Comparison of Toxicological Bioassays for Whiteflies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Application Route (Main Plot)
2.2. Bioassay Method (Subplot)
2.3. Insecticide Treatment (Sub-Subplot)
2.4. Data Analysis
3. Results
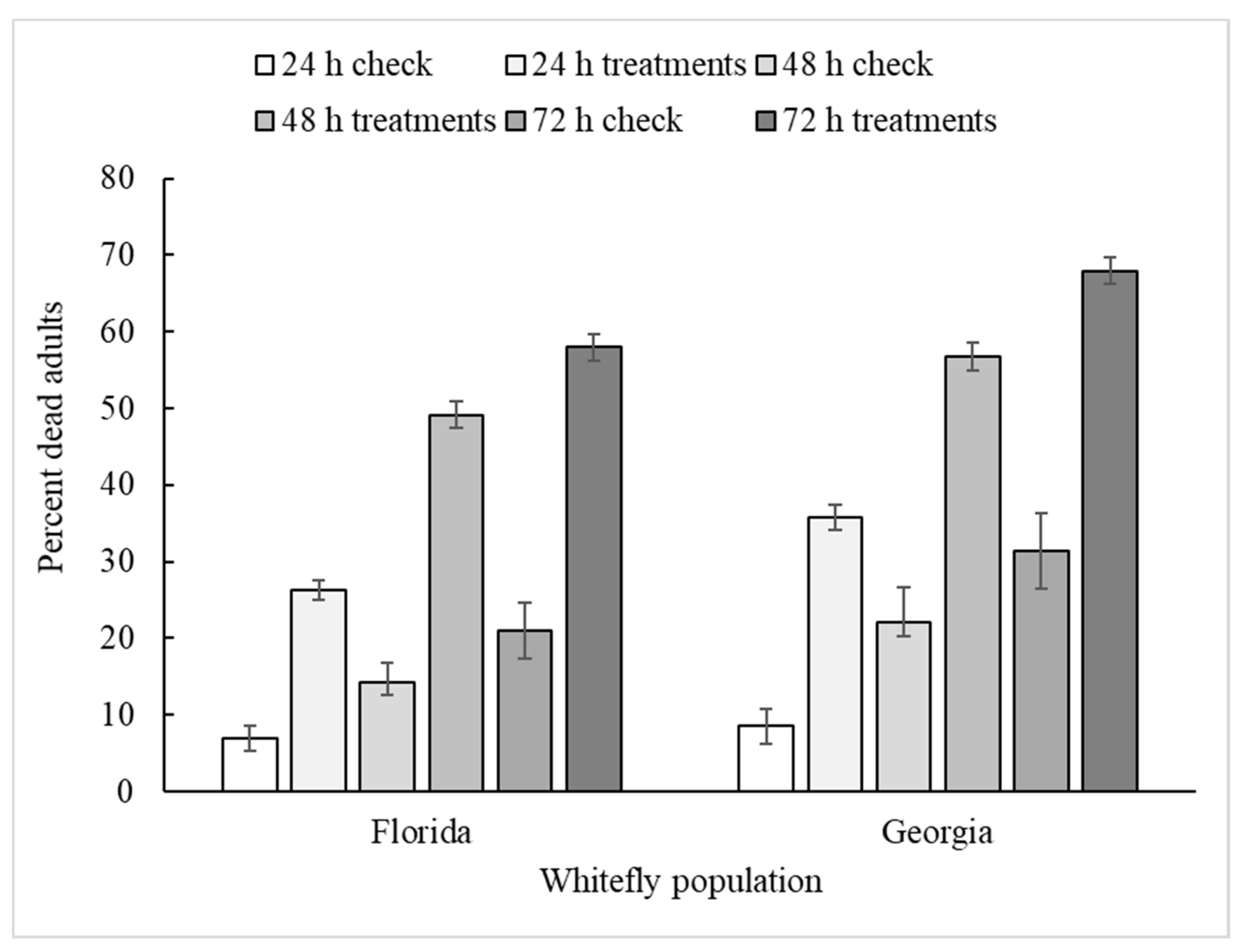
3.1. Application Route (Main Plot)
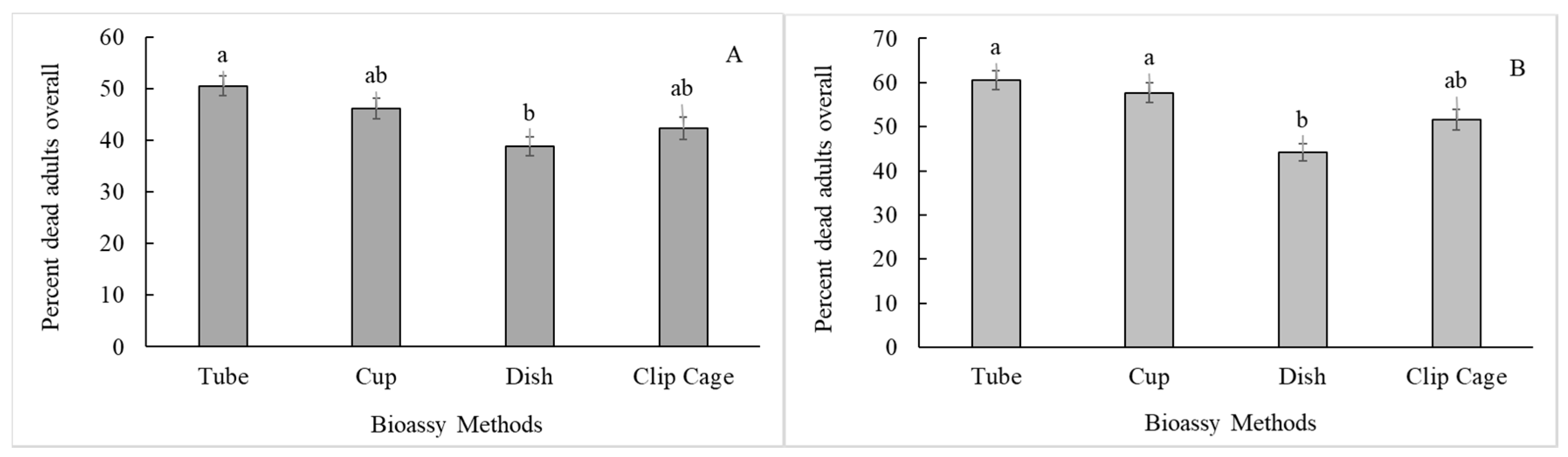
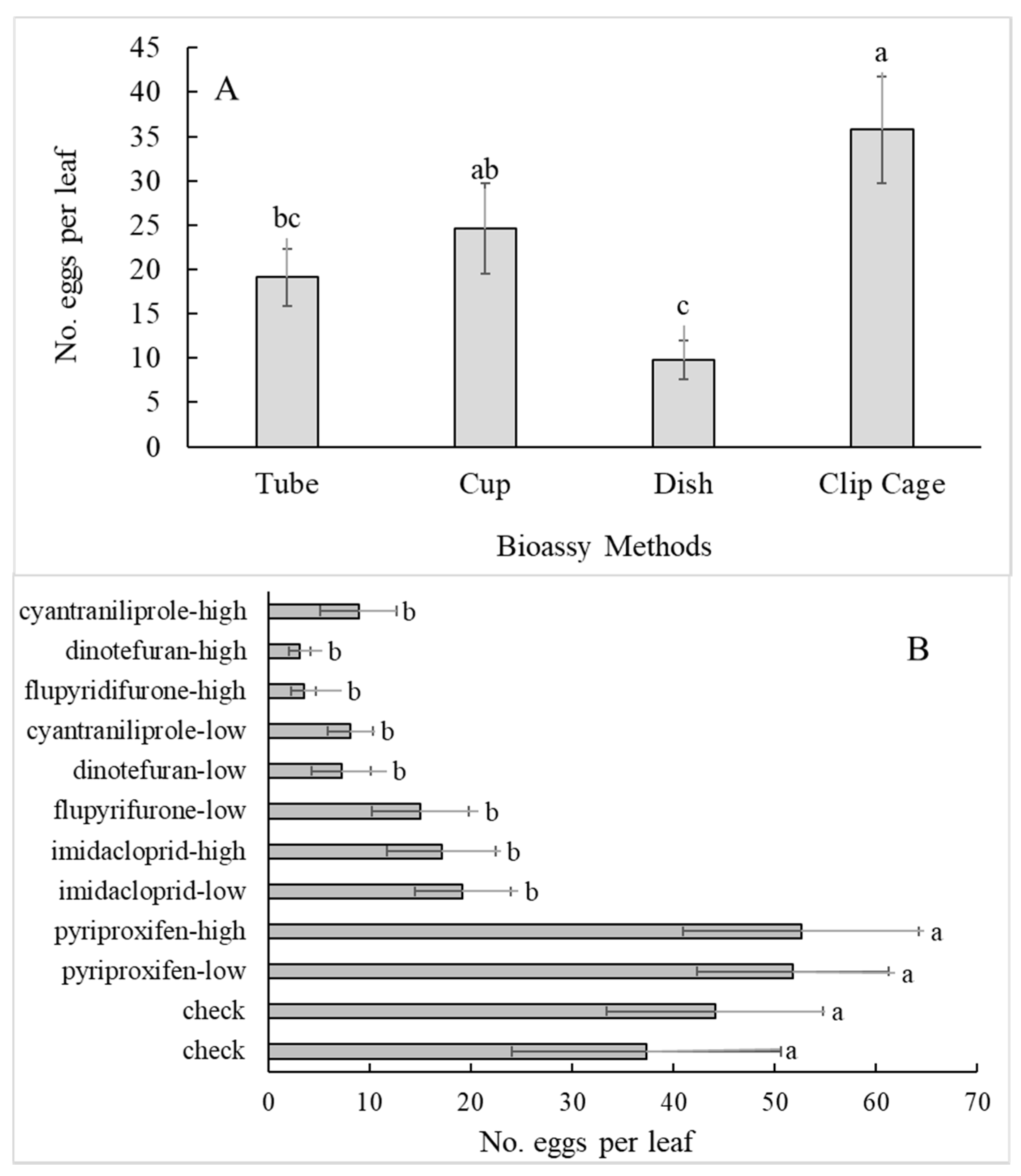
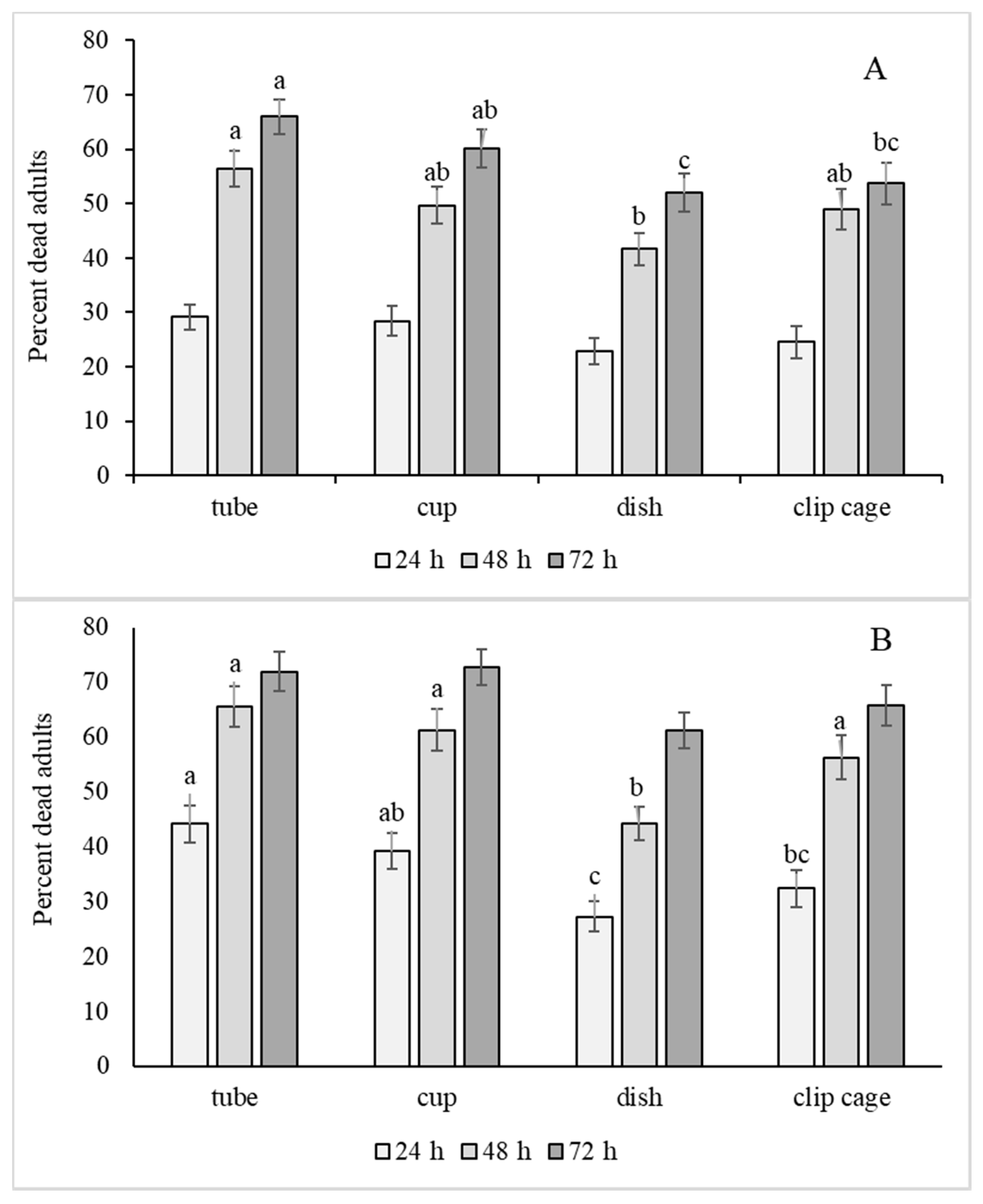
3.2. Bioassay Method (Subplot)
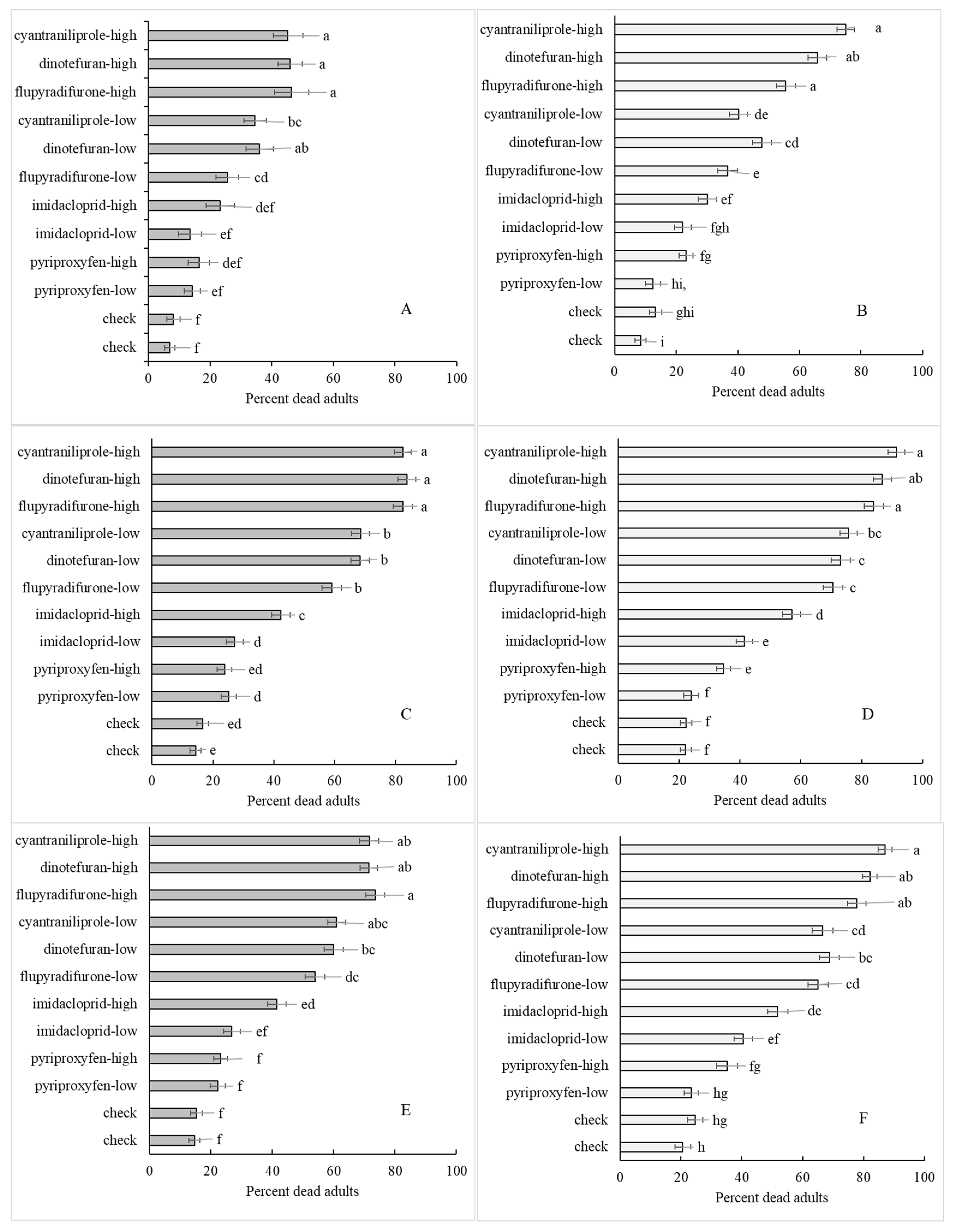
3.3. Insecticide Treatment (Sub-Subplot)
3.4. Interactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abd-Rabou, S.; Simmons, A.M. Survey of reproductive host plants of Bemisia tabaci (Hemiptera: Aleyrodidae) in Egypt, including new host records. Entomol. News 2010, 121, 456–465. [Google Scholar] [CrossRef]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant. Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Dittrich, V.; Uk, S.; Ernst, G.H. Chemical control and insecticide resistance of whiteflies. In Whiteflies: Their Bionomics, Pest Status, and Management, 1st ed.; Gerling, D., Ed.; Intercept Ltd.: Andover, Hants, UK, 1990; pp. 263–286. [Google Scholar]
- Denholm, I.; Cahill, M.; Byrne, F.J.; Devonshire, A.L. Progress with documenting and combatting insecticide resistance. In Bemisia 1995: Taxonomy, Biology, Damage, Control and Management, 1st ed.; Gerling, D., Mayer, R.T., Eds.; Intercept Ltd.: Andover, UK; Hants, UK, 1995; p. 702. [Google Scholar]
