Using Moderate Transgene Expression to Improve the Genetic Sexing System of the Australian Sheep Blow Fly Lucilia cuprina
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Rearing and Germ-Line Transformation
2.2. Plasmid Construction
2.3. RNA Isolation and qRT-PCR Analysis
2.4. Confocal Imaging to Assess Ovary-Specific Expression of tTA
2.5. Female Lethality Test and Embryo-Specific Lethality Assessments
2.6. Statistical Analysis
3. Results
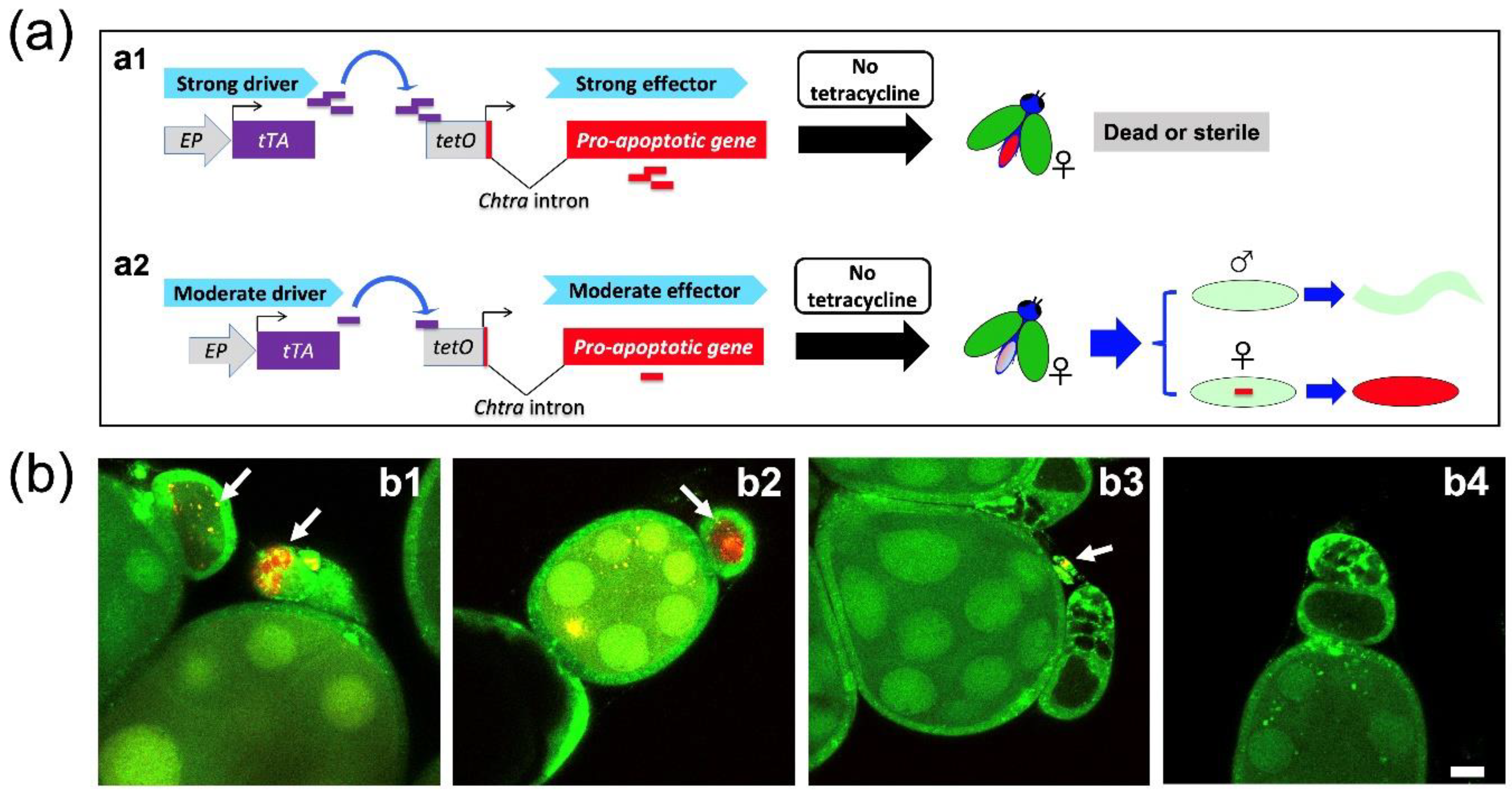
3.1. A Moderate Driver Line with Reduced Expression in Ovaries
3.2. The Combination of Selected Driver and Effector Lines Determines Female Fertility and Lethality
3.3. Female Embryo-Specific Lethality of DH Strains with EF3A
4. Discussion
5. Conclusions
Data Availability
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heath, A.C.; Bishop, D.M. Flystrike in New Zealand: An overview based on a 16-year study, following the introduction and dispersal of the Australian sheep blowfly, Lucilia cuprina Wiedemann (Diptera: Calliphoridae). Vet. Parasitol. 2006, 137, 333–344. [Google Scholar] [CrossRef]
- Smith, R. Battling the blowfly. In Beyond the Bale; Australian Wool Innovation: Sydney, NSW, Australia, 2007; p. 2. [Google Scholar]
- Williams, K.; Villet, M.H. Ancient and modern hybridization between Lucilia sericata and L. cuprina (Diptera: Calliphoridae). Eur. J. Entomol. 2013, 110, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Owings, C.G.; Picard, C.J. New Distribution Record for Lucilia cuprina (Diptera: Calliphoridae) in Indiana, United States. J. Insect Sci. 2018, 18, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Heath, A.C.G.; Bishop, D.M. Flystrike in New Zealand. Surveillance 1995, 22, 11–13. [Google Scholar]
- Tellam, R.L.; Bowles, V.M. Control of blowfly strike in sheep: Current strategies and future prospects. Int. J. Parasitol. 1997, 27, 261–273. [Google Scholar] [CrossRef]
