7.1. GC-EI-QqQ-MS System and Operating Conditions
2.7.2. LC-ESI-QqQ-MS System and Operating Conditions
2.7.3. GC-ECD Analysis
3. Results
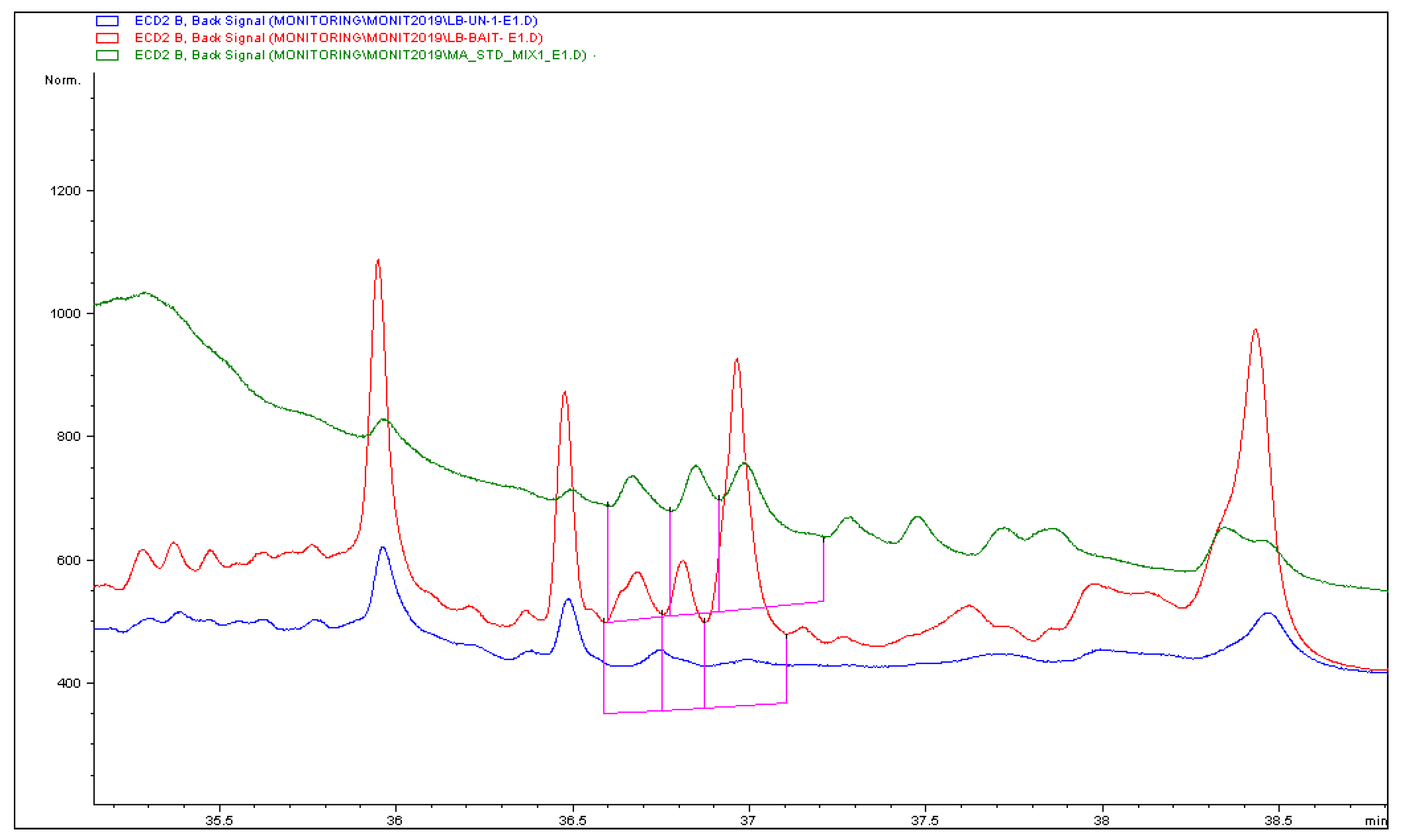
3.1. Determination of the Targeted Compounds and Validation of the Analytical Methods
3.2. Residues in Honeybees, Bumblebees and Honey Samples
3.3. Analysis of Olive Oil Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Foxhall, L. Olive Cultivation in Ancient Greece: Seeking the Ancient Economy; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Kandylis, P.; Vekiari, A.S.; Kanellaki, M.; Kamoun, N.G.; Msallem, M.; Kourkoutas, Y. Comparative study of extra virgin olive oil flavor profile of Koroneiki variety (Olea europaea var. Microcarpa alba) cultivated in Greece and Tunisia during one period of harvesting. LWT Food Sci. Technol. 2011, 44, 1333–1341. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Williams, M.; Chartzoulakis, K.; Harwood, J. Olive oil qualitative parameters after orchard irrigation with saline water. J. Agric. Food Chem. 2009, 57, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Michener, C.D. Biogeography of the bees. Ann. Missouri Bot. Gard. 1979, 66, 277–347. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World; The Johns Hopkins University Press: Baltimore, MD, USA, 2000. [Google Scholar]
- Petanidou, T.; Ellis, W.N. Pollinating fauna of a phryganic ecosystem: Composition and diversity. Biodivers. Lett. 1993, 1, 9–22. [Google Scholar] [CrossRef]
- Nielsen, A.; Steffan-Dewenter, I.; Westphal, C.; Messinger, O.; Potts, S.G.; Roberts, S.P.M.; Settele, J.; Szentgyorgyi, H.; Vaissiere, B.E.; Vaitis, M.; et al. Assessing bee species richness in two Mediterranean communities: Importance of habitat type and sampling techniques. Ecol. Res. 2011, 26, 969–983. [Google Scholar] [CrossRef]
- Giovanetti, M. Do bees like olive? A preliminary analysis of honey bee behaviour on flowers of the wind-pollinated species Olea europaea. Acta Hortic. 2018, 121–126. [Google Scholar] [CrossRef]
- Hagen, K.S.; Finney, G.L. A food supplement for effectively increasing the fecundity of certain tephritid species. J. Econ. Entomol. 1950, 43, 735. [Google Scholar] [CrossRef]
- Haniotakis, G.E. Olive pest control: Present status and prospects. In Proceedings of the European Meeting of the IOBC/WPRS Working Group “Integrated Protection of Olive Crops”, Chania, Greece, 29–31 May 2003. Bull. OILB/SROP 2005, 28, 1–9. [Google Scholar]
- Varikou, K.; Garantonakis, N.; Birouraki, A.; Gkilpathi, D.; Kapogia, E. Refreshing bait spots in an olive orchard for the control of Bactrocera oleae (Diptera: Tephritidae). Crop Prot. 2017, 92, 153–159. [Google Scholar] [CrossRef]