- Cahill, M.; Gorman, K.; Day, S.; Denholm, I.; Elbert, A.; Nauen, R. Baseline determination and detection of resistance to imidacloprid in Bemisia tabaci (Homoptera: Aleyrodidae). Bull. Entomol. Res. 1996, 86, 343–349. [Google Scholar] [CrossRef]
- Alon, M.; Benting, J.; Luecke, B.; Ponge, T.; Alon, F.; Morin, S. Multiple origins of pyrethroid resistance in sympatric biotypes of Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Biochem. Mol. Biol. 2006, 36, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.J.; Palumbo, J.C.; Prabhaker, N.; Horowitz, A.R.; Denholm, I. Ecological determinants of Bemisia tabaci resistance to insecticides. In Bemisia: Bionomics and Management of a Global Pest; Springer: Dordrecht, The Netherlands, 2009; pp. 423–465. [Google Scholar]
- Castle, S.J.; Prabhaker, N. Monitoring changes in Bemisia tabaci (Hemiptera: Aleyrodidae) susceptibility to neonicotinoid insecticides in Arizona and California. J. Econ. Entomol. 2013, 106, 1404–1413. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Li, X.; Dennehy, T.J.; Lei, C.; Wang, M.; Degain, B.A.; Nichols, R.L. Pyriproxyfen resistance of Bemisia tabaci (Homoptera: Aleyrodidae) biotype B: Metabolic mechanism. J. Econ. Entomol. 2010, 103, 158–165. [Google Scholar] [CrossRef]
