Different Pollinators’ Functional Traits Can Explain Pollen Load in Two Solitary Oil-Collecting Bees
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
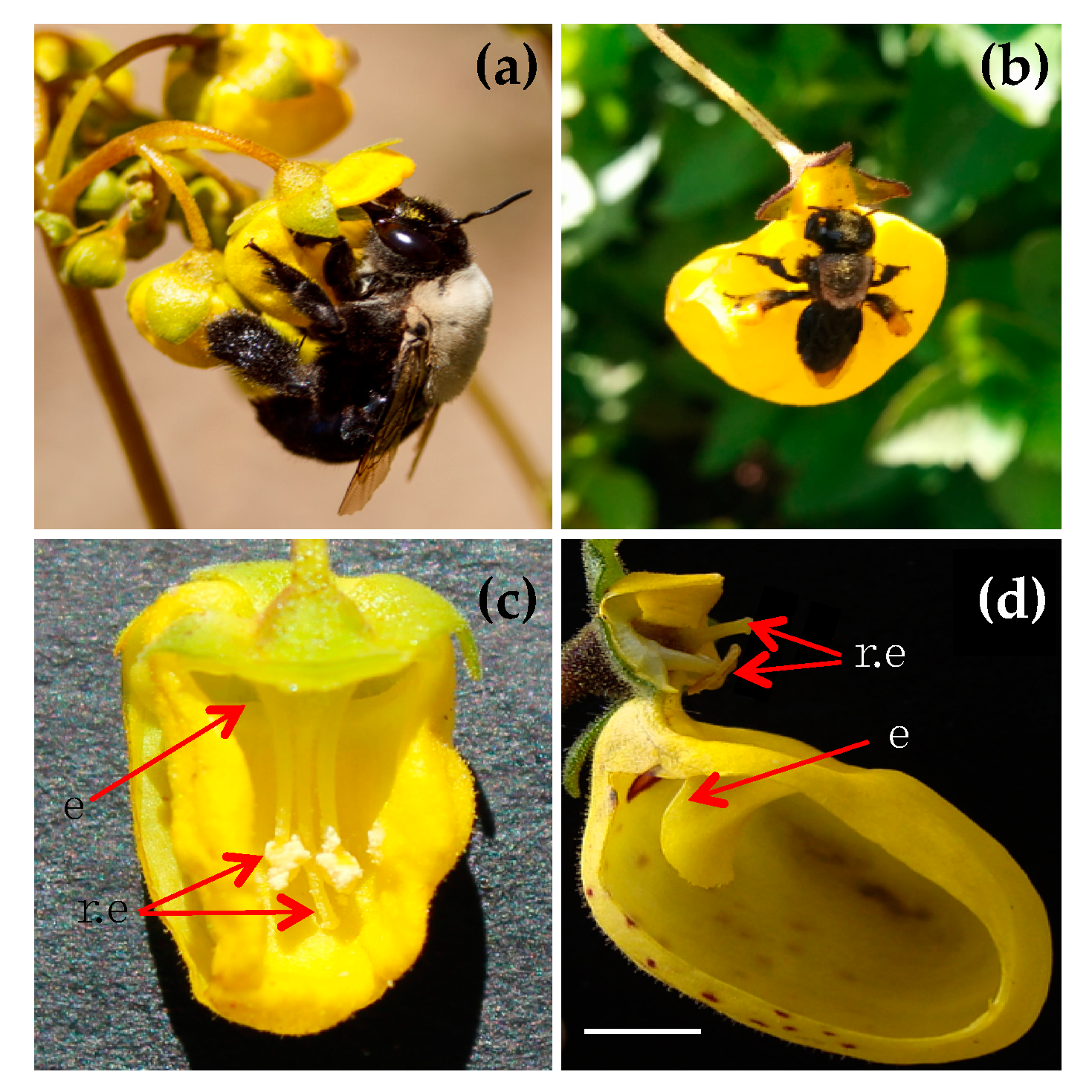
2.1. Study Site and Plant–Pollinator Species
2.2. Visitation Frequency and Handling Time
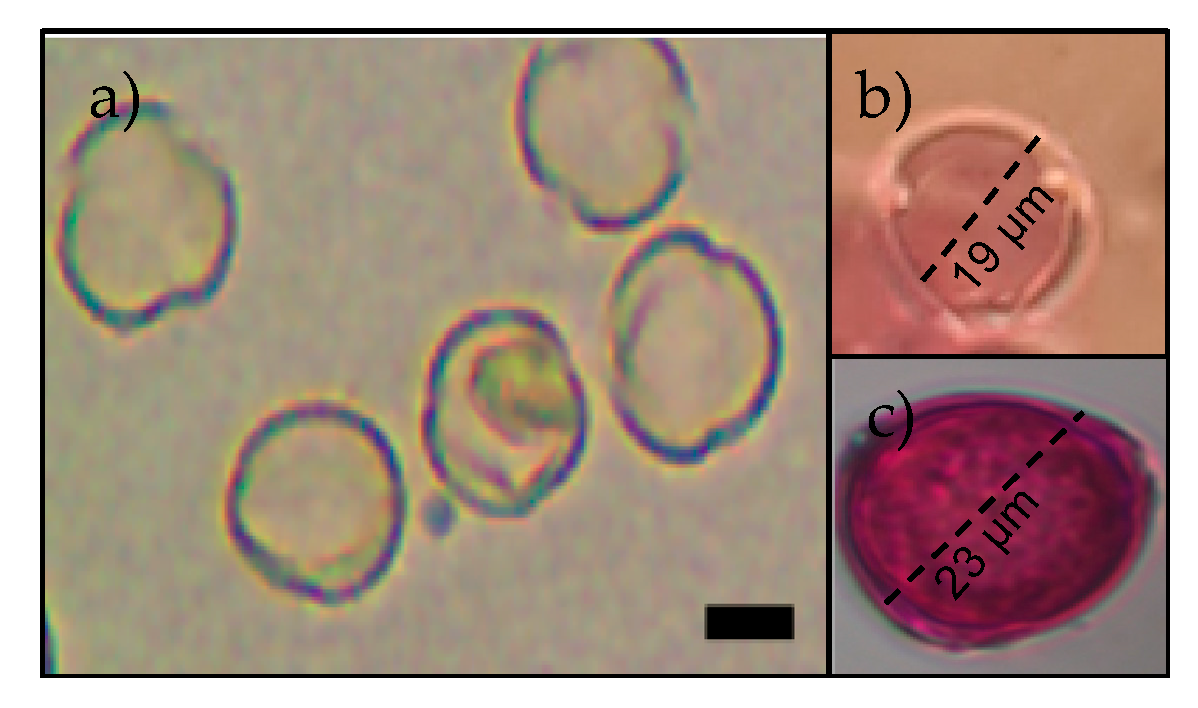
2.3. Floral Communities, Pollinators’ Pollen Load and Body Size
2.4. Data Analysis
3. Results
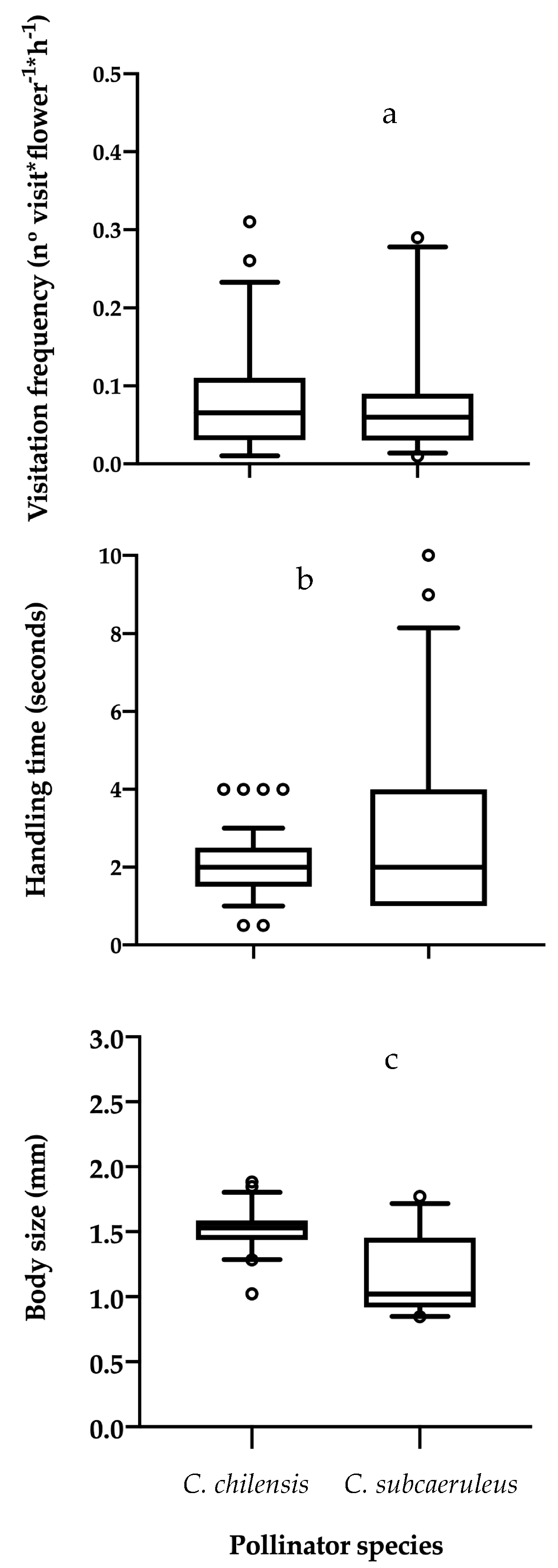
3.1. Visitation Frequency and Handling Time
3.2. Floral Communities, Pollinator’s Pollen Load and Body Size
4. Discussion and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Stebbins, G.L. Adaptive radiation of reproductive characteristics in angiosperms, I: Pollination mechanisms. Ann. Rev. Ecol. Syst. 1970, 1, 307–326. [Google Scholar] [CrossRef]
- Faegri, J.; van der Pijl, L. The Principles of Pollination Ecology; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Fenster, C.B.; Armbruster, W.S.; Wilson, P.; Dudash, M.R.; Thomson, J.D. Pollination syndromes and floral specialization. Ann. Rev. Ecol. Evol. Syst. 2004, 35, 375–403. [Google Scholar] [CrossRef]
- Murúa, M.; Espíndola, A. Pollination syndromes in a specialized plant-pollinator interaction: Does floral morphology predict pollinators in Calceolaria? Plant Biol. 2015, 17, 551–557. [Google Scholar] [CrossRef]
- Dellinger, A.S.; Artuso, S.; Pamperl, S.; Michelangeli, F.A.; Penneys, D.S.; Fernández-Fernández, D.M.; Alvear, M.; Almeda, F.; Armbruster, W.S.; Staedler, Y.; et al. Modularity increases rate of floral evolution and adaptive success for functionally specialized pollination systems. Commun. Biol. 2019, 2, 453. [Google Scholar] [CrossRef]
- Johnson, S.D.; Wester, P. Stefan Vogel’s analysis of floral syndromes in the South African flora: An appraisal based on 60 years of pollination studies. Flora 2017, 232, 200–206. [Google Scholar] [CrossRef]
- Murúa, M.; Ramírez, M.J.; González, A. Is the same pollinator species equally effective in different populations of the generalist herb Alstroemeria ligtu var. simsii? Gayana Bot. 2019, 76, 109–114. [Google Scholar] [CrossRef]
- Smith, C.; Weinman, L.; Gibbss, J.; Winfree, R. Specialist foragers in forest bee communities are small, social or emerge early. J. Anim. Ecol. 2019, 88, 1158–1167. [Google Scholar] [CrossRef]
