Landscape and Anthropogenic Factors Associated with Adult Aedes aegypti and Aedes albopictus in Small Cities in the Southern Great Plains
Simple Summary
Abstract
1. Introduction
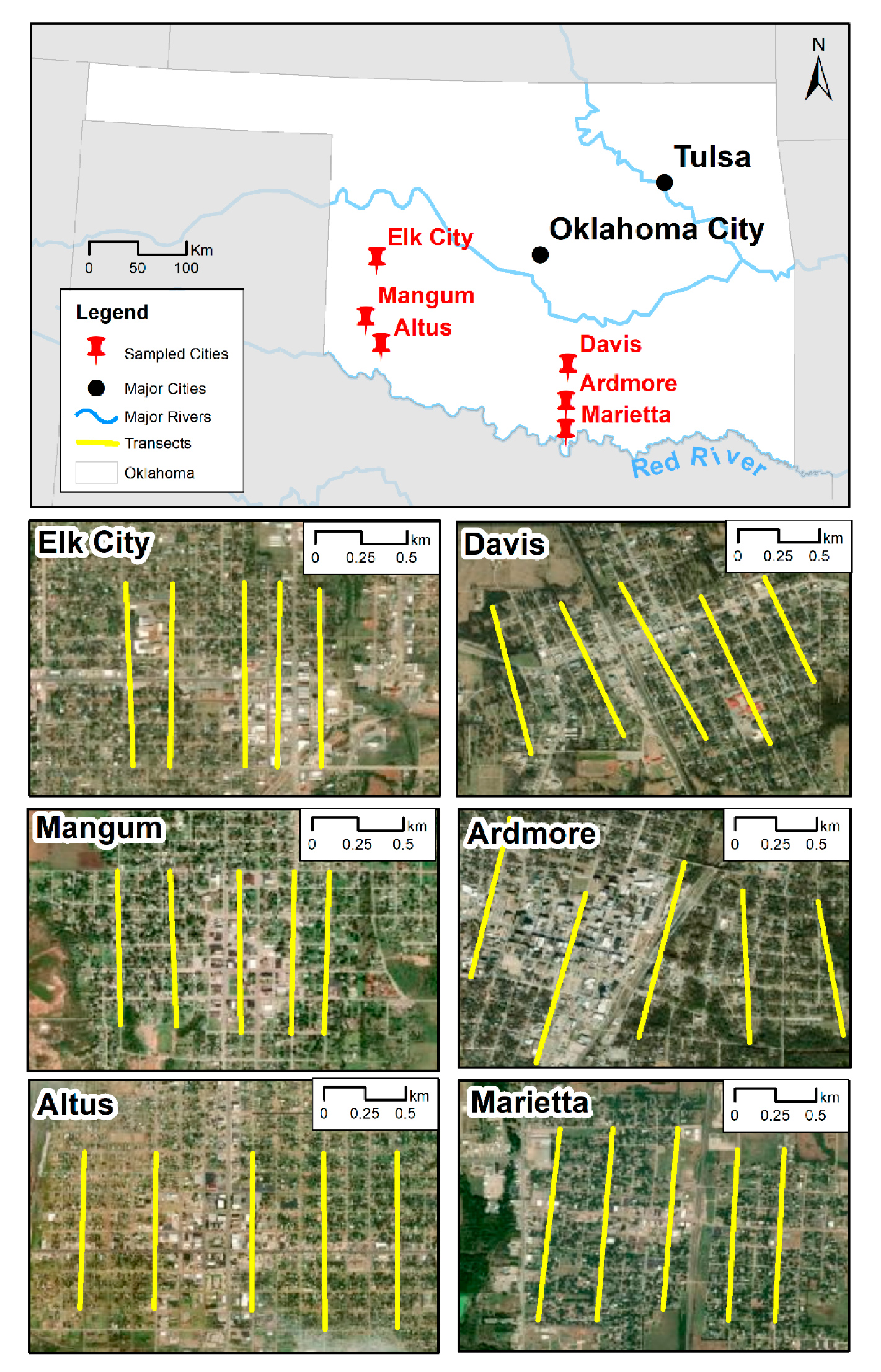
2. Materials and Methods
3. Results
3.1. Mosquito Collection
3.2. Aedes aegypti Confirmation Assay
3.3. Spatial Patterns of Mosquito Abundance
3.4. Site-Specific Assessment of Mosquito Presence and Abundance
3.4.1. Aedes aegypti
3.4.2. Aedes albopictus
3.4.3. Culex pipiens Complex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital signs: Trends in reported vectorborne disease cases–United States and territories, 2004–2016. Morb. Mortal Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Dougherty, E.R.; Getz, W. An ecological assessment of the pandemic threat of Zika virus. PLoS Negl. Trop. Dis. 2016, 10, e0004968. [Google Scholar] [CrossRef] [PubMed]
- Nsoesie, E.O.; Kraemer, M.U.; Golding, N.; Pigott, D.M.; Brady, O.J.; Moyes, C.L.; Johansson, M.A.; Gething, P.W.; Velayudhan, R.; Khan, K.; et al. Global distribution and environmental suitability for chikungunya virus, 1952 to 2015. Euro Surveill. 2016, 21, 30234. [Google Scholar] [CrossRef]
- Zohdy, S.; Morse, W.; Mathias, D.; Ashby, V.; Lessard, S. Detection of Aedes (Stegomyia) aegypti (Diptera: Culicidae) populations in southern Alabama following a 26-yr absence and public perceptions of the threat of Zika virus. J. Med. Entomol. 2018, 55, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Boyles, S.M.; Mavian, C.N.; Finol, E.; Ukhanova, M.; Stephenson, C.J.; Hamerlinck, G.; Kang, S.; Baumgartner, C.; Geesey, M.; Stinton, I.; et al. Under-the-radar Dengue virus infections in natural populations of Aedes aegypti mosquitoes. Msphere 2020, 5, e00316-20. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Moore, C.G. Aedes (Stegomyia) aegypti in the continental United States: A vector at the cool margin of its geographic range. J. Med. Entomol. 2013, 50, 467–478. [Google Scholar] [CrossRef]
- Hahn, M.; Eisen, L.; McAllister, J.; Savage, H.; Mutebi, J.; Eisen, R. Updated reported distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in the United States, 1995–2016. J. Med. Entomol. 2017, 54, 1420–1424. [Google Scholar] [CrossRef]
- Woods, A.J.; Omernik, J.M.; Butler, D.R.; Ford, J.G.; Henley, J.E.; Hoagland, B.W.; Arndt, D.S.; Moran, B.C. Ecoregions of Oklahoma. U.S. Geological Survey (map scale 1:1,250,000), Reston, VA 2005. Available online: ftp://newftp.epa.gov/EPADataCommons/ORD/Ecoregions/ok/ok_front.pdf (accessed on 12 October 2020).