- Scott, M.J.; Concha, J.B.; Welch, P.L.; Skoda, S.R. Research Advances in the Screwworm Eradication Program Over the Past 25 Years. Entomol. Exp. Appl. 2017, 164, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Foster, G.G.; Weller, G.L.; James, W.J.; Paschalidis, K.M.; McKenzie, L.J. Advances in sheep blowfly genetic control in Australia. In Management of Insect Pests: Nuclear and Related Molecular and Genetic Techniques; IAEA, Ed.; International Atomic Energy Agency: Vienna, Austria, 1993; pp. 299–312. [Google Scholar]
- Knipling, E.F. Sterile-male method of population control. Science 1959, 130, 902–904. [Google Scholar] [CrossRef]
- Foster, G.G. Chromosomal inversions and genetic control revisited: The use of inversions in sexing systems for higher Diptera. Theor. Appl. Genet. 1991, 81, 619–623. [Google Scholar] [CrossRef]
- Rendon, P.; McInnis, D.; Lance, D.; Stewart, J. Medfly (Diptera: Tephritidae) genetic sexing: Large-scale field comparison of males-only and bisexual sterile fly releases in Guatemala. J. Econ. Entomol. 2004, 97, 1547–1553. [Google Scholar] [CrossRef]
- Heinrich, J.C.; Scott, M.J. A repressible female-specific lethal genetic system for making transgenic insect strains suitable for a sterile-release program. Proc. Natl. Acad. Sci. USA 2000, 97, 8229–8232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.D.; Donnelly, C.A.; Wood, R.J.; Alphey, L.S. Insect population control using a dominant, repressible, lethal genetic system. Science 2000, 287, 2474–2476. [Google Scholar] [CrossRef] [PubMed]
- Schetelig, M.F.; Handler, A.M. A transgenic embryonic sexing system for Anastrepha suspensa (Diptera: Tephritidae). Insect Biochem. Mol. Biol. 2012, 42, 790–795. [Google Scholar] [CrossRef]
- Ogaugwu, C.E.; Schetelig, M.F.; Wimmer, E.A. Transgenic sexing system for Ceratitis capitata (Diptera: Tephritidae) based on female-specific embryonic lethality. Insect Biochem. Mol. Biol. 2013, 43, 1–8. [Google Scholar] [CrossRef]
- Yan, Y.; Scott, M.J. A transgenic embryonic sexing system for the Australian sheep blow fly Lucilia cuprina. Sci. Rep. 2015, 5, 16090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Linger, R.J.; Scott, M.J. Building early-larval sexing systems for genetic control of the Australian sheep blow fly Lucilia cuprina using two constitutive promoters. Sci. Rep. 2017, 7, 2538. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Vensko, S.P.I.; Belikoff, E.J.; Scott, M.J. Conservation and sex-specific splicing of the transformer gene in the Calliphorids Cochliomyia hominivorax, Cochliomyia macellaria and Lucilia sericata. PLoS ONE 2013, 8, e56303. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, P.W.; Pinkerton, A.C.; O’Brochta, D.A. Genetic transformation systems in insects. Annu. Rev. Entomol. 2001, 46, 317–346. [Google Scholar] [CrossRef]
- Schetelig, M.F.; Targovska, A.; Meza, J.S.; Bourtzis, K.; Handler, A.M. Tetracycline-suppressible female lethality and sterility in the Mexican fruit fly, Anastrepha ludens. Insect Mol. Biol. 2016, 25, 500–508. [Google Scholar] [CrossRef]
- Yan, Y.; Williamson, M.E.; Davis, R.J.; Andere, A.A.; Picard, C.J.; Scott, M.J. Improved transgenic sexing strains for genetic control of the Australian sheep blow fly Lucilia cuprina using embryo-specific gene promoters. Mol. Genet. Genom. 2020, 295, 287–298. [Google Scholar] [CrossRef]
- Edman, R.M.; Linger, R.J.; Belikoff, E.J.; Li, F.; Sze, S.H.; Tarone, A.M.; Scott, M.J. Functional characterization of calliphorid cell death genes and cellularization gene promoters for controlling gene expression and cell viability in early embryos. Insect Mol. Biol. 2015, 24, 58–70. [Google Scholar] [CrossRef]
- Bergmann, A.; Agapite, J.; McCall, K.; Steller, H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell 1998, 95, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Franz, G. Genetic Sexing Strains in Mediterranean Fruit Fly, an Example for Other Species Amenable to Large-Scale Rearing for the Sterile Insect Technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A., Eds.; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Papathanos, P.A.; Bossin, H.C.; Benedict, M.Q.; Catteruccia, F.; Malcolm, C.A.; Alphey, L.; Crisanti, A. Sex separation strategies: Past experience and new approaches. Malaria J. 2009, 8, S5. [Google Scholar] [CrossRef] [Green Version]
- Koga, R.; Tsuchida, T.; Sakurai, M.; Fukatsu, T. Selective elimination of aphid endosymbionts: Effects of antibiotic dose and host genotype, and fitness consequences. FEMS Microbiol. Ecol. 2007, 60, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Jasinskas, A.; Barbour, A.G. Antibotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS ONE 2007, 2, e405. [Google Scholar] [CrossRef]