- Feurbacher, E.; Fewell, J.H.; Roberts, S.P.; Smith, E.F.; Harrison, J.F. Effects of load type (pollen or nectar) and load mass on hovering metabolic rate and mechanical power output in the honeybee Apis mellifera. J. Exp. Biol. 2003, 206, 1855–1865. [Google Scholar] [CrossRef]
- Valverde, J.; Perfectti, F.; Gómez, J.M. Pollination effectiveness in a generalist plant: Adding the genetic component. New Phytol. 2019, 223, 354–365. [Google Scholar] [CrossRef]
- Ohashi, K. Consequences of floral complexity for bumblebee-mediated geitonogamous self-pollination in Salvia nipponica Miq. (Labiatae). Evolution 2002, 56, 2414–2423. [Google Scholar]
- Morales, C.L.; Traveset, A. Interspecific Pollen Transfer: Magnitude, prevalence and consequences for plant fitness. Crit. Rev. Plant. Sci. 2008, 27, 221–238. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Partch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Ins. Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef]
- Muchhala, N.; Brown, Z.; Armbruster, W.S.; Potts, M. Competition drives specialization in pollination systems through cost to male fitness. Am. Nat. 2010, 176, 732–743. [Google Scholar] [CrossRef]
- O’Neill, R.; O’Neill, K. Pollen load composition and size in the leaf-cutting bee Megachile rotundata (Hymenoptera: Megachilidae). Apidologie 2011, 42, 223–233. [Google Scholar] [CrossRef]
- Vásquez, D.; Aizen, M. Asymmetric specialization: A pervasive feature of plant–pollinator interactions. Ecology 2004, 85, 1251–1257. [Google Scholar] [CrossRef]
- Danforth, B.N.; Sipes, S.; Fang, J.; Brady, S.G. The history of early bee diversification based on five genes plus morphology. PNAS 2006, 103, 15118–15123. [Google Scholar] [CrossRef]
- Martins, D.J. Our Friends the Pollinators A Handbook of Pollinator Diversity and Conservation in East Africa; Nature Kenya, the East Africa Natural History Society National Museums of Kenya: Museum Hill, Kenya, 2014. [Google Scholar]
- Neef, J.L.; Simpson, B.B. Vogel’s great legacy: The oil flower and oil-collecting bee syndrome. Flora 2017, 232, 104–116. [Google Scholar] [CrossRef]
- Danforth, B.N.; Minckley, R.L.; Neff, J.L.; Fawcett, F. The Solitary Bees: Biology, Evolution, Conservation; Princeton University Press: Princeton, NJ, USA, 2019. [Google Scholar]