- Johnson, M.G.; Adams, J.; McDonald-Hamm, C.; Wendelboe, A.; Bradley, K.K. Seasonality and survival associated with three outbreak seasons of West Nile virus disease in Oklahoma—2003, 2007, and 2012. J. Med. Virol. 2015, 87, 1633–1640. [Google Scholar] [CrossRef]
- Murray, K.O.; Rodriguez, L.F.; Herrington, E.; Kharat, V.; Vasilakis, N.; Walker, C.; Turner, C.; Khuwaja, S.; Arafat, R.; Weaver, S.C.; et al. Identification of dengue fever cases in Houston, Texas, with evidence of autochthonous transmission between 2003 and 2005. Vector Borne Zoonotic Dis. 2013, 13, 835–845. [Google Scholar] [CrossRef]
- Howard, A.; Visintine, J.; Fergie, J.; Deleon, M. Two infants with presumed congenital Zika syndrome, Brownsville, Texas, USA, 2016-2017. Emerg. Infect. Dis. 2018, 24, 625–630. [Google Scholar] [CrossRef]
- Noden, B.H.; Coburn, L.; Wright, R.; Bradley, K. An updated checklist of the mosquitoes in Oklahoma including new state records and West Nile Virus vectors, 2003–2006. J. Am. Mosq. Cont. Assoc. 2015, 31, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Bradt, D.; Coburn, L.; Bradley, K.K.; Noden, B.H. First record of Aedes japonicus japonicus in Oklahoma, 2017. J. Am. Mosq. Control. Assoc. 2018, 34, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Bradt, D.; Wormington, J.D.; Long, J.M.; Hoback, W.W.; Noden, B.H. Differences in mosquito communities in six cities in Oklahoma. J. Med. Entomol. 2019, 56, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Bradt, D.L.; Bradley, K.K.; Hoback, W.W.; Noden, B.H. New records of Aedes aegypti in southern Oklahoma, 2016. J. Am. Mosq. Control. Assoc. 2017, 33, 56–59. [Google Scholar] [CrossRef]
- Farajollahi, A.; Nelder, M.P. Changes in Aedes albopictus (Diptera: Culicidae) populations in New Jersey and implications for arbovirus transmission. J. Med. Entomol. 2009, 46, 1220–1224. [Google Scholar] [CrossRef]
- Srinivasan, K.; Tapia, B.; Rodriguez, A.; Wood, R.; Salinas, J. Species abundance and temporal variation of arbovirus vectors in Brownsville, Texas. Rev. Panam. Salud. Publica 2017, 41, e28. [Google Scholar] [CrossRef]
- United States Census Bureau (USCB). 2010 Census Urban and Rural Classification and Urban Area Criteria. 2018. Available online: https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html (accessed on 4 September 2019).
- Sanders, J. Distribution of Container-Breeding Mosquitoes in Urban Areas of Southern Oklahoma. Master’s Thesis, Oklahoma State University, Stillwater, Oklahoma, 2019. Available online: https://search.proquest.com/docview/2270038312?pq-origsite=primo (accessed on 27 July 2020).
- Homer, C.; Dewitz, J.; Yang, L.; Jin, S.; Danielson, P.; Xian, G.; Coulston, J.; Herold, N.; Wickham, J.; Megown, K. Completion of the 2011 National Land Cover Database for the conterminous United States–Representing a decade of Land Cover Change information. Photogram. Eng. Remote Sens. 2015, 81, 345–354. [Google Scholar]
