Toxorhynchites Species: A Review of Current Knowledge
Abstract
:Simple Summary
Abstract
1. Introduction
2. General Biology
3. Taxonomic Classification
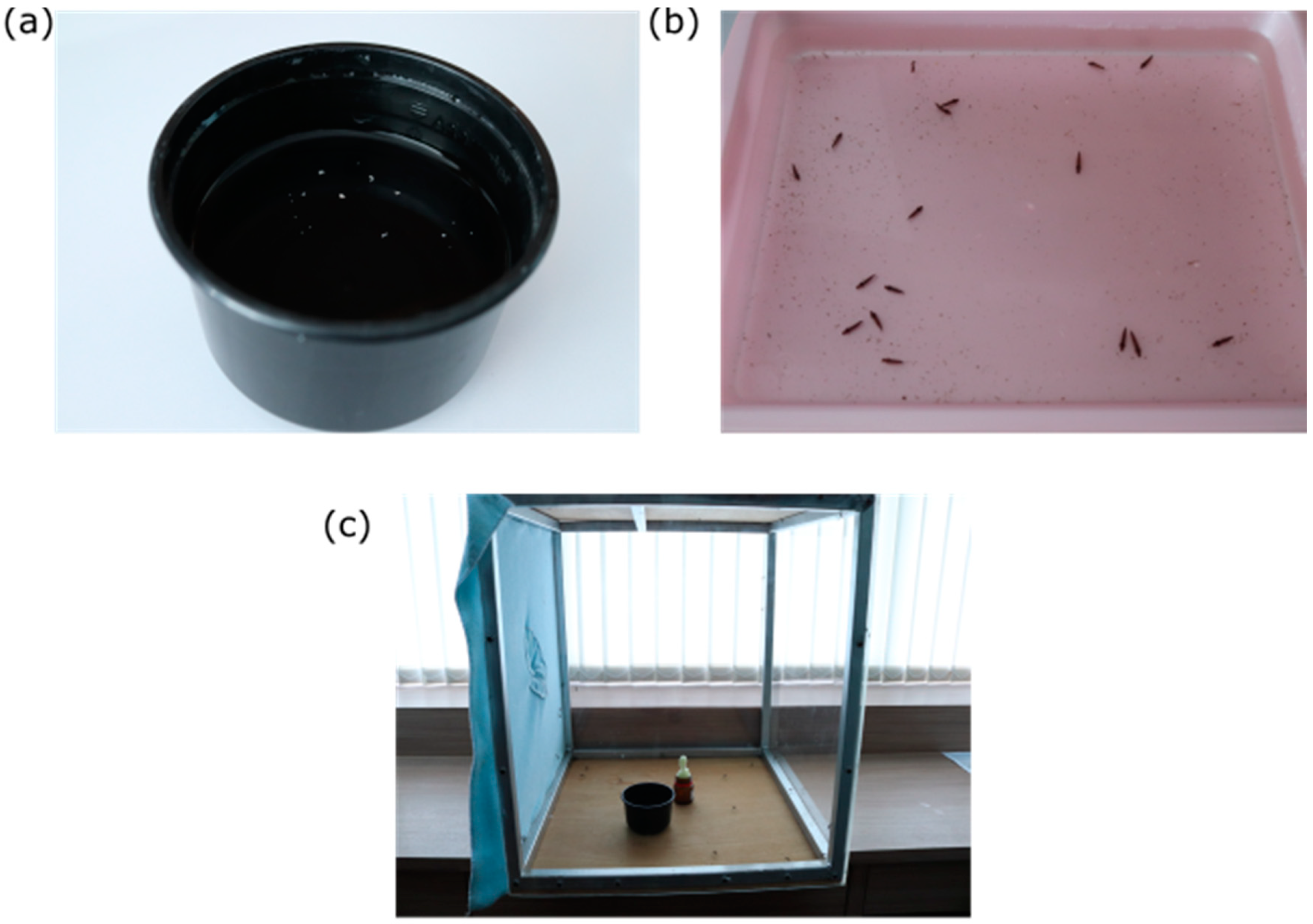
4. Development
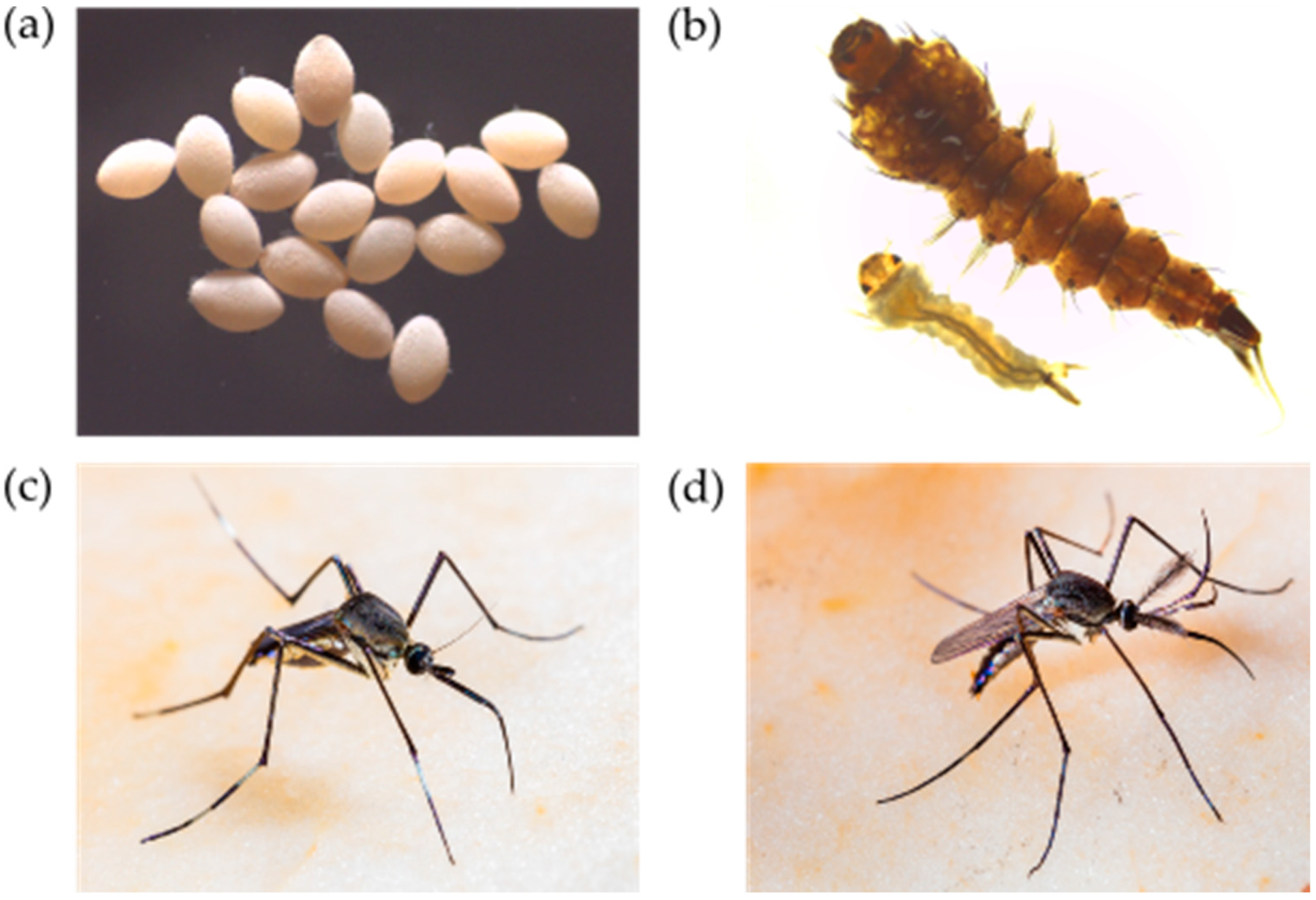
4.1. Eggs
4.2. Larvae
4.3. Adults
5. Olfaction
6. Interactions with Viruses
7. Interactions with Bacteria
8. Control Measures
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Dusfour, I.; Vontas, J.; David, J.-P.; Weetman, D.; Fonseca, D.M.; Corbel, V.; Raghavendra, K.; Coulibaly, M.B.; Martins, A.J.; Kasai, S.; et al. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl. Trop. Dis. 2019, 13, e0007615. [Google Scholar] [CrossRef] [PubMed]
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-Trotting Aedes aegypti and Aedes albopictus: Risk Factors for Arbovirus Pandemics. Vector-Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, N.M. Challenges and opportunities in controlling mosquito-borne infections. Nature 2018, 559, 490–497. [Google Scholar] [CrossRef]
- Kean, J.; Rainey, S.M.; McFarlane, M.; Donald, C.L.; Schnettler, E.; Kohl, A.; Pondeville, E. Fighting Arbovirus Transmission: Natural and Engineered Control of Vector Competence in Aedes Mosquitoes. Insects 2015, 6, 236–278. [Google Scholar] [CrossRef] [Green Version]
- Yen, P.-S.; Failloux, A.-B. A Review: Wolbachia-Based Population Replacement for Mosquito Control Shares Common Points with Genetically Modified Control Approaches. Pathogens 2020, 9, 404. [Google Scholar] [CrossRef]
- Flores, H.; Neill, S.L.O. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Dahmana, H.; Mediannikov, O.Y. Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens 2020, 9, 310. [Google Scholar] [CrossRef] [Green Version]
- Steffan, W.A.; Evenhuis, N.L. Biology of Toxorhynchites. Annu. Rev. Entomol. 1981, 26, 159–181. [Google Scholar] [CrossRef]
- Collins, L.E.; Blackwell, A. The biology of Toxorhynchites mosquitoes and their potential as biocontrol agents. Biocontrol News Inf. 2000, 21, 105–116. [Google Scholar]
- Focks, D.A. Toxorhynchites as biocontrol agents. J. Am. Mosq. Control Assoc. 2007, 23 (Suppl. 2), 118–127. [Google Scholar] [CrossRef]
- Schreiber, E.T. Toxorhynchites. J. Am. Mosq. Control Assoc. 2007, 23 (Suppl. 2), 129–132. [Google Scholar] [CrossRef]
- Alonso-Palomares, L.A.; Moreno-García, M.; Lanz-Mendoza, H.; Salazar, M.I. Molecular Basis for Arbovirus Transmission by Aedes aegypti Mosquitoes. Intervirology 2018, 61, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Horsfall, W.R. Mosquitoes—Their Bionomics and Relation to Disease. AIBS Bull. 1955, 5, 13–14. [Google Scholar]
- Clements, A. Sensory Reception and Behaviour. In The Biology of Mosquitoes; CAB International: Oxford, UK, 1999; Volume 2. [Google Scholar]
- MacDonald, W.W. Some ecological factors influencing the distribution of Malayan mosquitoes. In Proceedings of the Centenary And Bicentenary Congress Of Biology, Singapore, 2–9 December 1958; pp. 117–122. [Google Scholar]
- Harbach, R.E. The Culicidae (Diptera): A review of taxonomy, classification and phylogeny. Zootaxa 2007, 1668, 591–638. [Google Scholar] [CrossRef] [Green Version]
- Steffan, W.A.; Evenhuis, N.L.; Manning, D.L. Annotated bibliography of Toxorhynchites (Diptera: Culicidae). J. Med. Entomol. Suppl. 1980, 3, 1–140. [Google Scholar] [CrossRef] [PubMed]
- Steffan, W.A.; Evenhuis, N.L. Classification of the Subgenus Toxorhynchites (Diptera: Culicidae) I. Australasian, eastern Palaearctic, and Oriental species-groups1. J. Med. Entomol. 1985, 22, 421–446. [Google Scholar] [CrossRef]
- Evenhuis, N.L.; Steffan, W.A. Classification of the subgenus Toxorhynchites (Diptera: Culicidae) II. Revision of the Toxorhynchites acaudatus group. J. Med. Entomol. 1986, 23, 538–574. [Google Scholar] [CrossRef]