- Michener, C.D.; Moure, L.S. A study of the classification of the more primitive nonparasitic Anthophoridae bees (Hymenoptera, Apoidea). Bull. Am. Nat. Hist. 1957, 112, 395–425. [Google Scholar]
- Roig-alsina, A.; Michener, C.D. Studies of the Phylogeny and Classification of Long-Toungued Bees (Hymenoptera: Apoidea). Univ. Kans. Sci. Bull. 1993, 55, 123–162. [Google Scholar]
- Roig-alsina, A. Revision de las abejas colectoras de aceites del genero Chalepogenus Holmberg (Hymenoptera, Apidae, Tapinotaspidini). Rev. Mus. Argent. Cienc. Nat. 1999, 1, 67–101. [Google Scholar] [CrossRef]
- Vogel, S.P. Ölblumen und olsammelnde Bienen. Trop. Subtrop. Pflanzenwelt 1974, 7, 1–267. [Google Scholar]
- Sérsic, A.N. Pollination biology in the genus Calceolaria L. (Calceolariaceae). Stapfia 2004, 82, 122. [Google Scholar]
- Cocucci, A.A.; Sérsic, A.N.; Roig-Alsina, A. Oil-collecting structures in Tapinotaspidini (Hymenoptera: Apidae): Their diversity, function, and probable origin. Mitteilungen Münchner Entomologischen Gesellschaft 2000, 90, 51–74. [Google Scholar]
- Fuentes, E.R.; Montenegro, G.; Rundel, P.W.; Arroyo, M.T.K.; Ginacchio, R.; Jaksic, F.M. Functional approaches to biodiversity in the Mediterranean-type ecosystems of central Chile. In Mediterranean-Type Ecosystems: The Function of Biodiversity; Davis, G.W., Richardson, D.M., Eds.; Springer: New York, NY, USA, 1995. [Google Scholar]
- Di Castri, F.; Hajek, E.R. Bioclimatología de Chile; Vicerrectoria Académica, Universidad Católica de Chile: Santiago, Chile, 1976. [Google Scholar]
- Erdtman, G. The acetolysis method. Sven. Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Hingston, A.B. Is the introduced Bumblebee (Bombus terrestris) assisting the naturalization of Agapanthux praecox ssp. orientalis in Tasmania? Ecol. Manag. Restor. 2006, 7, 236–238. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 10 October 2020).
- Michener, C.D. The Bees of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2007. [Google Scholar]
- Genissel, A.; Aupinel, P.; Bressac, C.; Tasei, J.N.; Chevrier, C. Influence of pollen origin on performance of Bombus terrestris micro-colonies. Entomol. Exp. Appl. 2002, 104, 329–336. [Google Scholar] [CrossRef]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honeybees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Roulston, T.H.; Cane, J. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol. Ecol. 2002, 16, 49–65. [Google Scholar] [CrossRef]