- Hopperstad, K.; Reiskind, M. Recent changes in the local distribution of Aedes aegypti (Diptera: Culicidae) in south Florida, USA. J. Med. Entomol. 2016, 53, 836–842. [Google Scholar] [CrossRef]
- Weterings, R.; Umponstira, C.; Buckley, H. Container-breeding mosquitoes and predator community dynamics along an urban-forest gradient: The effects of habitat type and isolation. Basic Appl. Ecol. 2014, 15, 486–495. [Google Scholar] [CrossRef]
- Reiskind, M.; Griffin, R.; Janairo, M.; Hopperstad, K. Mosquitoes of field and forest: The scale of habitat segregation in a diverse mosquito assemblage. Med. Vet. Entomol. 2016, 31, 44–54. [Google Scholar] [CrossRef]
- Darsie, R.; Ward, R. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico, 2nd ed.; University Press of Florida: Gainesville, FL, USA, 2005. [Google Scholar]
- Harbach, R. Culex pipiens: Species versus species complex-taxonomic history and perspective. Am. Mosq. Control. Assoc. 2012, 28, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Da Costa-da-Silva, A.L.; Capurro, M.L.; Bracco, J.E. Genetic lineages in the yellow fever mosquito Aedes (Stegomyia) aegypti (Diptera: Culicidae) from Peru. Mem. Inst. Oswaldo Cruz. 2005, 100, 539–544. [Google Scholar] [CrossRef]
- Coulston, J.W.; Moisen, G.G.; Wilson, B.T.; Finco, M.V.; Cohen, W.B.; Brewer, C.K. Modeling percent tree canopy cover—A pilot study: Photogram. Eng. Remote Sens. 2012, 78, 715–727. [Google Scholar]
- Coulston, J.W.; Jacobs, D.M.; King, C.R.; Elmore, I.C. The influence of multi-season imagery on models of canopy cover—A case study: Photogram. Eng. Remote Sens. 2013, 79, 469–477. [Google Scholar]
- Walker, K.; Joy, T.; Kirk, C.; Ramberg, F. Human and environmental factors affecting Aedes aegypti distribution in an arid urban environment. J. Am. Mosq. Cont. Assoc. 2011, 27, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Peper, S.; Wilson-Fallon, A.; Haydett, K.; Greenberg, H.; Presley, S. First record of Aedes aegypti and Aedes albopictus in thirteen panhandle region counties of Texas, USA. J. Vector Ecol. 2017, 42, 352–354. [Google Scholar] [CrossRef]
- Greenberg, H.S.; Wilson-Fallon, A.N.; Peper, S.T.; Haydett, K.M.; Presley, S.M. New records of Aedes aegypti and Aedes albopictus in eight Texas counties, U.S.A. J. Vector Ecol. 2019, 44, 199–200. [Google Scholar] [CrossRef]
- Bureau, U. National Population Totals: 2010–2017. Available online: https://www.census.gov/programs-surveys/acs/guidance/comparing-acs-data/2017.html (accessed on 27 July 2020).