- Li, X.; Degain, B.A.; Harpold, V.S.; Marcon, P.G.; Nichols, R.L.; Fournier, A.J.; Naranjo, S.E.; Palumbo, J.C.; Ellsworth, P.C. Baseline susceptibilities of B- and Q-biotype Bemisia tabaci to anthranilic diamides in Arizona. Pest. Manag. Sci. 2012, 68, 83–91. [Google Scholar] [CrossRef]
- Ma, D.; Gorman, K.; Devine, G.; Luo, W.; Denholm, I. The biotype and insecticide-resistance status of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae), invading cropping systems in Xinjiang Uygur Autonomous Region, northwestern China. Crop. Prot. 2007, 26, 612–617. [Google Scholar] [CrossRef]
- Schuster, D.J.; Stansly, P.A.; Gilreath, P.R.; Polston, J.E. Management of Bemisia, TYLCV, and insecticide resistance in Florida vegetables. J. Insect Sci. 2008, 8, 43–44. [Google Scholar]
- Schuster, D.J.; Mann, R.S.; Toapanta, M.; Cordero, R.; Thompson, S.; Cyman, S.; Shurtleff, A.; Morris, R.F. Monitoring neonicotinoid resistance in biotype B of Bemisia tabaci in Florida. Pest. Manag. Sci. 2010, 66, 186–195. [Google Scholar] [CrossRef]
- Caballero, R.; Cyman, S.; Schuster, D. Monitoring insecticide resistance in biotype B of Bemisia tabaci (Hemiptera: Aleyrodidae) in Florida. Fla. Entomol. 2013, 96, 1243–1256. [Google Scholar] [CrossRef]
- Caballero, R.; Cyman, S.; Schuster, D. Baseline susceptibility of Bemisia tabaci, biotype B (Hemiptera: Aleyrodidae) to chlorantraniliprole in southern Florida. Fla. Entomol. 2013, 96, 1002–1008. [Google Scholar] [CrossRef]