- Edwards, F.W. Mosquitoes of the Ethiopian Region III—Culicine Adults and Pupae; British Museum (Natural History): London, UK, 1941. [Google Scholar]
- Barraud, P.J. The Fauna of British India, Including Ceylon and Burma. Diptera; Family Culicidae. Tribes Megarhinini and Culicini; Taylor and Francis: London, UK, 1934; Volume 5. [Google Scholar]
- Hopkins, G.H.E. Mosquitoes of the Ethiopian Region, I.—Larval Bionomics of Mosquitoes and Taxonomy of Culicine Larvae; British Museum (Natural History): London, UK, 1952. [Google Scholar]
- Service, M.W. Handbook to the Afrotropical Toxorhynchitine and Culicine Mosquitoes, Excepting Aedes and Culex; British Museum (Natural History): London, UK, 1990. [Google Scholar]
- Ribeiro, H. Research on the mosquito subfamily Toxorhynchitinae (Diptera: Culicidae) [sic]. I―The Afrotropical group brevipalpis (Adults) (1). Arq. Mus. Bocage 1991, 2, 31–62. [Google Scholar]
- Belkin, J.N.; Heinemann, S.J.; Page, W.A. The Culicidae of Jamaica (Mosquito studies. XXI). Contrib. Am. Entomol. Inst. 1970, 6, 1–458. [Google Scholar]
- Steffan, W.A. Systematics and biological control potential of Toxorhynchites (Diptera: Culicidae). Mosq. Syst. News Lett. 1975, 7, 59–68. [Google Scholar]
- Harbach, R.E.; Kitching, I.J. Phylogeny and classification of the Culicidae (Diptera). Syst. Entomol. 1998, 23, 327–370. [Google Scholar] [CrossRef]
- Ribeiro, H. Les Toxorhynchites Theobald de Madagascar (Diptera: Culicidae). Annales de la Société Entomologique de France (N.S.) 2004, 40, 243–257. [Google Scholar] [CrossRef]
- Tyagi, B.K.; Munirathinam, A.; Krishnamoorthy, R.; Baskaran, G.; Govindarajan, R.; Krishnamoorthi, R.; Mariappan, T.; Dhananjeyanand, K.J.; Venkatesh, A. A revision of genus Toxorhynchites Theobald, 1901, in the South-East Asian countries, with description of a new species Toxorhynchites (Toxorhynchites) darjeelingensis from West Bengal, India (Diptera, Culicidae). Halteres 2015, 6, 13–32. [Google Scholar]
- Theobald, F.V. A Monograph of the Culicidae or Mosquitoes; British Museum (Natural History): London, UK, 1901; Volume 1. [Google Scholar]
- Miller, B.R.; Crabtree, M.B.; Savage, H.M. Phylogenetic relationships of the Culicomorpha inferred from 18S and 5.8S ribosomal DNA sequences. (Diptera:Nematocera). Insect Mol. Biol. 1997, 6, 105–114. [Google Scholar] [CrossRef]
- Shepard, J.J.; Andreadis, T.G.; Vossbrinck, C.R. Molecular phylogeny and evolutionary relationships among mosquitoes (Diptera: Culicidae) from the northeastern United States based on small subunit ribosomal DNA (18S rDNA) sequences. J. Med. Entomol. 2006, 43, 443–454. [Google Scholar] [CrossRef]
- Reidenbach, K.R.; Cook, S.; Bertone, M.A.; Harbach, R.E.; Wiegmann, B.M.; Besansky, N.J. Phylogenetic analysis and temporal diversification of mosquitoes (Diptera: Culicidae) based on nuclear genes and morphology. BMC Evol. Biol. 2009, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.F.; Machado, L.C.; De Paula, M.B.; Vieira, C.J.D.S.P.; de Morais Bronzoni, R.V.; de Melo-Santos, M.A.V.; da Luz Wallau, G. Time and mode of Culicidae evolutionary history. BioRxiv 2019. [Google Scholar] [CrossRef]
- Mitchell, A.; Sperling, F.A.; Hickey, D.A. Higher-level phylogeny of mosquitoes (Diptera: Culicidae): mtDNA data support a derived placement for Toxorhynchites. Insects Syst. Evol. 2002, 33, 163–174. [Google Scholar]
- Belkin, J.N. The Mosquitoes of the South Pacific (Diptera, Culicidae); University of California Press: Berkeley/Los Angeles, CA, USA, 1962; Volume 1–2. [Google Scholar]
- Harbach, R.E. Comparative and functional morphology of the mandibles of some fourth stage mosquito larvae (Diptera: Culicidae). Zoomorphologie 1977, 87, 217–236. [Google Scholar] [CrossRef]
- Harbach, R.E. Comparative structure of the labiohypopharynx of fourth stage mosquito larvae (Diptera: Culicidae), with comments on larval morphology, evolution and feeding habits. Mosq. Syst. 1978, 10, 301–333. [Google Scholar]