- Macior, L.W. Behavioral aspects of coadaptations between flowers and insect pollinators. Ann. Mo. Bot. Gard. 1974, 61, 760–769. [Google Scholar] [CrossRef]
- Thorp, R.W. The collection of pollen by bees. Plant Syst. Evol. 2000, 222, 211–223. [Google Scholar] [CrossRef]
- Westerkamp, C.; Claßen-Bockhoff, R. Bilabiate flowers: The ultimate response to bees? Ann. Bot. 2007, 100, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Sérsic, A.N. Observaciones sobre el mecanismo floral de Calceolaria (Scrophulariaceae). Kurtziana 1991, 21, 153–164. [Google Scholar]
- Blüthgen, N.; Klein, A.M. Functional complementarity and specialization: The role of biodiversity in plant-pollinator interactions. Basic Appl. Ecol. 2011, 12, 282–291. [Google Scholar] [CrossRef]
- Ivey, C.T.; Martinez, P.; Wyatt, R. Variation in pollinator effectiveness in Swamp Milkweed, Asclepias incarnata (Apocynaceae). Am. J. Bot. 2003, 90, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Veiga, J.C.; Menezez, C.; Venturieri, G.C.; Contrera, F.A. The bigger, the smaller: Relationship between body size and food stores in the stingless bee Melipona flavolineata. Apidologie 2013, 44, 324–333. [Google Scholar] [CrossRef]
- Stavert, J.R.; Liñán-Cembrano, G.; Beggs, J.R.; Howlett, B.G.; Pattemore, D.E.; Bartomeus, I. Hairiness: The missing link between pollinators and pollination. PeerJ 2016, 4, e2779. [Google Scholar] [CrossRef]
- Ramalho, M.; Imperatriz-Fonseca, V.L.; Giannini, T.C. Within-colony size variation of foragers and pollen load capacity in the stingless bee Melipona quadrifasciata anthidioides Lepeletier (Apidae, Hymenoptera). Apidologie 1998, 29, 221–228. [Google Scholar] [CrossRef]
- Maubecin, C.; Boero, L.; Sérsic, A. Specialization in pollen collection, pollination interactions and phenotypic variation of the oil-collecting bee Chalepogenus cocuccii. Apidologie 2020. [Google Scholar] [CrossRef]