- Varikou, K.; Garantonakis, N.; Birouraki, A. Exposure of Bombus terrestris L. to three different active ingredients and two application methods for olive pest control. Entomol. Gen. 2019, 39, 53–60. [Google Scholar] [CrossRef]
- Gray, A.; Brodschneider, R.; Adjlane, N.; Ballis, A.; Brusbardis, V.; Charrire, J.D.; Chlebo, R.; Coffey, M.F.; Cornelissen, B.; da Costa, C.A.; et al. Loss rates of honey bee colonies during winter 2017/18 in 36 countries participating in the COLOSS survey, including effects of forage sources. J. Apicult. Res. 2019, 58, 479–485. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Tzouganaki, Z.D.; Machera, K. Chromatographic determination of monoterpenes and other acaricides in honeybees: Prevalence and possible synergies. Sci. Total Environ. 2018, 625, 96–105. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Anagnostopoulos, C.; Anastasiadou, P.; Machera, K. Pesticide residues in honeybees, honey and bee pollen by LC-MS/MS screening: Reported death incidents in honeybees. Sci. Total Environ. 2014, 485–486, 633–642. [Google Scholar] [CrossRef]
- Faucon, J.P.; Mathieu, L.; Ribiere, M.; Martel, A.C.; Drajnudel, P.; Zeggane, S.; Aurieres, C.; Aubert, M.F.A. Honey bee winter mortality in France in 1999 and 2000. Bee World 2002, 83, 14–23. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemuller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- van Engelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef]
- van Engelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Pettis, J. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar] [CrossRef]
- Vanengelsdorp, D.; Underwood, R.; Caron, D.; Hayes, J. An estimate of managed colony losses in the winter of 2006–2007: A report commissioned by the apiary inspectors of America. Am. Bee J. 2007, 147, 599–603. [Google Scholar]
- de Miranda, J.R.; Cordoni, G.; Budge, G. The acute bee paralysis virus-Kashmir bee virus-Israeli acute paralysis virus complex. J. Invertebr. Pathol. 2010, 103, S30–S47. [Google Scholar] [CrossRef]
- Hatjina, F.; Bouga, M.; Karatasou, A.; Kontothanasi, A.; Charistos, L.; Emmanouil, C.; Emmanouil, N.; Maistros, A.D. Data on honey bee losses in Greece: A preliminary note. J. Apicult. Res. 2010, 49, 116–118. [Google Scholar] [CrossRef]
- Watanabe, M.E. Colony collapse disorder: Many suspects, no smoking gun. BioScience 2008, 58, 384–388. [Google Scholar] [CrossRef]
- Chauzat, M.P.; Carpentier, P.; Martel, A.C.; Bougeard, S.; Cougoule, N.; Porta, P.; Lachaize, J.; Madec, F.; Aubert, M.; Faucon, J.P. Influence of pesticide residues on honey bee (Hymenoptera: Apidae) colony health in France. Environ. Entomol. 2009, 38, 514–523. [Google Scholar] [CrossRef]