- Wiegmann, B.M.; Trautwein, M.D.; Winkler, I.S.; Barr, N.B.; Kim, J.W.; Lambkin, C.; Bertone, M.A.; Cassel, B.K.; Bayless, K.M.; Heimberg, A.M.; et al. Episodic radiations in the fly tree of life. Proc. Natl. Acad. Sci. USA 2011, 108, 5690–5695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, R.B.; Smith, S.M. Oogenesis in Toxorhynchites rutilus (Diptera: Culicidae). Can. J. Zool. 1978, 56, 136–139. [Google Scholar] [CrossRef]
- Trimble, R.M. Laboratory observations on oviposition by the predaceous tree-hole mosquito, Toxorhynchites rutilus septentrionalis (Diptera: Culicidae). Can. J. Zool. 1979, 57, 1104–1108. [Google Scholar] [CrossRef]
- Sahlén, G. Eggshell ultrastructure in four mosquito genera (Diptera, culicidae). J. Am. Mosq. Control Assoc. 1996, 12, 263–270. [Google Scholar]
- Belkin, J.N.; Hogue, C.L.; Galindo, P.; Aiken, T.H.G.; Schick, R.X.; Powder, W.A. Mosquito studies (Diptera, Culicidae). II. Methods for the collection, rearing and preservation of mosquitoes. Contrib. Am. Entomol. Inst. 1965, 1, 20–78. [Google Scholar]
- Jenkins, D.W.; Carpenter, S.J. Ecology of the tree-hole breeding mosquitoes of Nearctic North America. Ecol. Monogr. 1945, 16, 31–47. [Google Scholar] [CrossRef]
- Muspratt, J. The bionomics of an African Megarhinus and its possible use in biological control. Bull. Entomol. Res. 1951, 42, 355–370. [Google Scholar] [CrossRef]
- Breland, O.P. The Biology and the Immature Stages of the Mosquito, Megarhinus Septentrionalis Dyar & Knab1,3. Ann. Entomol. Soc. Am. 1949, 42, 38–47. [Google Scholar]
- Trpis, M. Development and Predatory Behavior of Toxorhynchites brevipalpis (Diptera: Culicidae) in Relation to Temperature 1. Environ. Entomol. 1972, 1, 537–546. [Google Scholar] [CrossRef]
- Focks, D.A.; Seawright, J.A.; Hall, D.W. Field Survival, Migration and Ovipositional Characteristics of Laboratory-Reared Toxorhynchites Rutilus Rutilus (Diptera: Culicidae)1. J. Med. Entomol. 1979, 16, 121–127. [Google Scholar] [CrossRef]
- Lounibos, L.P. Temporal and spatial distribution, growth and predatory behavior of Toxorhynchites brevipalpis on the Kenya coast. J. Anim. Ecol. 1979, 48, 213–236. [Google Scholar] [CrossRef]
- Campos, R.E.; Lounibos, L.P. Life Tables of Toxorhynchites rutilus (Diptera: Culicidae) in Nature in Southern Florida. J. Med. Entomol. 2000, 37, 385–392. [Google Scholar] [CrossRef]
- Schiller, A.; Allen, M.; Coffey, J.; Fike, A.; Carballo, F. Updated Methods for the Production of Toxorhynchites rutilus septentrionalis (Diptera, Culicidae) for Use as Biocontrol Agent Against Container Breeding Pest Mosquitoes in Harris County, Texas. J. Insect Sci. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.W. Pathogens, parasites and predators of medically important arthropods. annotated list and bibliography. Bull. World Health Organ. 1964, 30, 1–150. [Google Scholar]
- Laird, M. Some natural enemies of mosquitoes in the vicinity of Palmalmal, New Britain. Trans. R. Soc. N. Z. 1947, 76, 453–476. [Google Scholar]
- Parker, D.J. The biology of the tree-holes of point pelee national park, ontario: II. first record of toxorhynchites rutilus septentrionalis in canada (diptera: Culicidae). Can. Entomol. 1977, 109, 93–94. [Google Scholar] [CrossRef]
- Linley, J.R.; Duzak, D. Egg cannibalism and carnivory among three species of Toxorhynchites. J. Am. Mosq. Control Assoc. 1989, 5, 359–362. [Google Scholar]
- Zuharah, W.F.; Fadzly, N.; Yusof, N.A.; Dieng, H. Risky behaviors: Effects of Toxorhynchites splendens (Diptera: Culicidae) predator on the behavior of three mosquito species. J. Insect Sci. 2015, 15, 128. [Google Scholar] [CrossRef] [Green Version]
- Goettle, B.J.; Adler, P.H. Elephant (or treehole) predatory mosquito. South Carol. State Doc. Depos. 2005. Available online: https://fliphtml5.com/vtuj/hzsv/basic (accessed on 28 September 2015).