- Caballero, R.; Schuster, D.J.; Peres, N.A.; Mangandi, J.; Hasing, T.; Trexler, F.; Kalb, S.; Portillo, H.E.; Marcon, P.C.; Annan, I.B. Effectiveness of cyantraniliprole for managing Bemisia tabaci (Hemiptera: Aleyrodidae) and interfering with transmission of Tomato yellow leaf curl virus on tomato. J. Econ. Entomol. 2015, 108, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.A.; Giurcanu, M.C. Residual effects of new insecticides on egg and nymph densities of Bemisia tabaci (Hemiptera: Aleyrodidae). Fla. Entomol. 2013, 96, 504–511. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.D.; Che, W.N.; Chen, L.U.O. First report of field resistance to cyantraniliprole, a new anthranilic diamide insecticide, on Bemisia tabaci MED in China. J. Integr. Agric. 2018, 17, 158–163. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest. Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Schuster, D.; Spurgeon, S.; Riley, D. Imidacloprid resistance monitoring and management in whitefly. In Proceedings of the 2008 Southeastern Regional Vegetable Conference, 1st ed.; Savannah, G.A., Kelley, W.T., Eds.; University of Georgia: Athens, GA, USA, 2008; p. 702. [Google Scholar]
- Thompson, C. UGA CAES Team Researching Whiteflies Statewide. Available online: https://extension.uga.edu/story.html?storyid=7602 (accessed on 1 September 2020).