- Chauzat, M.P.; Faucon, J.P.; Martel, A.C.; Lachaize, J.; Cougoule, N.; Aubert, M. A survey of pesticide residues in pollen loads collected by honey bees in France. J. Econ. Entomol. 2006, 99, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ostiguy, N.; Drummond, F.A.; Aronstein, K.; Eitzer, B.; Ellis, J.D.; Spivak, M.; Sheppard, W.S. Honey bee exposure to pesticides: A four-year nationwide study. Insects 2019, 10, 13. [Google Scholar] [CrossRef]
- Zioga, E.; Kelly, R.; White, B.; Stout, J.C. Plant protection product residues in plant pollen and nectar: A review of current knowledge. Environ. Res. 2020, 189, 109873. [Google Scholar] [CrossRef]
- Botias, C.; David, A.; Hill, E.M.; Goulson, D. Quantifying exposure of wild bumblebees to mixtures of agrochemicals in agricultural and urban landscapes. Environ. Pollut. 2017, 222, 73–82. [Google Scholar] [CrossRef]
- David, A.; Botias, C.; Abdul-Sada, A.; Goulson, D.; Hill, E.M. Sensitive determination of mixtures of neonicotinoid and fungicide residues in pollen and single bumblebees using a scaled down QuEChERS method for exposure assessment. Anal. Bioanal. Chem. 2015, 407, 8151–8162. [Google Scholar] [CrossRef]
- EFSA-Guidance. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 2013, 11, 3295. [Google Scholar]
- Barganska, Z.; Lambropoulou, D.; Namiesnik, J. Problems and challenges to determine pesticide residues in bumblebees. Crit. Rev. Anal. Chem. 2018, 48, 447–458. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.; Miliadis, G.E. Development and validation of an easy multiresidue method for the determination of multiclass pesticide residues using GC-MS/MS and LC-MS/MS in olive oil and olives. Talanta 2013, 112, 1–10. [Google Scholar] [CrossRef]
- SANTE. SANTE/11945/2015. In Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; SANTE: Brussels, Belgium, 2015. [Google Scholar]
- SANTE. SANTE/11813/2017. In Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; SANTE: Brussels, Belgium, 2017. [Google Scholar]
- Alcantara, D.B.; Fernandes, T.S.M.; Nascimento, H.O.; Lopes, A.F.; Menezes, M.G.G.; Lima, A.C.A.; Carvalho, T.V.; Grinberg, P.; Milhome, M.A.L.; Oliveira, A.H.B.; et al. Diagnostic detection systems and QuEChERS methods for multiclass pesticide analyses in different types of fruits: An overview from the last decade. Food Chem. 2019, 298. [Google Scholar] [CrossRef]
- Tankiewicz, M. Determination of selected priority pesticides in high water fruits and vegetables by modified QuEChERS and GC-ECD with GC-MS/MS confirmation. Molecules 2019, 24, 417. [Google Scholar] [CrossRef] [PubMed]
- LD50-Dimethoate. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/244.htm (accessed on 1 November 2019).