- Corbet, P.S.; Griffiths, A. Observations on the aquatic stages of two species of Toxorhynchites (Diptera: Culicidae) in Uganda. Proc. R. Entomol. Soc. Lond. (A) 1963, 38, 125–135. [Google Scholar] [CrossRef]
- Bellamy, S.K.; Alto, B.W. Mosquito responses to trait- and density-mediated interactions of predation. Oecologia 2018, 187, 233–243. [Google Scholar] [CrossRef]
- Chandrasegaran, K.; Juliano, S.A. How Do Trait-Mediated Non-lethal Effects of Predation Affect Population-Level Performance of Mosquitoes? Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonesh, J.R.; Blaustein, L. Predator-Induced Shifts in Mosquito Oviposition Site Selection: A Meta-Analysis and Implications for Vector Control. Isr. J. Ecol. Evol. 2010, 56, 263–279. [Google Scholar] [CrossRef] [Green Version]
- Blaustein, L.; Kiflawi, M.; Eitam, A.; Mangel, M.; Cohen, J.E. Oviposition habitat selection in response to risk of predation in temporary pools: Mode of detection and consistency across experimental venue. Oecologia 2004, 138, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Silberbush, A.; Blaustein, L. Oviposition habitat selection by a mosquito in response to a predator: Are predator-released kairomones air-borne cues? J. Vector Ecol. 2008, 33, 208–211. [Google Scholar] [CrossRef]
- Kesavaraju, B.; Damal, K.; Juliano, S.A. Threat-Sensitive Behavioral Responses to Concentrations of Water-Borne Cues from Predation. Ethology 2007, 113, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Coon, K.L.; Valzania, L.; Brown, M.R.; Strand, M.R. Predaceous Toxorhynchites mosquitoes require a living gut microbiota to develop. Proc. Biol. Sci. 2020, 287, 20192705. [Google Scholar] [CrossRef] [Green Version]
- Focks, D.A.; Sackett, S.R.; Dame, D.A.; Bailey, D.L. Toxorhynchites rutilus rutilus (Diptera: Culicidae): Field studies on dispersal and oviposition in the context of the biocontrol of urban container-breeding mosquitoes. J. Med. Entomol. 1983, 20, 383–390. [Google Scholar] [CrossRef]
- Focks, D.A.; Sackett, S.R.; Dame, D.A.; Bailey, D.L. Ability of Toxorhynchites amboinensis (Doleschall) (Diptera: Culicidae) to locate and oviposit in artificial containers in an urban environment. Environ. Entomol. 1983, 12, 1073–1077. [Google Scholar] [CrossRef]
- Collins, L.E.; Blackwell, A. Colour cues for oviposition behaviour in Toxorhynchites moctezuma and Toxorhynchites amboinensis mosquitoes. J. Vector Ecol. 2000, 25, 127–135. [Google Scholar] [PubMed]
- Collins, L.E.; Blackwell, A. Olfactory cues for oviposition behavior in Toxorhynchites moctezuma and Toxorhynchites amboinensis (Diptera: Culicidae). J. Med. Entomol. 2002, 39, 121–126. [Google Scholar] [CrossRef]
- Hubbard, S.F.; Chadee, D.D.; O’Malley, S.L.C. Oviposition container preference of Toxorhynchites moctezuma mosquitoes in Trinidad (Diptera: Culicidae). J. Fla. Mosq. Control Assoc. 1991, 62, 12–17. [Google Scholar]
- Linley, J.R. Diel Rhythm and Lifetime Course of Oviposition in Toxorhynchites Amboinensis (Diptera: Culicidae)1. J. Med. Entomol. 1987, 24, 99–105. [Google Scholar] [CrossRef]
- Corbet, P.S. Observations on Toxorhynchites brevipalpis conradti Grünb. (Dipteria: Culicida) in Uganda. Bull. Entomol. Res. 1963, 54, 9–17. [Google Scholar] [CrossRef]
- Crans, W.J.; Slaff, M.E. Growth and behavior of colonized Toxrhynchites rutilus septentrionalis. Mosq. News 1977, 37, 207–211. [Google Scholar]
- Godoy, R.S.; Fernandes, K.M.; Martins, G.F. Midgut of the non-hematophagous mosquito Toxorhynchites theobaldi (Diptera, Culicidae). Sci. Rep. 2015, 5, 15836. [Google Scholar] [CrossRef] [Green Version]
- Jariyapan, N.; Choochote, W.; Jitpakdi, A.; Bates, P.A. Salivary gland of Toxorhynchites splendens Wiedemann (Diptera: Culicidae): Ultrastructural morphology and electrophoretic protein profiles. J. Med. Entomol. 2004, 41, 569–574. [Google Scholar] [CrossRef]
- Calvo, E.; Pham, V.M.; Ribeiro, J.M.C. An insight into the sialotranscriptome of the non-blood feeding Toxorhynchites amboinensis mosquito. Insect Biochem. Mol. Biol. 2008, 38, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Pascini, T.V.; Albeny, D.S.; Ramalho-Ortigão, M.; Vilela, E.F.; Serrão, J.E.; Martins, G.F. Changes in the fat body during the post-embryonic development of the predator Toxorhynchites theobaldi (Dyar & Knab) (Diptera: Culicidae). Neotrop. Entomol. 2011, 40, 456–461. [Google Scholar]
- Davis, E.E.; Bowen, M.F. Sensory physiological basis for attraction in mosquitoes. J. Am. Mosq. Control Assoc. 1994, 10, 316–325. [Google Scholar]
- Takken, W.; Knols, B.G. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu. Rev. Entomol. 1999, 44, 131–157. [Google Scholar] [CrossRef] [PubMed]
- Zwiebel, L.J.; Takken, W. Olfactory regulation of mosquito-host interactions. Insect Biochem. Mol. Biol. 2004, 34, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Wolff, G.H.; Riffell, J.A. Olfaction, experience and neural mechanisms underlying mosquito host preference. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Rinker, D.C.; Pitts, R.J.; Rokas, A.; Zwiebel, L.J. Divergent and conserved elements comprise the chemoreceptive repertoire of the nonblood-feeding mosquito Toxorhynchites amboinensis. Genome Biol. Evol. 2014, 6, 2883–2896. [Google Scholar] [CrossRef] [PubMed]