- Staetz, C.A.; Boyler, K.A.; Gage, E.V.; Riley, D.G.; Wolfenbarger, D.A. Vial bioassay for contact insecticides for adult whiteflies, Bemisia tabaci. In Proceedings of the Beltwide Cotton Production Conference, Memphis, TN, USA, 6–10 January 1992; Herber, D.J., Richter, D.A., Eds.; National Cotton Council of America: Cordova, TN, USA, 1992; pp. 704–707. [Google Scholar]
- IRAC Susceptibility Test Method 015. Available online: https://www.irac-online.org/methods/trialeurodes-vaporariorum-bemisia-tabaci-adult/ (accessed on 1 September 2020).
- IRAC Susceptibility Test Method 008. Available online: https://www.irac-online.org/methods/bemisia-tabaci-adults/ (accessed on 1 September 2020).
- Van Lenteren, J.C.; Noldus, L.P.J.J. Whitefly-plant relationships: Behavioural and ecological aspects. In Whiteflies: Their Bionomics, Pest Status, and Management, 1st ed.; Gerling, D., Ed.; Intercept Ltd.: Andover, UK; Hants, UK, 1990; pp. 47–89. [Google Scholar]
- Yu, S. The Toxicology and Biochemistry of Insecticides, 2nd ed.; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Sparks, A.N., Jr.; Riley, D.G. Horticultural crops commercial vegetable insect control. In 2020 Georgia Pest Management Handbook; Commercial Edition; Spec. Bull. 28.; Guillebeau, P., Ed.; Univ. of Georgia Coop. Ext. Service: Athens, GA, USA, 2020; pp. 309–363. [Google Scholar]
- SAS Institute. SAS® Enterprise Guide 8.1: User’s Guide; SAS Institute: Cary, NC, USA, 2019; Available online: https://documentation.sas.com/?docsetId=egug&docsetTarget=titlepage.htm&docsetVersion=8.1&locale=en (accessed on 1 September 2020).
