Taxonomic and Functional Response of Millipedes (Diplopoda) to Urban Soil Disturbance in a Metropolitan Area
Abstract
1. Introduction
- (1)
- To assess and compare millipede assemblages on the two sides of the city divided by the river Danube as a barrier;
- (2)
- To investigate the taxonomic and functional diversity, and compositional response of millipedes to urban intensity;
- (3)
- To identify the major soil properties shaping the structure of taxonomic and functional communities.
2. Materials and Methods
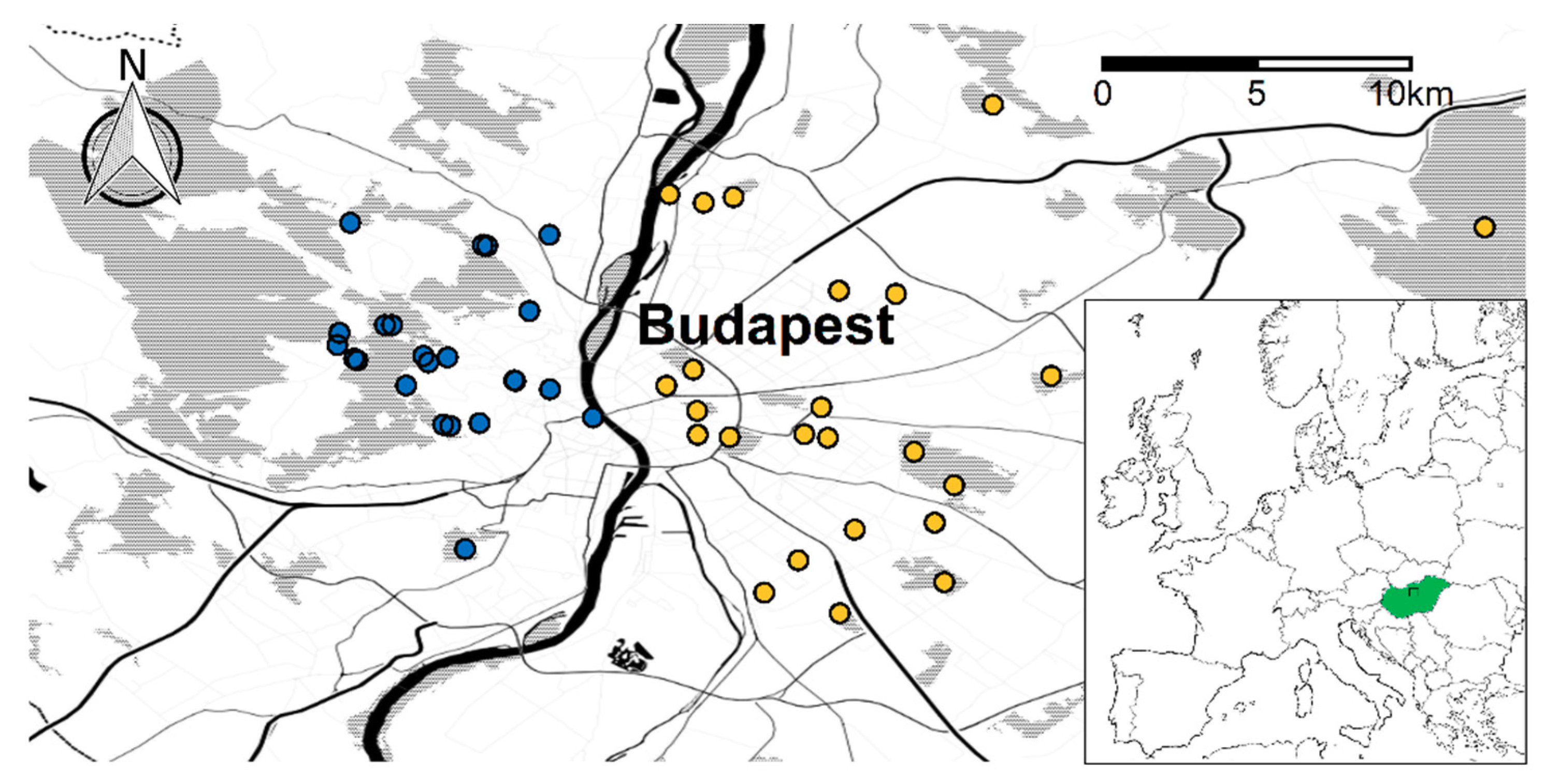
2.1. Study Sites and Design
2.2. Diplopoda
2.2.1. Sampling and Species Identification
2.2.2. Taxonomic and Functional Diversity