- Reisen, W. Landscape epidemiology of vector-borne disease. Rev. Entomol. 2010, 55, 461–483. [Google Scholar] [CrossRef]
- Damal, K.; Murrel, E.; Juliano, S.; Conn, J.; Loew, S. Phylogeography of Aedes aegypti (Yellow Fever mosquito) in south Florida: mtDNA evidence for human-aided dispersal. Am. J. Trop. Med. Hyg. 2013, 89, 482–488. [Google Scholar] [CrossRef]
- Metzger, M.; Yoshimizu, M.H.; Padgett, K.; Hu, R.; Kramer, V. Detection and establishment of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) mosquitoes in California, 2011–2015. J. Med. Entomol. 2017, 54, 533–543. [Google Scholar] [CrossRef]
- Eiras, A.E.; Buhagiar, T.S.; Ritchie, S.A. Development of the gravid Aedes trap for the capture of adult female container-exploiting mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Hurst, T.; Quoc, H.; Unlu, I.; Freebairn, C.; Faraji, A.; Ritchie, S. Field comparison of the Gravid Aedes Trap (GAT) and BG-Sentinel Trap for monitoring Aedes albopictus (DipteraL Culicidae) population and notes on indoor GAT collections in Vietnam. J. Med. Entomol. 2017, 54, 340–348. [Google Scholar] [PubMed]
- Braks, M.; Honorio, N.; Lounibos, L.; Lourenceo-De-Oliveria, R.; Juliano, A. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (DIptera: Culicidae), in Brazil. Ann. Entomol. Soc. Am. 2003, 97, 130–139. [Google Scholar] [CrossRef]
- Tsuda, Y.; Suwonkerd, W.; Chawprom, S.; Prajakwong, S.; Takagii, M. Different spatial distribution of Aedes aegypti and Aedes albopictus along an urban-rural gradient and the relating environmental factors examined in three villages in northern Thailand. J. Am. Mosq. Cont. Assoc. 2006, 22, 222–228. [Google Scholar] [CrossRef]
- Reiskind, M.H.; Lounibos, L.P. Spatial and temporal patterns of abundance of Aedes aegypti L. (Stegomyia aegypti) and Aedes albopictus (Skuse) [Stegomyia albopictus (Skuse)] in southern Florida. Med. Vet. Entomol 2013, 27, 421–429. [Google Scholar] [CrossRef]
- Myer, M.H.; Fizer, C.M.; Mcpherson, K.R.; Neale, A.C.; Pilant, A.N.; Rodriguez, A.; Whung, P.Y.; Johnston, J.M. Mapping Aedes aegypti (Diptera: Culicidae) and Aedes albopictus vector mosquito distribution in Brownsville, TX. J. Med. Entomol. 2020, 57, 231–240. [Google Scholar] [CrossRef]
- Buckner, E.A.; Blackmore, M.S.; Golladay, S.W.; Covich, A.P. Weather and landscape factors associated with adult mosquito abundance in southwestern Georgia, U.S.A. J. Vector Ecol. 2011, 36, 269–278. [Google Scholar] [CrossRef]
- Little, E.; Biehler, D.; Leisnham, P.T.; Jordan, R.; Wilson, S.; LaDeau, S.L. Socio-ecological mechanisms supporting high densities of Aedes albopictus (Diptera: Culicidae) in Baltimore, MD. J. Med. Entomol. 2017, 54, 1183–1192. [Google Scholar] [CrossRef]
- Murdock, C.; Evans, M.; McClanahan, T.; Miazgowicz, K.; Tesla, B. Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit Arboviral disease. PLoS Neg. Trop. Dis. 2017, 11, e0005640. [Google Scholar] [CrossRef]