- Prabhaker, N.D.; Coudriet, D.L.; Meyerdirk, D.E. Insecticide resistance in the sweetpotato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 1985, 78, 748–752. [Google Scholar] [CrossRef]
- Wolfenbarger, D.A.; Riley, D.G.; Staetz, C.A.; Herzog, G.A. Response of silverleaf whitefly (Homoptera: Aleyrodidae) to bifenthrin and endosulfan by vial bioassay in Florida, Georgia and Texas. J. Entomol. Sci. 1998, 33, 412–420. [Google Scholar] [CrossRef]
- Elbert, A.; Nauen, R. Bioassays for imidacloprid for resistance monitoring against the whitefly Bemisia tabaci. In Brighton Crop Protection Conference: Pests and Diseases. In Proceedings of the Brighton Crop Protection Conference on Pests and Diseases, Brighton, UK, 18–21 November 1996; British Crop Protection Council: Farnham, UK, 1996. [Google Scholar]
- Castle, S.J.; Merten, P.; Prabhaker, N. Comparative susceptibility of Bemisia tabaci to imidacloprid in field- and laboratory-based bioassays. Pest. Manag. Sci. 2014, 70, 1538–1546. [Google Scholar] [CrossRef]
- Chen, J.C.; Wang, Z.H.; Cao, L.J.; Gong, Y.J.; Hoffmann, A.A.; Wei, S.J. Toxicity of seven insecticides to different developmental stages of the whitefly Bemisia tabaci MED (Hemiptera: Aleyrodidae) in multiple field populations of China. Ecotoxicology 2018, 27, 742–751. [Google Scholar] [CrossRef]
- Sain, S.K.; Monga, D.; Kumar, R.; Nagrale, D.T.; Kranthi, S.; Kranthi, K. Comparative effectiveness of bioassay methods in identifying the most virulent entomopathogenic fungal strains to control Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). Egypt. J. Biol. Pest. Control 2019, 29, 31. [Google Scholar] [CrossRef] [Green Version]
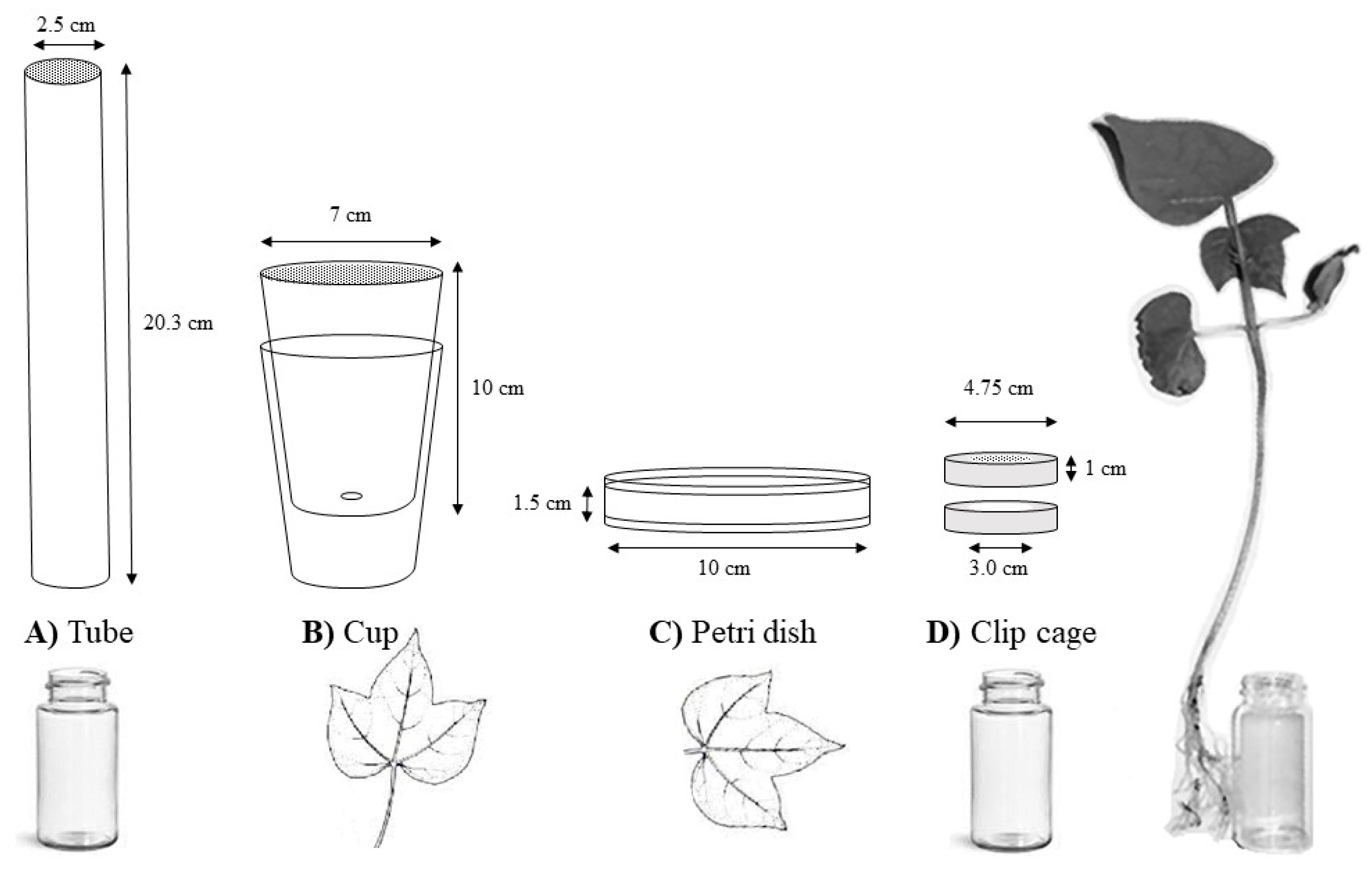
| Treatment | Florida Population | Georgia Population | ||||
|---|---|---|---|---|---|---|
| DF 1 | F | P > F | DF | F | P > F | |
| Application Route | 1, 26.5 | 0.29 | 0.598 | 1, 23.7 | 0.27 | 0.606 |
| Bioassay | 3, 26.6 | 3.18 | 0.040 | 3, 23.9 | 8.29 | <0.001 |
| Insecticide | 11, 296 | 41.5 | <0.001 | 11, 354 | 39.1 | <0.001 |
| Hour | 2, 915 | 222 | <0.001 | 2, 798 | 338 | <0.001 |
| Application × Bioassay | 3, 26.5 | 0.82 | 0.492 | 3, 23.2 | 1.95 | 0.149 |
| Application × Insecticide | 11, 315 | 1.88 | 0.041 | 11, 404 | 5.97 | <0.001 |
| Application × Bioassay × Insecticide | 33, 315 | 1.15 | 0.269 | 33, 399 | 1.12 | 0.307 |
| Bioassay × Insecticide | 33, 313 | 1.20 | 0.213 | 33, 394 | 2.69 | <0.001 |
| Hour × Application | 2, 911 | 0.03 | 0.969 | 2, 760 | 1.08 | 0.339 |
| Hour × Bioassay | 6, 914 | 2.21 | 0.040 | 6, 769 | 3.96 | <0.001 |