- LD50-λ-Cyhalothrin. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/415.htm (accessed on 1 November 2019).
- Fried, G.; Villers, A.; Porcher, E. Assessing non-intended effects of farming practices on field margin vegetation with a functional approach. Agric. Ecosyst. Environ. 2018, 261, 33–44. [Google Scholar] [CrossRef]
- Grella, M.; Marucco, P.; Manzone, M.; Gallart, M.; Balsari, P. Effect of sprayer settings on spray drift during pesticide application in poplar plantations (Populus spp.). Sci. Total Environ. 2017, 578, 427–439. [Google Scholar] [CrossRef]
- Grella, M.; Gallart, M.; Marucco, P.; Balsari, P.; Gil, E. Ground deposition and airborne spray drift assessment in vineyard and orchard: The influence of environmental variables and sprayer settings. Sustainability 2017, 9, 728. [Google Scholar] [CrossRef]
- Kenna, D.; Cooley, H.; Pretelli, I.; Ramos Rodrigues, A.; Gill, S.D.; Gill, R.J. Pesticide exposure affects flight dynamics and reduces flight endurance in bumblebees. Ecol. Evol. 2019, 9, 5637–5650. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chen, Y.; Simonds, R. The effects of pesticides on queen rearing and virus titers in honey bees (Apis mellifera L.). Insects 2013, 4, 71–89. [Google Scholar] [CrossRef]
- López, J.H.; Krainer, S.; Engert, A.; Schuehly, W.; Riessberger-Gallé, U.; Crailsheim, K. Sublethal pesticide doses negatively affect survival and the cellular responses in American foulbrood-infected honeybee larvae. Sci. Rep. 2017, 7, 40853. Available online: https://www.nature.com/articles/srep40853#supplementary-information (accessed on 5 January 2018). [CrossRef]
- Rendon, P.A.; Jeronimo, F.; Ibarra, J.; Alverez, V.C. Effectiveness of Success 0.02CB for the Control of Fruit Flies and its Effect on Bees Apis mellifera L.; U.S. Dep. Agric./APHIS/PPQ, Methods Development Station: Washington, DC, USA, 2000.
- Mangan, R.L.; Moreno, A.T. Honey bee foraging preferences, effects of sugars, and fruit fly toxic bait components. J. Econ. Entomol. 2009, 102, 1472–1481. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Papaj, D.R.; Hendrichs, J.; Wong, T.T.Y. Behavioral-responses of Ceratitis-capitata flies to bait spray droplets and natural food. Entomol. Exp. Appl. 1992, 64, 247–257. [Google Scholar] [CrossRef]
- Roessler, Y. Insecticidal bait and cover sprays. In World Crop Pests: Fruit Flies, Their Biology, Natural Enemies and Control; Robinson, B.S., Hooper, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 3B, pp. 329–337. [Google Scholar]
- Messing, R.H.; Seiler, S.J. Malathion leakage from fruit fly male-annihilation traps on Kauai, Hawaii. Bull. Environ. Contam. Toxicol. 1993, 51, 193–198. [Google Scholar] [CrossRef]
- Asquith, A.; Messing, R.H. Attraction of Hawaiian ground litter invertebrates to protein hydrolysate bait. Environ. Entomol. 1992, 21, 1022–1028. [Google Scholar] [CrossRef]
- Ichinohe, F.; Hashimoto, T.; Nakasone, S. Faunal survey of insects and spiders killed by protein hydrolysate insecticide bait for control of melon fly. Res. Bull. Plant Prot. Serv. Jpn. 1977, 14, 64–70. [Google Scholar]