- Evans, M.V.; Hintz, C.W.; Jones, L.; Shiau, J.; Solano, N.; Drake, J.M.; Murdock, C.C. Microclimate and larval habitat density predict adult Aedes albopictus abundance in urban areas. Am. J. Trop. Med. Hyg. 2019, 101, 362–370. [Google Scholar] [CrossRef]
- Becker, B.; Leisnham, P.T.; LaDeau, S.L. A tale of two city blocks: Differences in immature and adult mosquito abundances between socioeconomically different urban blocks in Baltimore (Maryland, USA). Int. J. Environ. Res. Public Health 2014, 11, 3256–3270. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Apperson, C.S.; Ghosh, S.K.; Cheshire, H.M.; Zeichner, B.C. Spatial analysis of Aedes albopictus (Diptera: Culicidae) oviposition in suburban neighborhoods of a Piedmont community in North Carolina. J. Med. Entomol. 2006, 43, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Shragai, T.; Harrington, L.C. Aedes albopictus (Diptera: Culicidae) on an invasive edge: Abundance, spatial distribution, and habitat usage of larvae and pupae across urban and socioeconomic environmental gradients. J. Med. Entomol. 2019, 56, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Noden, B.H.; Coburn, L.; Wright, R.; Bradley, K. Updated distribution of Aedes albopictus in Oklahoma, and implications in arbovirus transmission. J. Am. Mosq. Cont. Assoc. 2015, 31, 93–96. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Buhagiar, T.S.; Townsend, M.; Hoffmann, A.; van Den Hurk, A.F.; McMahon, J.L.; Eiras, A.E. Field validation of the gravid Aedes trap (GAT) for collection of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 210–219. [Google Scholar] [CrossRef]
- Williams, C.R.; Long, S.A.; Russell, R.C.; Ritchie, S.A. Field efficacy of the BG-Sentinel compared with CDC Backpack Aspirators and CO2-baited EVS traps for collection of adult Aedes aegypti in Cairns, Queensland, Australia. J. Am. Mosq. Control. Assoc. 2006, 22, 296–300. [Google Scholar] [CrossRef]
- Cilek, J.E.; Weston, J.R.; Richardson, A.G. Comparison of adult mosquito abundance from Biogents-2 Sentinel and Biogents Gravid Aedes Traps in northeastern Florida. J. Am. Mosq. Control. Assoc. 2017, 33, 358–360. [Google Scholar] [CrossRef]
- Landau, K.I.; van Leeuwen, W.J. Fine scale spatial urban land cover factors associated with adult mosquito abundance and risk in Tucson, Arizona. J. Vector Ecol. 2012, 37, 407–418. [Google Scholar] [CrossRef]

| Explanatory Variables | Descriptor |
|---|---|
| #_containers | Number of containers in visibility from site location |
| # dogs | Number of visible canines around site location |
| dog presence/absence | Presence/absence of visible resident canine(s) around site |
| clutter density | Low, medium, high |
| percent vegetation | No_veg (level 1), Low_Veg (level 2), Med_veg (level 3), High_veg (level 4) |
| Urban_100 | Total amount of impervious surface within 100 m of the site |
| Urban_250 | Total amount of impervious surface within 250 m of the site |
| Tree_100 | Total amount of tree canopy cover within 100 m of the site |