- Dekel, A.; Yakir, E.; Bohbot, J.D. The evolutionarily conserved indolergic receptors of the non-hematophagous elephant mosquito Toxorhynchites amboinensis. Insect Biochem. Mol. Biol. 2019, 110, 45–51. [Google Scholar] [CrossRef]
- Dekel, A.; Pitts, R.J.; Yakir, E.; Bohbot, J.D. Evolutionarily conserved odorant receptor function questions ecological context of octenol role in mosquitoes. Sci. Rep. 2016, 6, 37330. [Google Scholar] [CrossRef] [Green Version]
- Dekel, A.; Yakir, E.; Bohbot, J.D. The sulcatone receptor of the strict nectar-feeding mosquito Toxorhynchites amboinensis. Insect Biochem. Mol. Biol. 2019, 111, 103174. [Google Scholar] [CrossRef]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef]
- Baak-Baak, C.M.; Rodríguez-Ramírez, A.D.; García-Rejón, J.E.; Ríos-Delgado, S.; Torres-Estrada, J.L. Development and laboratory evaluation of chemically-based baited ovitrap for the monitoring of Aedes aegypti. J. Vector Ecol. 2013, 38, 175–181. [Google Scholar] [CrossRef]
- Lindh, J.M.; Kännaste, A.; Knols, B.G.; Faye, I.; Borg-Karlson, A.K. Oviposition responses of Anopheles gambiae s.s. (Diptera: Culicidae) and identification of volatiles from bacteria-containing solutions. J. Med. Entomol. 2008, 45, 1039–1049. [Google Scholar]
- Pelletier, J.; Hughes, D.T.; Luetje, C.W.; Leal, W.S. An odorant receptor from the southern house mosquito Culex pipiens quinquefasciatus sensitive to oviposition attractants. PLoS ONE 2010, 5, e10090. [Google Scholar] [CrossRef] [Green Version]
- Cork, A. Olfactory basis of host location by mosquitoes and other haematophagous Diptera. Ciba Found. Symp. 1996, 200, 71–84. [Google Scholar] [PubMed]
- Nyasembe, V.O.; Tchouassi, D.P.; Pirk, C.W.W.; Sole, C.L.; Torto, B. Host plant forensics and olfactory-based detection in Afro-tropical mosquito disease vectors. PLoS Negl. Trop. Dis. 2018, 12, e0006185. [Google Scholar] [CrossRef] [PubMed]
- Lequime, S.; Lambrechts, L. Vertical transmission of arboviruses in mosquitoes: A historical perspective. Infect. Genet. Evol. 2014, 28, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, L. The use of Toxorhynchites mosquitoes to detect and propagate dengue and other arboviruses. Am. J. Trop. Med. Hyg. 1981, 30, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.; Shroyer, D.A. Comparative Susceptibility of Five Species of Toxorhynchites Mosquitoes to Parenteral Infection with Dengue and other Flaviviruses. Am. J. Trop. Med. Hyg. 1985, 34, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.M.; Harrison, B.A.; Nisalak, A.; Scott, R.M.; Burke, D.S. Evaluation of Toxorhynchites Splendens (Diptera: Culicidae) as a Bioassay Host for Dengue Viruses1. J. Med. Entomol. 1982, 19, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, N.; Kimura, T.; Ohyama, A. Multiplication and distribution of type 2 dengue and Japanese encephalitis viruses in Toxorhynchites splendens after intrathoracic inoculation. Arch. Virol. 1987, 97, 37–47. [Google Scholar] [CrossRef]
- Zeller, H.G.; Mitchell, C.J. Replication of certain recently classified viruses in Toxorhynchites amboinensis mosquitoes and in mosquito and mammalian cell lines, with implications for their arthropod-borne status. Res. Virol. 1989, 140, 563–570. [Google Scholar] [CrossRef]
- Tesh, R.B.; McLean, R.G.; Shroyer, D.A.; Calisher, C.H.; Rosen, L. Ross River virus (Togaviridae: Alphavirus) infection (epidemic polyarthritis) in American Samoa. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 426–431. [Google Scholar] [CrossRef]
- Scherer, W.F.; Chin, J. Sensitivity of Toxorhynchites amboinensis mosquitoes versus chicken embryonic cell cultures for assays of Venezuelan encephalitis virus. J. Clin. Microbiol. 1981, 13, 947–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, L. Carbon dioxide sensitivity in mosquitoes infected with sigma, vesicular stomatitis, and other rhabdoviruses. Science 1980, 207, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B. Infectivity and Pathogenicity of Nodamura Virus for Mosquitoes. J. Gen. Virol. 1980, 48, 177–182. [Google Scholar] [CrossRef]
- Tesh, R.B. Establishment of two cell lines from the mosquito Toxorhynchites Amboinensis (Diptera: Culicidae) and their susceptibility to infcetion with arboviruses. J. Med. Entomol. 1980, 17, 338–343. [Google Scholar] [CrossRef]
- Kuno, G. A continuous cell line of a nonhematophagous mosquito, Toxorhynchites amboinensis. In Vitro 1980, 16, 915–917. [Google Scholar] [CrossRef]
- Kuno, G. Replication of Dengue, Yellow Fever, St, Louis encephalitis and vesticular stomatitis virus in a cell line (TRA-171) derived from Toxorhynchites amboinensis. In Vitro 1981, 17, 1011–1015. [Google Scholar] [CrossRef]
- Kuno, G. A method for the isolation continuous cell lines from toxorhynchites amboinensis. J. Med. Entomol. 1981, 18, 140–144. [Google Scholar] [CrossRef]
- Kuno, G. Persistent infection of a nonvector mosquito cell line (TRA-171) with dengue viruses. Intervirology 1982, 18, 45–55. [Google Scholar] [CrossRef]