- Yuval, B.; Ben-Ami, E.; Behar, A.; Ben-Yosef, M.; Jurkevitch, E. The Mediterranean fruit fly and its bacteria—potential for improving sterile insect technique operations. J. Appl. Entomol. 2013, 1, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Schetelig, M.F.; Nirmala, X.; Handler, A.M. Pro-apoptotic cell death genes, hid and reaper, from the tephritid pest species, Anastrepha suspensa. Apoptosis 2011, 16, 759–768. [Google Scholar] [CrossRef]
- Horn, C.; Wimmer, E.A. A transgene-based, embryo-specific lethality system for insect pest management. Nat. Biotechnol. 2003, 21, 64–70. [Google Scholar] [CrossRef]
- Li, F.; Wantuch, H.A.; Linger, R.J.; Belikoff, E.J.; Scott, M.J. Transgenic sexing system for genetic control of the Australian sheep blow fly Lucilia cuprina. Insect Biochem. Mol. Biol. 2014, 51, 80–88. [Google Scholar] [CrossRef]
- Schwirz, J.; Yan, Y.; Franta, Z.; Schetelig, M.F. Bicistronic expression and differential localization of proteins in insect cells and Drosophila suzukii using picornaviral 2A peptides. Insect Biochem. Mol. Biol. 2020, 119, 103324. [Google Scholar] [CrossRef]
- Schetelig, M.F.; Yan, Y.; Zhao, Y.; Handle, A.M. Genomic targeting by recombinase-mediated cassette exchange in the spotted wing drosophila, Drosophila suzukii. Insect Mol. Biol. 2018, 1–9. [Google Scholar] [CrossRef]
- Davis, R.J.; Belikoff, E.J.; Scholl, E.H.; Li, F.; Scott, M.J. no blokes is essential for male viability and X chromosome gene expression in the Australian sheep blowfly. Curr. Biol. 2018, 28, 1987–1992.e1983. [Google Scholar] [CrossRef] [Green Version]
- Paulo, D.F.; Williamson, M.E.; Arp, A.P.; Li, F.; Sagel, A.; Skoda, S.R.; Sanchez-Gallego, J.; Vasquez, M.; Quintero, G.; de Leon, A.A.P.; et al. Specific gene disruption in the major livestock pests Cochliomyia hominivorax and Lucilia cuprina Using CRISPR/Cas9. G3 2019, 9, 3045–3055. [Google Scholar] [CrossRef] [Green Version]