- Díaz, S.; Symstad, M.J.; Chapin, F.S.; Wardle, D.A.; Huenneke, L.F. Functional diversity revealed by removal experiments. TREE 2003, 18, 140–146. [Google Scholar]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Park, M.G.; Raguso, R.A.; Losey, J.E.; Danforth, B.N. Per-visit pollinator performance and regional importance of wild Bombus and Andrena (Melandrena) compared to the managed honey bee in New York apple orchards. Apidologie 2015, 47, 145–160. [Google Scholar] [CrossRef]
- Russo, L.; Park, M.G.; Blitzer, E.J.; Danforth, B.N. Flower handling behavior and abundance determines the relative contribution of pollinators to seed set in apple orchards. Agric. Ecosyst. Environ. 2017, 246, 102–108. [Google Scholar] [CrossRef]
| Pollinator Species | Calceolaria sp. | A. emarginata | Plantago sp. | Phacelia sp. | Others * |
|---|---|---|---|---|---|
| C. chilensis | 2705 ± 204 (59.9%) | 1092 ± 106 (1.6%) | 44 ± 20.40 (25.8%) | 2528 ± 201 (11.7%) | 47 ± 13 (0.9%) |
| C. subcaeruleus | 76,629 ± 7200 (42.2%) | 1992 ± 380 (17%) | 32,945 ± 4171 (0.7%) | 15,007 ± 1372 (39.4%) | 1180 ± 195 (0.7%) |
| Pollinator Species | Source | Estimate | t-Value | P |
|---|---|---|---|---|
| C. chilensis | VF | −1.332 | −0.405 | 0.687 |
| HT | 0.034 | 0.515 | 0.609 | |
| BS | −0.519 | −1.975 | 0.055 * | |
| VF × HT | −0.075 | −0.115 | 0.908 | |
| VF × BS | 0.811 | 0.33 | 0.743 | |
| C. subcaeruleus | VF | 0.804 | 0.881 | 0.389 |
| HT | 0.108 | 3.382 | <0.001 * | |
| BS | 0.511 | 4.292 | <0.001 * | |
| VF × HT | 0.089 | 0.436 | 0.667 | |
| VF × BS | −1.129 | −1.108 | 0.281 | |
| HT × BS | −0.106 | −3.754 | <0.001 * |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murúa, M. Different Pollinators’ Functional Traits Can Explain Pollen Load in Two Solitary Oil-Collecting Bees. Insects 2020, 11, 685. https://doi.org/10.3390/insects11100685
Murúa M. Different Pollinators’ Functional Traits Can Explain Pollen Load in Two Solitary Oil-Collecting Bees. Insects. 2020; 11(10):685. https://doi.org/10.3390/insects11100685
Chicago/Turabian StyleMurúa, Maureen. 2020. "Different Pollinators’ Functional Traits Can Explain Pollen Load in Two Solitary Oil-Collecting Bees" Insects 11, no. 10: 685. https://doi.org/10.3390/insects11100685
APA StyleMurúa, M. (2020). Different Pollinators’ Functional Traits Can Explain Pollen Load in Two Solitary Oil-Collecting Bees. Insects, 11(10), 685. https://doi.org/10.3390/insects11100685