| Tree_250 | Total amount of tree canopy cover within 250 m of the site |
| Week | Sampling period |
| Residential | Site location in a neighborhood |
| Industrial | Site location at a business or industrial area |
| Agricultural | Site location in an agricultural setting |
| Rural Sites | Site location in the outer limits surrounded by open fields, not in neighborhood |
| Species | City | ||||||
|---|---|---|---|---|---|---|---|
| Eastern Transect | Western Transect | ||||||
| Marietta | Ardmore | Davis | Altus | Mangum | Elk City | Total | |
| Aedes aegypti | 197 | 90 | 1 | 253 | 4 | 0 | 547 |
| Ae. albopictus | 345 | 649 | 1266 | 384 | 1715 | 432 | 4791 |
| Ae. epactius | 9 | 16 | 35 | 35 | 13 | 11 | 119 |
| Ae. sollicitans | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| Ae. triseriatus | 0 | 23 | 25 | 1 | 0 | 6 | 55 |
| Ae. vexans | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Anopheles pseudopunctipennis | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| An. punctipennis | 0 | 0 | 2 | 0 | 2 | 2 | 6 |
| An. quadrimaculatus | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Culex erraticus | 2 | 0 | 9 | 1 | 1 | 2 | 15 |
| Cx. nigripalpus | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Cx. pipiens (complex) | 264 | 108 | 562 | 50 | 40 | 21 | 1045 |
| Cx. tarsalis | 0 | 0 | 0 | 0 | 3 | 1 | 4 |
| Cx. territans | 0 | 0 | 3 | 0 | 0 | 4 | 7 |
| Psorophora ciliata | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Ps. cyanescens | 0 | 0 | 1 | 4 | 1 | 9 | 15 |
| Ps. ferox | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Toxorhynchites. rutilus | 3 | 5 | 5 | 0 | 0 | 0 | 13 |
| Total | 820 | 892 | 1909 | 730 | 1782 | 495 | 6628 |
| Independent Variable | Estimate | Std Error | ChiSq | CL | p | |
|---|---|---|---|---|---|---|
| Eastern transect | ||||||
| Ardmore | Intercept | 1.6940914 | 0.322488 | 27.6 | . | <0.0001 * |
| R2 = 0.1983 | Weeks 1 and 2 vs. others | 1.1771011 | 0.324021 | 13.2 | 0.61–1.92 | 0.0003 * |
| Low Vegetation | 0.7154231 | 0.220261 | 10.55 | 0.29–1.16 | 0.0012 * | |
| Marietta | ||||||
| R2 = 0.1900 | Intercept | 0.73964512 | 0.2161211 | 11.71 | . | 0.0006 * |
| Weeks 1 and 2 vs. others | 1.20626465 | 0.2161211 | 31.15 | 0.81–1.66 | <0.0001 * | |
| Western transect | ||||||
| Altus | Intercept | 0.8293264 | 0.273737 | 9.18 | . | 0.0024 * |
| R2 = 0.2599 | Weeks 1 and 2 vs. others | 1.3700909 | 0.242377 | 31.95 | 0.92–1.88 | <0.0001 * |
| Residential sites | 0.5367054 | 0.231508 | 5.37 | 0.093–1.01 | 0.0204 * | |
| No Vegetation | 0.489398 | 0.226246 | 4.68 | 0.06–0.95 | 0.0305 * |
| Eastern Transect | Western Transect | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent variable | Estimate | Std Error | CI (95%) | p | Independent variable | Estimate | Std Error | CI (95%) | p | ||
| Davis | Elk City | ||||||||||
| R2 = 0.0936 | Intercept | −1.2978944 | 0.5758527 | . | 0.0242 * | R2 = 0.1003 | Intercept | −1.3910586 | 0.501634 | . | 0.0056 * |