- Anstead, C.A.; Korhonen, P.K.; Young, N.D.; Hall, R.S.; Jex, A.R.; Murali, S.C.; Hughes, D.S.; Lee, S.F.; Perry, T.; Stroehlein, A.J.; et al. Lucilia cuprina genome unlocks parasitic fly biology to underpin future interventions. Nat. Commun. 2015, 6, 7344. [Google Scholar] [CrossRef] [Green Version]
- Scott, M.J.; Benoit, J.B.; Davis, R.J.; Bailey, S.T.; Varga, V.; Martinson, E.O.; Hickner, P.V.; Syed, Z.; Cardoso, G.A.; Torres, T.T.; et al. Genomic analyses of a livestock pest, the New World screwworm, find potential targets for genetic control programs. Commun. Biol. 2020, 3, 424. [Google Scholar] [CrossRef]
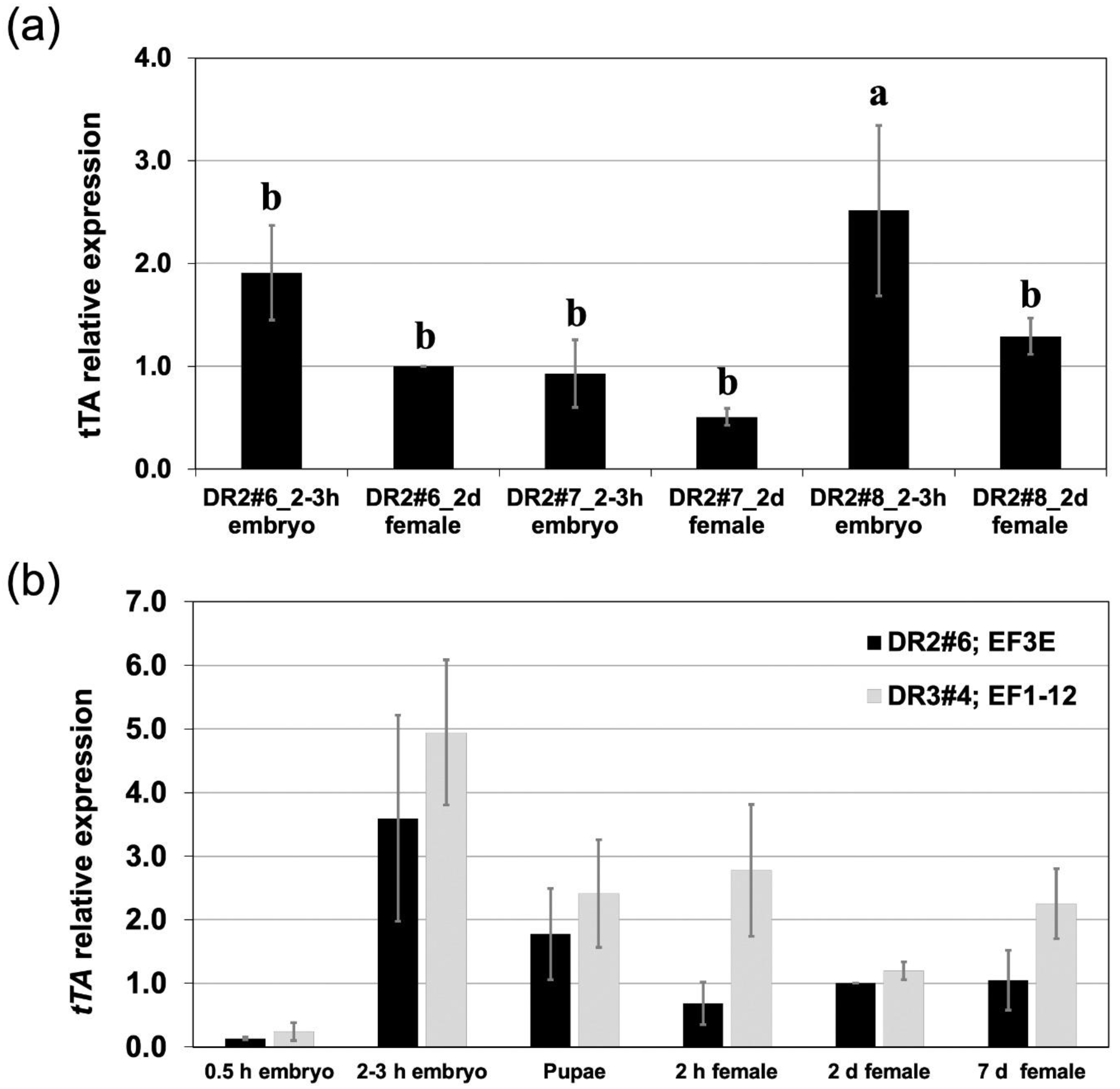


| Strains | Tet Water a | Egg Clutches b | #Pupae c | #Male | #Female | Female (%) |
|---|---|---|---|---|---|---|
| Wildtype | 0 | 4–8 | 843 ± 105 | 402 ± 46 | 376 ± 33 | 48.3 |
| DR2#7; EF3F | 0 | 4–8 | 503 ± 46 | 248 ± 28 | 160 ± 18 | 39.2 |
| DR2#7; EF3A | 0 | 4–8 | 143 ± 28 | 112 ± 25 | 0 | 0 |
| DR2#7; EF3A | 1 | 4–8 | 193 | 159 | 0 | 0 |
| DR2#7; EF3A | 3 | 4–8 | 197 | 153 | 0 | 0 |
| DR2#8; EF3F | 0 | 4–8 | 359 ± 38 | 176 ± 42 | 101 ± 11 | 36.3 |
| DR2#8; EF3A | 0 | No eggs | ||||
| DR2#8; EF3A | 1 | 1 | 5 | 3 | 0 | 0 |
| DR2#8; EF3A | 3 | 1–3 | 41 ± 7 | 31 ± 2 | 0 | 0 |
| DR2#8; EF3A | 50 | 4–8 | 249 ± 14 | 197 ± 9 | 0 | 0 |
| DR2#8; EF1-13 | 1 | No eggs | ||||
| DR2#8; EF1-13 | 3 | No eggs | ||||
| DR2#8; EF1-13 | 10 | No eggs | ||||
| DR2#8; EF1-13 | 20 | No eggs | ||||
| DR2#8; EF1-13 | 100 | 1–3 | 321 | 296 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Williamson, M.E.; Scott, M.J. Using Moderate Transgene Expression to Improve the Genetic Sexing System of the Australian Sheep Blow Fly Lucilia cuprina. Insects 2020, 11, 797. https://doi.org/10.3390/insects11110797
Yan Y, Williamson ME, Scott MJ. Using Moderate Transgene Expression to Improve the Genetic Sexing System of the Australian Sheep Blow Fly Lucilia cuprina. Insects. 2020; 11(11):797. https://doi.org/10.3390/insects11110797
Chicago/Turabian StyleYan, Ying, Megan E. Williamson, and Maxwell J. Scott. 2020. "Using Moderate Transgene Expression to Improve the Genetic Sexing System of the Australian Sheep Blow Fly Lucilia cuprina" Insects 11, no. 11: 797. https://doi.org/10.3390/insects11110797