- Smith, D.; Nannan, L. Yeast autolysate bait sprays for control of Queensland fruit fly on passion fruit in Queeensland. Qld. J. Agric. Anim. Sci. 1988, 45, 169–177. [Google Scholar]
- Thomas, D.B. Nontarget insects captured in fruit fly (Diptera: Tephritidae) surveillance traps. J. Econ. Entomol. 2003, 96, 1732–1737. [Google Scholar] [CrossRef]
- Troetschler, R.G. Effects on nontarget arthropods of malathion bait sprays used in California to eradicate the Mediterranean fruit-fly, Ceratitis-capitata (Wiedemann) (Dystera, Tephritidae). Environ. Entomol. 1983, 12, 1816–1822. [Google Scholar] [CrossRef]
- Ehler, L.E.; Endicott, P.C. Effect of malathion-bait sprays on biological-control of insect pests of olive, citrus, and walnut. Hilgardia 1984, 52, 1–47. [Google Scholar] [CrossRef][Green Version]
- Gary, N.E.; Mussen, E.C. Impact of Mediterranean fruit-fly malathion bait spray on honey bees. Environ. Entomol. 1984, 13, 711–717. [Google Scholar] [CrossRef]
- Porrini, C.; Sabatini, A.; Girotti, S.; Ghini, S.; Medrzycki, P.; Grillenzoni, F.; Bortolotti, L.; Gattavecchia, E.; Celli, G. Honey bees and bee products as monitors of the environmental contamination. APIACTA 2003, 38, 63–70. [Google Scholar]
- Porrini, C.; Caprio, E.; Tesoriero, D.; Di Prisco, G. Using honey bee as bioindicator of chemicals in Campanian agroecosystems (South Italy). Bull. Insectol. 2014, 67, 137–146. [Google Scholar]
- Goretti, E.; Pallottini, M.; Rossi, R.; La Porta, G.; Gardi, T.; Goga, B.T.C.; Elia, A.C.; Galletti, M.; Moroni, B.; Petroselli, C.; et al. Heavy metal bioaccumulation in honey bee matrix, an indicator to assess the contamination level in terrestrial environments. Environ. Pollut. 2020, 256. [Google Scholar] [CrossRef]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A worldwide survey of neonicotinoids in honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef]
| Year Tested | Dates of Bait Sprays | * Applied a.i. + Hydr.pr. | * Dates of Cover Sprays | * Applied a.i. | Sampling Dates |
|---|---|---|---|---|---|
| 2017 | 14/7 | λ-cyhalothrin | 11/7 | β-cyfluthrin, dimethoate | 6/7 13/7 22/7 |
| 28/8 | Saccharopolyspora spinosa (S. spinosa) | 2/8 | β-cyfluthrin, dimethoate | N.S. | |
| 22/9 | λ-cyhalothrin | 11/9 | dimethoate | N.S. | |
| 16/10 | thiacloprid | 1/10 | dimethoate | N.S. | |
| 2018 | 30/6 | β-cyfluthrin | 2/7 | β-cyfluthrin, dimethoate | 28/6 3/7 |
| 10/7 | β-cyfluthrin | 2/8 | β-cyfluthrin, dimethoate | 12/7 19/7 26/7 | |
| 1/8 | dimethoate | 3/9 | dimethoate | 8/8 | |
| 21/8 | dimethoate | dimethoate | N.S. | ||
| 20/9 | dimethoate | 10/9 24/9 11/10 17/10 |
| Recovery ± RSD% | Inter-d Precision | ||||||
|---|---|---|---|---|---|---|---|
| n = 3 | RSD%, n = 3 | ||||||
| Analyte | LOQ (mg/kg) | LOQ | 10LOQ | 100LOQ | LOQ | 10LOQ | 100LOQ |
| Deltamethrin | 0.005 | 70 ± 19 | 70 ± 10 | 74 ± 6.0 | 10 | 11 | 15 |
| Etofenprox | 88 ± 17 | 82 ± 14 | 85 ± 10 | 8 | 15 | 17 | |
| Tefluthrin | 78 ± 19 | 88 ± 17 | 95 ± 10 | 11 | 15 | 18 | |
| λ-Cyhalothrin | 77 ± 11 | 82 ± 11 | 81 ± 18 | 13 | 17 | 20 | |
| Acrinathrin | 80 ± 10 | 81 ± 17 | 88 ± 9.2 | 6 | 8 | 15 | |
| tau-Fluvalinate | 82 ± 3.9 | 76 ± 7.1 | 75 ± 8.0 | 14 | 11 | 15 | |
| Bifenthrin | 85 ± 3.8 | 79 ± 7.9 | 82 ± 10 | 12 | 8 | 18 | |