| Hour × Insecticide | 22, 913 | 2.75 | <0.001 | 22, 766 | 2.91 | <0.001 |
| Treatment | Florida Population | Georgia Population | ||||
|---|---|---|---|---|---|---|
| DF 1 | F | P > F | DF | F | P > F | |
| Application Route | 1, 2 | 0.16 | 0.729 | 1, 3 | 3.74 | 0.149 |
| Bioassay | 3, 6 | 3.53 | 0.089 | 3, 9 | 2.39 | 0.136 |
| Insecticide | 11, 220 | 6.34 | <0.001 | 11, 264 | 3.12 | <0.001 |
| Application * Bioassay | 3, 6 | 0.25 | 0.859 | 3, 9 | 0.38 | 0.773 |
| Application * Insecticide | 11, 220 | 0.97 | 0.474 | 11, 264 | 1.74 | 0.065 |
| Application * Bioassay * Insecticide | 33, 220 | 0.95 | 0.554 | 33, 264 | 0.58 | 0.968 |
| Bioassay * Insecticide | 33, 220 | 1.44 | 0.065 | 33, 264 | 0.77 | 0.817 |
| Treatment | Florida Population | Georgia Population | ||||
|---|---|---|---|---|---|---|
| DF 1 | F | P > F | DF | F | P > F | |
| Application Route | 1, 2 | 1.30 | 0.372 | 1, 3 | 4.12 | 0.135 |
| Bioassay | 3, 6 | 0.820 | 0.529 | 3, 9 | 0.78 | 0.533 |
| Insecticide | 11, 220 | 1.16 | 0.317 | 11, 264 | 0.91 | 0.527 |
| Application * Bioassay | 3, 6 | 1.46 | 0.316 | 3, 9 | 0.78 | 0.533 |
| Application * Insecticide | 11, 220 | 0.85 | 0.595 | 11, 264 | 0.79 | 0.646 |
| Application * Bioassay * Insecticide | 33, 220 | 0.72 | 0.866 | 33, 264 | 0.73 | 0.860 |
| Bioassay * Insecticide | 33, 220 | 0.87 | 0.673 | 33, 264 | 0.97 | 0.526 |
| Method | Insecticide Treatment | Florida Population | Georgia Population |
|---|---|---|---|
| Tube | check | 25.3 ± 3.15 d 1 | 13.8 ± 2.84 d |
| check | 13.5 ± 1.91 d | 21.6 ± 2.89 d | |
| pyriproxyfen—low | 20.7 ± 4.22 d | 28.4 ± 5.40 d | |
| pyriproxyfen—high | 21.7 ± 3.27 d | 23.2 ± 3.5 d | |
| imidacloprid—low | 32.9 ± 4.66 c,d | 53.6 ± 6.62 c | |
| imidacloprid—high | 55.4 ± 6.42 b,c | 65.5 ± 6.02 b,c | |
| flupyradifurone—low | 64.3 ± 5.73 a,b | 78.0 ± 5.76 a,b,c | |
| dinotefuran—low | 73.7 ± 5.02 a,b | 85.0 ± 4.51 a,b,c | |
| cyantraniliprole—low | 69.0 ± 5.31 a,b | 79.3 ± 5.26 a,b,c | |
| flupyradifurone—high | 82.1 ± 3.94 a | 87.7 ± 4.31 a,b | |
| dinotefuran—high | 77.2 ± 4.73 a,b | 95.8 ± 1.56 a | |
| cyantraniliprole—high | 70.7 ± 6.29 a,b | 94.4 ± 2.12 a | |
| Cup | check | 9.26 ± 3.00 e | 19.6 ± 3.88 e |
| check | 14.5 ± 3.29 d,e | 23.3 ± 3.18 e | |
| pyriproxyfen—low | 21.7 ± 5.21 c,d,e | 17.3 ± 4.29 e | |
| pyriproxyfen—high | 18.1 ± 3.57 c,d,e | 45.6 ± 7.66 c,d,e | |
| imidacloprid—low | 26.9 ± 4.98 c,d,e | 44.5 ± 5.93 d,e | |
| imidacloprid—high | 42.4 ± 5.53 b,c,d | 56.8 ± 6.40 b,c,d | |
| flupyradifurone—low | 50.5 ± 5.72 b,c | 73.4 ± 6.10 a,b,c | |