- Kuno, G. Dengue virus replication in a polyploid mosquito cell culture grown in serum-free medium. J. Clin. Microbiol. 1982, 16, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Kuno, G. Arbovirus Replication in Cell Cultures of Nonhematophagous Mosquitoes. In Arboviruses in Arthropod Cells In Vitro; Yunker, C.E., Ed.; CRC Press: Boca Raton, FL, USA, 1987; Volume 2. [Google Scholar]
- Munderloh, U.G.; Kurtti, T.J.; Maramorosch, K. Anopheles stephensi and Toxorhynchites amboinensis: Aseptic rearing of mosquito larvae on cultured cells. J. Parasitol. 1982, 68, 1085–1091. [Google Scholar] [CrossRef]
- Legrand, F.S.; Hotta, S. Susceptility of Cloned Toxorhynchites amboinensis Cells to Dengue and Chikungunya Viruses. Microbiol. Immunol. 1983, 27, 101–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, K.L.; Price, B.D.; Eckerle, L.D.; Ball, L.A. Nodamura virus nonstructural protein B2 can enhance viral RNA accumulation in both mammalian and insect cells. J. Virol. 2004, 78, 6698–6704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuno, G.; Gubler, D.J.; Vélez, M.; Oliver, A. Comparative sensitivity of three mosquito cell lines for isolation of dengue viruses. Bull. World Health Organ. 1985, 63, 279–286. [Google Scholar]
- Tesh, R.B. A method for the isolation and identification of dengue viruses, using mosquito cell cultures. Am. J. Trop. Med. Hyg. 1979, 28, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Jirakanjanakit, N.; Khin, M.M.; Yoksan, S.; Bhamarapravati, N. The use of Toxorhynchites splendens for identification and quantitation of serotypes contained in the tetravalent live attenuated dengue vaccine. Vaccine 1999, 17, 597–601. [Google Scholar] [CrossRef]
- Sun, W.; Nisalak, A.; Gettayacamin, M.; Eckels, K.H.; Putnak, J.R.; Vaughn, D.W.; Innis, B.L.; Thomas, S.J.; Endy, T.P. Protection of Rhesus Monkeys against Dengue Virus Challenge after Tetravalent Live Attenuated Dengue Virus Vaccination. J. Infect. Dis. 2006, 193, 1658–1665. [Google Scholar] [CrossRef]
- Nitatpattana, N.; Le Flohic, G.; Thongchai, P.; Nakgoi, K.; Palaboodeewat, S.; Khin, M.; Gonzalez, J.-P. Elevated Japanese Encephalitis Virus Activity Monitored by Domestic Sentinel Piglets in Thailand. Vector-Borne Zoonotic Dis. 2011, 11, 391–394. [Google Scholar] [CrossRef]
- Nitatpattana, N.; Chaiyo, K.; Rajakam, S.; Poolam, K.; Chansiprasert, K.; Pesirikan, N.; Buree, S.; Rodpai, E.; Yoksan, S. Complete Genome Sequence of a Zika Virus Strain Isolated from the Serum of an Infected Patient in Thailand in 2006. Genome Announc. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Dhanda, V.; Thenmozhi, V.; Kumar, N.P.; Hiriyan, J.; Arunachalam, N.; Balasubramanian, A.; Ilango, A.; Gajanana, A. Virus isolation from wild-caught mosquitoes during a Japanese encephalitis outbreak in Kerala in 1996. Indian, J. Med. Res. 1997, 106, 4–6. [Google Scholar]
- Donald, C.L.; Varjak, M.; Aguiar, E.R.G.R.; Marques, J.T.; Sreenu, V.B.; Schnettler, E.; Kohl, A. Antiviral RNA Interference Activity in Cells of the Predatory Mosquito. Viruses 2018, 10, 694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafee, N.; AbuBakar, S. AbuBakar Effects of Dengue 2 virus Inoculation on Toxorhynchites splendens Larvae. J. Entomol. 2006, 3, 89–94. [Google Scholar]
- Pattanakitsakul, S.N.; Boonnak, K.; Auethavornanan, K.; Jairungsri, A.; Duangjinda, T.; Puttatesk, P.; Thongrungkiat, S.; Malasit, P. A new densovirus isolated from the mosquito Toxorhynchites splendens (Wiedemann) (Diptera: Culicidae). Southeast Asian J. Trop. Med. Public Health 2007, 38, 283–293. [Google Scholar]
- O’Neill, S.L.; Kittayapong, P.; Braig, H.R.; Andreadis, T.G.; Gonzalez, J.P.; Tesh, R.B. Insect densoviruses may be widespread in mosquito cell lines. J. Gen. Virol. 1995, 76 Pt 8, 2067–2074. [Google Scholar] [CrossRef]
- Albeny-Simões, D.; Murrell, E.G.; Elliot, S.L.; Andrade, M.R.; Lima, E.; Juliano, S.A.; Vilela, E.F. Attracted to the enemy: Aedes aegypti prefers oviposition sites with predator-killed conspecifics. Oecologia 2014, 175, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, P.Y. Status of Toxorhynchites in Hawaii. Proc. Hawaii Entomol. Soc. 1963, 18, 291–294. [Google Scholar]
- Nakagawa, P.Y.; Hirst, J.M. Current efforts in mosquito control in Hawaii. Mosq. News 1959, 19, 64–69. [Google Scholar]
- Annis, B.; Nalim, S.; Boewono, D.T. Toxorhynchites amboinensis larvae released in domestic containers fail to control dengue vectors in a rural village in central Java. J. Am. Mosq. Control Assoc. 1990, 6, 75–78. [Google Scholar] [PubMed]
- Annis, B.; Krisnowardojo, S.; Atmosoedjono, S.; Supardi, P. Suppression of larval Aedes aegypti populations in household water storage containers in Jakarta, Indonesia, through releases of first-instar Toxorhynchites splendens larvae. J. Am. Mosq. Control Assoc. 1989, 5, 235–238. [Google Scholar]
- Hubbard, S.F.; O’Malley, S.L.C.; Russo, R. The functional response of Toxorhynchites rutilus rutilus to changes in the population density of its prey Aedes aegypti. Med. Vet. Entomol. 1988, 2, 279–283. [Google Scholar] [CrossRef]
- Brady, O.J.; Hay, S.I. The Global Expansion of Dengue: How Aedes aegypti Mosquitoes Enabled the First Pandemic Arbovirus. Annu. Rev. Entomol. 2020, 65, 191–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef] [Green Version]