| Week 2 vs. others | 0.79691911 | 0.2202347 | 0.37–1.24 | 0.0003 * | Weeks 1 and 2 vs. others | 0.51198048 | 0.1800807 | 0.16–0.87 | 0.0045 * | ||
| Urban_250 | 4.14610868 | 2.0561731 | 0.22–8.33 | 0.0438 * | Urban_100 | 3.44103666 | 1.5014318 | 0.56–6.48 | 0.0219 * | ||
| Med_Veg | 0.63290445 | 0.2090189 | 0.24–1.06 | 0.0025 * | |||||||
| Ardmore | Mangum | ||||||||||
| R2 = 0.0826 | Intercept | 0.82541703 | 0.3351642 | . | 0.0138 * | R2 = 0.2351 | Intercept | −0.8020088 | 0.2138956 | . | 0.0002 * |
| Weeks 2 and 5 vs. others | 0.75887535 | 0.2294639 | 0.33–1.24 | 0.0009 * | Weeks 1 and 2 vs. others | 1.27743253 | 0.2138956 | 0.88–1.72 | <0.0001 * | ||
| Tree_100 | −1.4247312 | 0.6611617 | −2.75–−0.15 | 0.0312 * | |||||||
| Marietta | Altus | ||||||||||
| R2 = 0.2792 | Intercept | −1.5599924 | 0.7086991 | . | 0.0277 * | R2 = 0.0828 | Intercept | 0.84384565 | 0.3757964 | . | 0.0247 * |
| Weeks 1 and 2 vs. others | 1.15226096 | 0.3362427 | 0.53–1.87 | 0.0006 * | Week 1 and 2 vs. others | 0.62962077 | 0.1793985 | 0.28–0.99 | 0.0004 * | ||
| Dog_Presence | −0.7307708 | 0.2656929 | −1.27–−0.22 | 0.0060 * | Tree 250 | −3.0729878 | 1.4726262 | −6.04–−0.23 | 0.0369 * | ||
| Num_Containers | 1.10144786 | 0.299501 | 0.56–1.74 | 0.0002 * | |||||||
| GAT Traps | |||||||||||
| Eastern Transect | Western Transect | ||||||||||
| Independent variable | Estimate | Std Error | CI (95%) | p | Independent Variable | Estimate | Std Error | CI (95%) | p | ||
| Davis | Intercept | −0.392052 | 0.2283544 | −0.902, 0.017 | 0.0183 * | Elk City | Intercept | 0.8746491 | 0.3687235 | 0.14, 1.60 | 0.0192 * |
| Week 1 | −1.075915 | 0.3761278 | −1.94, −0.42 | 0.0003 * | Urban_100 | −3.664891 | 1.3454824 | −6.44, −1.10 | 0.0034 * | ||
| Week 2 | 0.3292866 | 0.21952 | −0.12, 0.75 | 0.1466 | |||||||
| Week 3 | 0.2616456 | 0.2057748 | −0.16, 0.66 | 0.2058 | |||||||
| Week 4 | 0.5852899 | 0.1690119 | 0.25, 0.93 | 0.0003 * | |||||||
| Med Clutter | −0.274869 | 0.0949419 | −0.46, −0.08 | 0.0055 * | |||||||
| Industrial | 0.4647822 | 0.2129124 | 0.08, 0.95 | 0.0076 * | |||||||
| Ardmore | Intercept | −0.692742 | 0.2574502 | −1.32, −0.24 | 0.0001 * | Mangum | Intercept | −0.940648 | 0.2611862 | −1.47, −0.43 | <0.0001 * |
| Week 1 | −1.794448 | 0.7779615 | −3.99, −0.62 | <0.0001 * | Week 1 | −0.925338 | 0.3232383 | 0.0, −0.35 | 0.0004 * | ||
| Week 2 | 0.2019992 | 0.3152251 | −0.42, 0.89 | 0.5933 | Week 2 | −0.466729 | 0.2444934 | −0.98, −0.02 | 0.0470 * | ||
| Week 3 | 0.8659301 | 0.2364756 | 0.45, 0.0 | <0.0001 * | Week 3 | 1.0037865 | 0.1340088 | 0.75, 1.28 | <0.0001 * | ||
| Week 4 | 0.8187788 | 0.246799 | 0.38, 1.42 | <0.0001 * | Week 4 | 0.121549 | 0.1655347 | −0.21, 0.45 | 0.4094 | ||
| Tree_100 | 0.7141316 | 0.3545683 | 0.01, 0.0 | 0.0479 * | Tree_100 | 2.1172071 | 0.5942898 | 0.94, 3.31 | 0.0004 * | ||
| Marietta | Intercept | −0.237518 | 0.1406443 | −0.53, 0.02 | 0.0289 * | Altus | Intercept | −1.220092 | 0.4427689 | −2.40, −0.50 | <0.0001 * |
| Week 1 | −0.120377 | 0.3841283 | −0.99, 0.55 | 0.6942 | Week 1 | −0.223027 | 0.2880166 | −0.83, 0.32 | 1 | ||