| dinotefuran—low | 67.1 ± 5.75 a,b | 77.4 ± 4.91 a,b | |
| cyantraniliprole—low | 65.4 ± 5.33 a,b | 70.5 ± 7.01 a,b,c,d | |
| flupyradifurone—high | 77.7 ± 5.54 a | 85.9 ± 4.42 a | |
| dinotefuran—high | 81.1 ± 3.99 a | 89.3 ± 3.23 a | |
| cyantraniliprole—high | 77.1 ± 4.44 a | 90.5 ± 3.60 a | |
| Petri dish | check | 8.97 ± 2.52 d | 25.8 ± 5.74 c |
| check | 13.2 ± 3.61 d | 30.0 ± 7.03 b,c | |
| pyriproxyfen—low | 25.6 ± 5.16 c,d | 24.5 ± 4.75 c | |
| pyriproxyfen—high | 27.2 ± 5.84 c,d | 36.3 ± 5.94 b,c | |
| imidacloprid—low | 14.6 ± 3.01 d | 32.7 ± 5.47 b,c | |
| imidacloprid—high | 31.6 ± 5.09 b,c,d | 38.5 ± 5.88 a,b,c | |
| flupyradifurone—low | 55.7 ± 6.22 a,b,c | 57.3 ± 7.52 a,b | |
| dinotefuran—low | 48.4 ± 5.54 a,b,c | 48.4 ± 6.69 a,b,c | |
| cyantraniliprole—low | 54.6 ± 5.73 a,b,c | 49.1 ± 5.87 a,b,c | |
| flupyradifurone—high | 62.4 ± 5.60 a,b | 58.6 ± 7.09 a,b | |
| dinotefuran—high | 61.2 ± 5.24 a | 58.2 ± 4.76 a,b | |
| cyantraniliprole—high | 65.8 ± 7.04 a | 69.7 ± 6.31 a | |
| Clip cage | check | 15.4 ± 4.51 d | 23.6 ± 7.01 e |
| check | 19.8 ± 5.52 c,d | 23.8 ± 5.00 e | |
| pyriproxyfen—low | 21.2 ± 5.49 c,d | 23.1 ± 3.63 e | |
| pyriproxyfen—high | 26.0 ± 5.19 b,c,d | 35.7 ± 8.74 c,d,e | |
| imidacloprid—low | 33.3 ± 7.89 b,c,d | 31.4 ± 5.14 d,e | |
| imidacloprid—high | 36.5 ± 6.22 b,c,d | 46.0 ± 7.53 c,d,e | |
| flupyradifurone—low | 45.7 ± 7.21 a,b,c d | 51.7 ± 6.65 c,d,e | |
| dinotefuran—low | 50.9 ± 7.23 a,b,c | 64.1 ± 6.82 b,c | |
| cyantraniliprole—low | 53.8 ± 6.34 a,b | 67.1 ± 8.10 b,c,d | |
| flupyradifurone—high | 71.9 ± 7.96 a | 79.0 ± 5.79 a,b | |
| dinotefuran—high | 66.1 ± 7.88,a | 85.2 ± 4.80 a,b | |
| cyantraniliprole—high | 72.6 ± 6.71,a | 94.4 ± 3.10 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparks, T.C.; Riley, D.G.; Simmons, A.M.; Guo, L. Comparison of Toxicological Bioassays for Whiteflies. Insects 2020, 11, 789. https://doi.org/10.3390/insects11110789
Sparks TC, Riley DG, Simmons AM, Guo L. Comparison of Toxicological Bioassays for Whiteflies. Insects. 2020; 11(11):789. https://doi.org/10.3390/insects11110789
Chicago/Turabian StyleSparks, Tanner C., David G. Riley, Alvin M. Simmons, and Liangzhen Guo. 2020. "Comparison of Toxicological Bioassays for Whiteflies" Insects 11, no. 11: 789. https://doi.org/10.3390/insects11110789
APA StyleSparks, T. C., Riley, D. G., Simmons, A. M., & Guo, L. (2020). Comparison of Toxicological Bioassays for Whiteflies. Insects, 11(11), 789. https://doi.org/10.3390/insects11110789