- Panicker, K.N.; Bai, M.G. Field release of Toxorhynchites splendens (Diptera; Culicidae) in controlling container breeding mosquitoes in a coastal village. Indian J. Med. Res. 1983, 77, 339–341. [Google Scholar]
- Marinee, L.; Chuah, K.; Yap, H.H. Studies on biological control potentials of Toxorhynchites splendens (Diptera: Culicidae). Trop. Biomed. 1984, 1, 145–150. [Google Scholar]
- Vongtangswad, S.; Tirabutana, C.; Thongkum, B. The biological control of Aedes aegypti on Sa-Med Island, Rayong Province by means of Toxorhynchites splendens, a predatory mosquito larva. J. Med. Assoc. Thai. 1983, 66, 8–12. [Google Scholar] [PubMed]
- Nyamah, M.A.; Sulaiman, S.; Omar, B. Field observation on the efficacy of Toxorhynchites splendens (Wiedemann) as a biocontrol agent against Aedes albopictus (Skuse) larvae in a cemetery. Trop. Biomed. 2011, 28, 312–319. [Google Scholar] [PubMed]
- Engber, B.; Sone, P.F.; Pillai, J.S. The occurrence of Toxorhynchites amboinensis in Western Samoa. Mosq. News 1978, 38, 295–296. [Google Scholar]
- Toohey, M.K.; Goettel, M.S.; Takagi, M.; Ram, R.C.; Prakash, G.; Pillai, J.S. Field studies on the introduction of the mosquito predator Toxorhynchites amboinensis (Diptera: Culicidae) into Fiji. J. Med. Entomol. 1985, 22, 102–110. [Google Scholar] [CrossRef]
- Focks, D.A.; Sackett, S.R.; Kloter, K.O.; Dame, D.A.; Carmichael, G.T. The integrated use of Toxorhynchites amboinensis and ground-level ULV insecticide application to suppress Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1986, 23, 513–519. [Google Scholar] [CrossRef]
- Focks, D.A.; Sackett, S.R.; Dame, D.A.; Bailey, D.L. Effect of weekly releases of Toxorhynchites amboinensis (Doleschall) on Aedes aegypti (L.) (Diptera: Culicidae) in New Orleans, Louisiana. J. Econ. Entomol. 1985, 78, 622–626. [Google Scholar] [CrossRef]
- Focks, D.A.; Sackett, S.R.; Bailey, D.L. Field experiments on the control of Aedes aegypti and Culex quinquefasciatus by Toxorhynchites rutilus rutilus (Diptera: Culicidae). J. Med. Entomol. 1982, 19, 336–339. [Google Scholar] [CrossRef]
- Bailey, D.L.; Jones, R.G.; Simmonds, P.R. Effects of indigenous Toxorhynchites rutilus rutilus on Aedes aegypti breeding in tire dumps. Mosq. News 1983, 43, 33–37. [Google Scholar]
- Gerberg, E.J.; Visser, W.M. Preliminary field trial for the biological control of Aedes aegypti by means of Toxorhynchites brevipalpis, a predatory mosquito larva. Mosqu. News 1978, 38, 197–200. [Google Scholar]
- Sempala, S.D.K. Interactions between immature Aedes africanus (Theobald) and larvae of two predatory species of Toxorhynchites (Diptera: Culicidae) in Zika Forest, Uganda. Bull. Entomol. Res. 1983, 73, 19–24. [Google Scholar] [CrossRef]
- Chadee, D.D. Toxorhynchites moctezuma, a potential biological control agent in Trinidad and Tobago, W. I. J. Am. Mosq. Control Assoc. 1985, 1, 376–378. [Google Scholar]
- Tikasingh, E.S.; Eustace, A. Suppression of Aedes aegypti by predatory Toxorhynchites moctezuma in an island habitat. Med. Vet. Entomol. 1992, 6, 272–280. [Google Scholar] [CrossRef]
- Rawlins, S.C.; Clark, G.G.; Martinez, R. Effects of single introduction of Toxorhynchites moctezuma upon Aedes aegypti on a Caribbean Island. J. Am. Mosq. Control Assoc. 1991, 7, 7–10. [Google Scholar]
- Aditya, G.; Ash, A.; Saha, G.K. Predatory activity of Rhantus sikkimensis and larvae of Toxorhynchites splendens on mosquito larvae in Darjeeling, India. J. Vector Borne Dis. 2006, 43, 66–72. [Google Scholar]
- Focks, D.A.; Seawright, J.A.; Hall, D.W. Laboratory Rearing of Toxorhynchites-Rutilus-Rutilus (Coquillett) on a Non-Living Diet. Mosq. News 1978, 38, 325–328. [Google Scholar]
- Riviere, F.; OPichon, G.; Duval, J.; Thirel, R.; Toudic, A. Introduction of Tx. amboinensis (Doleschall. 1857) (Diptera: Culicidae) in French Polynesia. Cah. ORSTOM Ser. Ent. Med. Parasitol. 1979, 17, 225–234. [Google Scholar]
- Trimble, R.M.; Smith, S.M. Geographic variation in development time and predation in the tree-hole mosquito, Toxorhynchites rutilus septentrionalis (Diptera: Culicidae). Can. J. Zool. 1978, 56, 2156–2165. [Google Scholar] [CrossRef]
- Toma, T.; Miyagi, I. Laboratory evaluation of Toxorhynchites splendens (Diptera: Culicidae) for predation of Aedes albopictus mosquito larvae. Med. Vet. Entomol. 1992, 6, 281–289. [Google Scholar] [CrossRef]
- Faithpraise, F.O.; Idung, J.; Usibe, B.; Chatwin, C.R.; Young, R.; Birch, P. Natural control of the mosquito population via Odonata and Toxorhynchites. Int. J. Innov. Res. Sci. Eng. Technol. 2014, 3, 12898–12911. [Google Scholar]
- Choochote, W.; Jitpakdi, A.; Suntaravitun, T.; Junkum, A.; Rongsriyam, K.; Chaithong, U. A Note on Laboratory Colonization of Toxorhynchites splendens by Using an Artificial Mating Technique and Autogenous Aedes togoi Larva as Prey. J. Trop. Med. Parasitol. 2002, 25, 47–50. [Google Scholar]
- Chowanadisai, L.; Benjaphong, N.; Phanthumachinda, B. Laboratory observations on Toxorhynchites splendens Wiedemann in Thailand. Southeast Asian J. Trop. Med. Public Health 1984, 15, 337–341. [Google Scholar]