| Week 2 | −0.048433 | 0.2632948 | −0.60, 0.44 | 1.0000 | Week 2 | −0.290156 | 0.3293038 | −1.00, 0.31 | 0.5914 | ||
| Week 3 | 0.4270776 | 0.2148863 | 0.0, 0.84 | 0.0415 * | Week 3 | 1.1361697 | 0.1737104 | 0.81, 1.50 | <0.0001 * | ||
| Week 4 | 0.3346308 | 0.2207547 | −0.11, 0.76 | 0.1168 | Week 4 | −1.217254 | 0.4490468 | 0 | 0.0004 * | ||
| Industrial | 0.6660607 | 0.4247139 | 0.0, 1.82 | 0.0081 * | |||||||
| Sentinel Traps | |||||||||||
| Eastern Transect | Western Transect | ||||||||||
| Independent variable | Estimate | Std Error | CI (95%) | p | Independent variable | Estimate | Std Error | CI (95%) | p | ||
| Davis | Intercept | 0.4772758 | 0.1650725 | 0.12, 0.78 | 0.0190 * | Elk City | Intercept | −1.194775 | 0.4853312 | 0.0, −0.30 | 0.0033 * |
| Rural sites | 0.3347099 | 0.1650725 | 0.03, 0.69 | 0.0212 * | # Containers | 0.390101 | 0.1235389 | 0 | 0.0012 * | ||
| Tree_250 | 3.2080804 | 1.0549301 | 0 | 0.0009 * | |||||||
| Ardmore | Intercept | 1.1541507 | 0.2134228 | 0.73, 1.57 | <0.0001 * | Mangum | Intercept | 0.5314689 | 0.1603245 | 0.19, 0.82 | 0.0192 * |
| Urban_100 | −1.656097 | 0.7662041 | −3.23, 0.0 | 0.0194 * | Week 1 | 0.0062933 | 0.2959498 | −0.62, 0.56 | 1 | ||
| Week 2 | 0.1366674 | 0.4018702 | −0.77, 0.84 | 1 | |||||||
| Week 3 | 0.5005273 | 0.2160474 | 0.08, 0.93 | 0.0131 * | |||||||
| Week 4 | −1.174995 | 0.411993 | −2.12, −0.46 | 0.0006 * | |||||||
| Marietta | Intercept | 1.2546588 | 0.3241852 | 0.54, 1.83 | 0.0028 * | Altus | Intercept | −0.582725 | 0.3565931 | −1.36, 0.07 | 0.0337 * |
| # Containers | −0.555369 | 0.1793199 | −0.92, −0.20 | 0.0031 * | Tree_100 | 4.8654761 | 1.6249095 | 1.83, 8.38 | 0.0007 * | ||
| Dog_Pres | 0.452842 | 0.2334301 | 0.0, 0.98 | 0.0241 * | |||||||
| No Veg | −0.414189 | 0.1757211 | −0.76, −0.06 | 0.0241 * | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanders, J.D.; Talley, J.L.; Frazier, A.E.; Noden, B.H. Landscape and Anthropogenic Factors Associated with Adult Aedes aegypti and Aedes albopictus in Small Cities in the Southern Great Plains. Insects 2020, 11, 699. https://doi.org/10.3390/insects11100699
Sanders JD, Talley JL, Frazier AE, Noden BH. Landscape and Anthropogenic Factors Associated with Adult Aedes aegypti and Aedes albopictus in Small Cities in the Southern Great Plains. Insects. 2020; 11(10):699. https://doi.org/10.3390/insects11100699
Chicago/Turabian StyleSanders, Jordan D., Justin L. Talley, Amy E. Frazier, and Bruce H. Noden. 2020. "Landscape and Anthropogenic Factors Associated with Adult Aedes aegypti and Aedes albopictus in Small Cities in the Southern Great Plains" Insects 11, no. 10: 699. https://doi.org/10.3390/insects11100699
APA StyleSanders, J. D., Talley, J. L., Frazier, A. E., & Noden, B. H. (2020). Landscape and Anthropogenic Factors Associated with Adult Aedes aegypti and Aedes albopictus in Small Cities in the Southern Great Plains. Insects, 11(10), 699. https://doi.org/10.3390/insects11100699