| Cypermethrin | 80 ± 10 | 82 ± 15 | 101 ± 11 | 6 | 7 | 9 | |
| Esfenvalerate | 82 ± 10 | 71 ± 5.2 | 72 ± 5.0 | 13 | 13 | 17 | |
| β-Cyfluthrin | 85 ± 11 | 91 ± 12 | 93 ± 8.6 | 10 | 8 | 14 | |
| Permethrin | 71 ± 4.7 | 70 ± 5.3 | 65 ± 9.2 | 6 | 7 | 8 | |
| 2017 | Analyte and Concentrations Range (mg/kg) | Findings | ||||
| Matrix | Dimethoate | Omethoate | β-Cyfluthrin | λ-Cyhalothrin | Positive * | Negative |
| Honeybees | 0.020–2.300 | 0.23 | nd ** | 0.14 | 6 | 9 |
| Honey | 0.0017 | nd | nd | nd | 1 | 8 |
| 2018 | Analyte and Concentrations Range (mg/kg) | |||||
| Matrix | Dimethoate | Omethoate | β-Cyfluthrin | λ-Cyhalothrin | Positive * | Negative |
| Honeybees | <LOQ–0.013 | 0.0024–0.020 | 0.0035–0.630 | <LOQ–0.010 | 24 | 22 |
| Bumblebees | 0.0041–0.700 | 0.0013–0.059 | nd | 0.0051 | 6 | 5 |
| Honey | 0.0057–0.022 | 0.0051–0.031 | nd | 0.048 | 12 | 15 |
| 2017 | Analyte and Concentrations Range (mg/kg) | ||||
| Field Treatment | Dimethoate | Thiacloprid | Chlorpyrifos | β-Cyfluthrin | λ-Cyhalothrin |
| Bait spray | 0.014–0.64 | 0.011–0.028 | 0.015–0.017 | nd * | nd |
| Cover spray | 0.01–0.11 | nd | nd | nd | nd |
| Non-treated | nd | nd | nd | nd | nd |
| 2018 | Analyte and Concentrations Range (mg/kg) | ||||
| Field Treatment | Dimethoate | Thiacloprid | Chlorpyrifos | β-Cyfluthrin | λ-Cyhalothrin |
| Bait spray | nd | nd | nd | 0.050–0.056 | 0.016–0.019 |
| Cover spray | nd | nd | nd | nd | 0.010–0.023 |
| Non-treated | nd | nd | nd | nd | 0.022–0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varikou, K.; Kasiotis, K.M.; Bempelou, E.; Manea-Karga, E.; Anagnostopoulos, C.; Charalampous, A.; Garantonakis, N.; Birouraki, A.; Hatjina, F.; Machera, K. A Pesticide Residues Insight on Honeybees, Bumblebees and Olive Oil after Pesticidal Applications against the Olive Fruit Fly Bactrocera oleae (Diptera: Tephritidae). Insects 2020, 11, 855. https://doi.org/10.3390/insects11120855
Varikou K, Kasiotis KM, Bempelou E, Manea-Karga E, Anagnostopoulos C, Charalampous A, Garantonakis N, Birouraki A, Hatjina F, Machera K. A Pesticide Residues Insight on Honeybees, Bumblebees and Olive Oil after Pesticidal Applications against the Olive Fruit Fly Bactrocera oleae (Diptera: Tephritidae). Insects. 2020; 11(12):855. https://doi.org/10.3390/insects11120855
Chicago/Turabian StyleVarikou, Kyriaki, Konstantinos M. Kasiotis, Eleftheria Bempelou, Electra Manea-Karga, Chris Anagnostopoulos, Angeliki Charalampous, Nikos Garantonakis, Athanasia Birouraki, Fani Hatjina, and Kyriaki Machera. 2020. "A Pesticide Residues Insight on Honeybees, Bumblebees and Olive Oil after Pesticidal Applications against the Olive Fruit Fly Bactrocera oleae (Diptera: Tephritidae)" Insects 11, no. 12: 855. https://doi.org/10.3390/insects11120855
APA StyleVarikou, K., Kasiotis, K. M., Bempelou, E., Manea-Karga, E., Anagnostopoulos, C., Charalampous, A., Garantonakis, N., Birouraki, A., Hatjina, F., & Machera, K. (2020). A Pesticide Residues Insight on Honeybees, Bumblebees and Olive Oil after Pesticidal Applications against the Olive Fruit Fly Bactrocera oleae (Diptera: Tephritidae). Insects, 11(12), 855. https://doi.org/10.3390/insects11120855