- Focks, D.A.; Boston, M.D. A quantified mass-rearing technique for Toxorhynchites rutilus rutilus (Coquillett). Mosq. News 1979, 39, 616–619. [Google Scholar]
- Digma, J.R.; Sumalde, A.C.; Salibay, C.C. Laboratory evaluation of predation of Toxorhynchites amboinensis (Diptera:Culicidae) on three mosquito vectors of arboviruses in the Philippines. Biol. Control 2019, 137, 104009. [Google Scholar] [CrossRef]
- Mohamad, N.; Zuharah, W.F. Influence of container design on predation rate of potential biocontrol agent, Toxorhynchites splendens (Diptera: Culicidae) against dengue vector. Trop. Biomed. 2014, 31, 166–173. [Google Scholar]
- Padgett, P.D.; Focks, D.A. Laboratory Observations on the Predation of Toxorhynchites Rutilus Rutilus on Aedes Aegypti (Diptera: Culicidae). J. Med. Entomol. 1980, 17, 466–472. [Google Scholar] [CrossRef]
- Frank, J.H.; Curtis, G.A.; O’Meara, G.F. On the Bionomics of Bromeliad-Inhabiting Mosquitoes, X. Toxorhynchites r. rutilus as a predator of Wyeomyia vanduzeei (Diptera: Culicidae)1. J. Med. Entomol. 1984, 21, 149–158. [Google Scholar] [CrossRef]
- Agudelo-Silva, F.; Spielman, A. Paradoxical Effects of Simulated Larviciding on Production of Adult Mosquitoes*. Am. J. Trop. Med. Hyg. 1984, 33, 1267–1269. [Google Scholar] [CrossRef]
- Shaalan, E.A.; Canyon, D.V. Aquatic insect predators and mosquito control. Trop. Biomed. 2009, 26, 223–261. [Google Scholar] [PubMed]
- Focks, D.A.; Kloter, K.O.; Carmichael, G.T. The impact of sequential ultra-low volume ground aerosol applications of malathion on the population dynamics of Aedes aegypti (L.). Am. J. Trop. Med. Hyg. 1987, 36, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, E.T.; Jones, C.J. Evaluation of Inoculative Releases of Toxorhynchites splendens (Diptera: Culicidae) in Urban Environments in Florida. Environ. Entomol. 1994, 23, 770–777. [Google Scholar] [CrossRef]
- Hurst, T.P.; Pittman, G.; O’Neill, S.L.; Ryan, P.A.; Nguyen, H.L.; Kay, B.H. Impacts of Wolbachia infection on predator prey relationships: Evaluating survival and horizontal transfer between wMelPop infected Aedes aegypti and its predators. J. Med. Entomol. 2012, 49, 624–630. [Google Scholar] [CrossRef] [Green Version]
- Alkhaibari, A.M.; Maffeis, T.; Bull, J.C.; Butt, T.M. Combined use of the entomopathogenic fungus, Metarhizium brunneum, and the mosquito predator, Toxorhynchites brevipalpis, for control of mosquito larvae: Is this a risky biocontrol strategy? J. Invertebr. Pathol. 2018, 153, 38–50. [Google Scholar] [CrossRef]
- Costa, M.S.; Santana, A.E.; Oliveira, L.L.; Zanuncio, J.C.; Serrão, J.E. Toxicity of squamocin on Aedes aegypti larvae, its predators and human cells. Pest Manag. Sci. 2017, 73, 636–640. [Google Scholar] [CrossRef]
- Kumar, P.M.; Murugan, K.; Madhiyazhagan, P.; Kovendan, K.; Amerasan, D.; Chandramohan, B.; Dinesh, D.; Suresh, U.; Nicoletti, M.; Alsalhi, M.S.; et al. Biosynthesis, characterization, and acute toxicity of Berberis tinctoria-fabricated silver nanoparticles against the Asian tiger mosquito, Aedes albopictus, and the mosquito predators Toxorhynchites splendens and Mesocyclops thermocyclopoides. Parasitol. Res. 2016, 115, 751–759. [Google Scholar] [CrossRef]
- Nordin, O.; Donald, W.; Ming, W.H.; Ney, T.G.; Mohamed, K.A.; Halim, N.A.A.; Winskill, P.; Hadi, A.A.; Muhammad, Z.S.; Lacroix, R.; et al. Oral Ingestion of Transgenic RIDL Ae. aegypti Larvae Has No Negative Effect on Two Predator Toxorhynchites Species. PLoS ONE 2013, 8, e58805. [Google Scholar] [CrossRef]
- Lacey, L.A. Larvicidal Activity of Bacillus Pathogens Against Toxorhynchites Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 1983, 20, 620–624. [Google Scholar] [CrossRef]
| Species (Subgenus) | Geographical Range | Oviposition Preferences | Target Species | Examples of Successful Application |
|---|---|---|---|---|
| Tx. splendens (Toxorhynchites) | Asia, Oceania | Artificial containers Tree holes Cut bamboo Leaf axils | Ae. aegypti Ae. albopictus Cx. quinquefasciatus | [133,134,135,136] |
| Tx. amboinensis (Toxorhynchites) | Asia, Oceania, North America | Artificial containers Tree holes Cut bamboo Leaf axils | Ae. polynesiensis Ae. aegypti | [137,138,139,140] |
| Tx. rutilus rutilus (Lynchiella) | North America | Artificial containers Tree holes Bromeliads | Ae. aegypti Cx. quinquefasciatus | [50,141,142] |
| Tx. brevipalpis (Toxorhynchites) | Africa | Artificial containers Leaf axils Tree holes | Ae. aegypti | [49,143] |
| Tx. brevipalpis conradti (Toxorhynchites) | Africa | Artificial containers Leaf axils Tree holes | Ae. africanus | [144] |
| Tx. moctezuma (Lynchiella) | Central America | Tree holes Cut bamboo | Ae. aegypti | [145,146,147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donald, C.L.; Siriyasatien, P.; Kohl, A. Toxorhynchites Species: A Review of Current Knowledge. Insects 2020, 11, 747. https://doi.org/10.3390/insects11110747
Donald CL, Siriyasatien P, Kohl A. Toxorhynchites Species: A Review of Current Knowledge. Insects. 2020; 11(11):747. https://doi.org/10.3390/insects11110747
Chicago/Turabian StyleDonald, Claire L., Padet Siriyasatien, and Alain Kohl. 2020. "Toxorhynchites Species: A Review of Current Knowledge" Insects 11, no. 11: 747. https://doi.org/10.3390/insects11110747