2.3. Environmental Factors
2.3.1. Landscape Structure Characteristics
2.3.2. Soil Properties
2.4. Statistical Analyses
3. Results
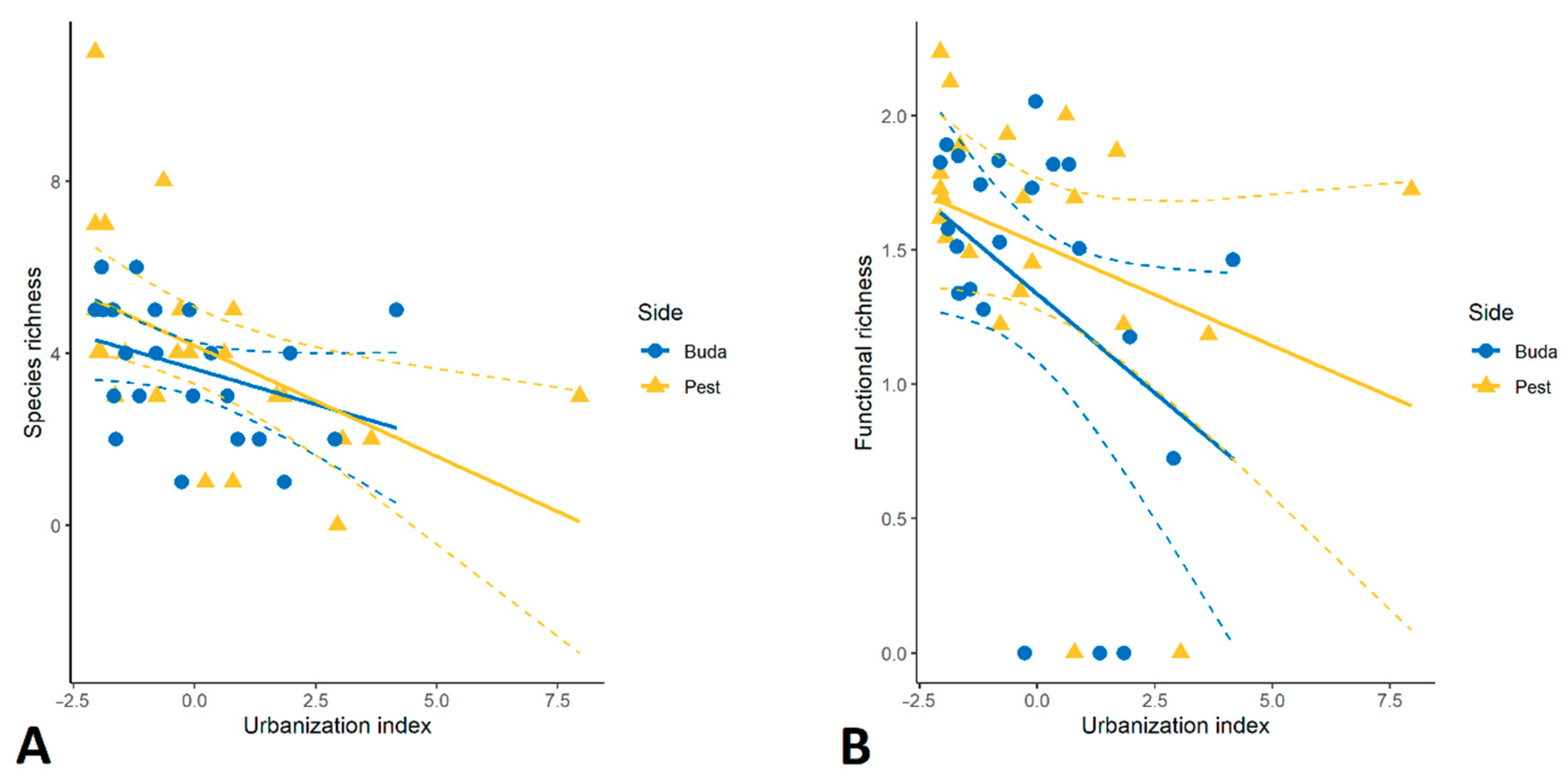
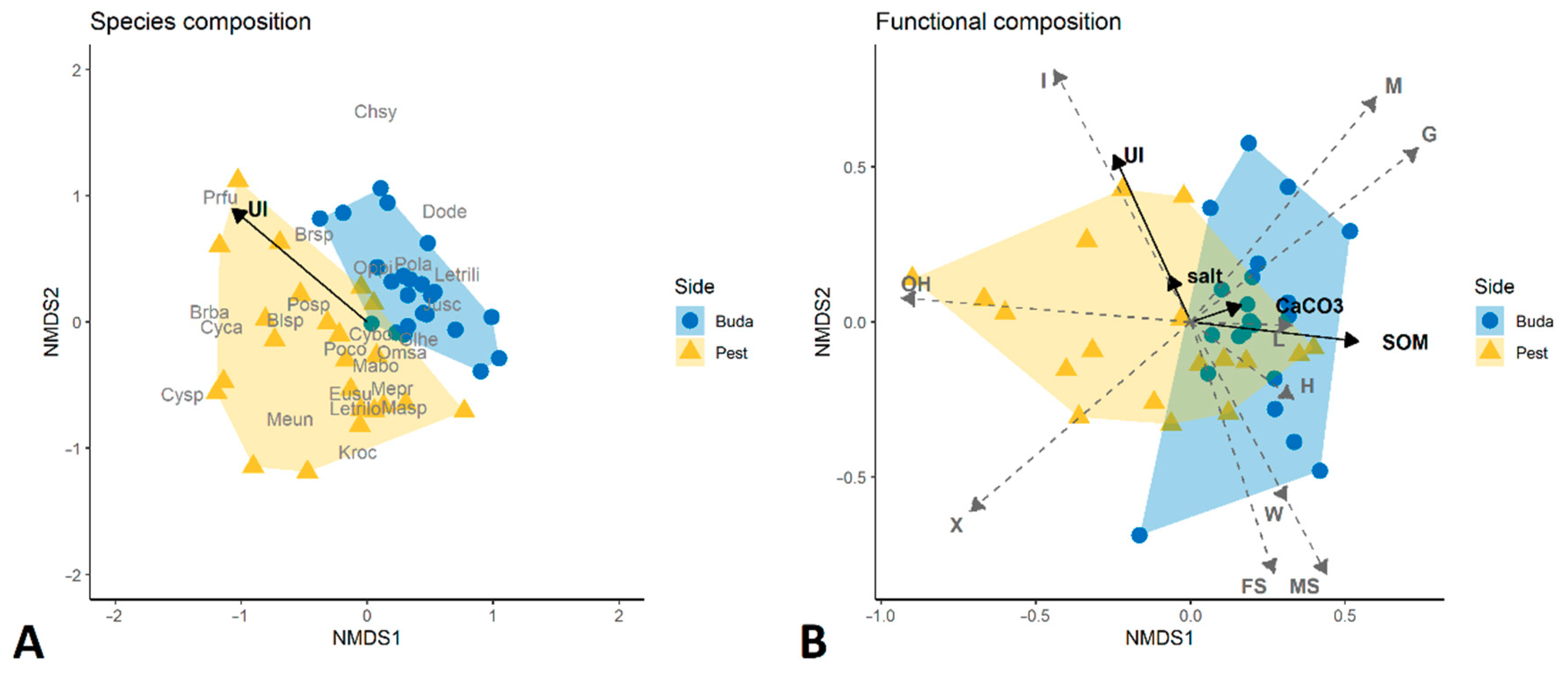
3.1. Taxonomic Diversity and Species Composition of Diplopoda Assemblages
3.2. Traits Analysis and Functional Diversity
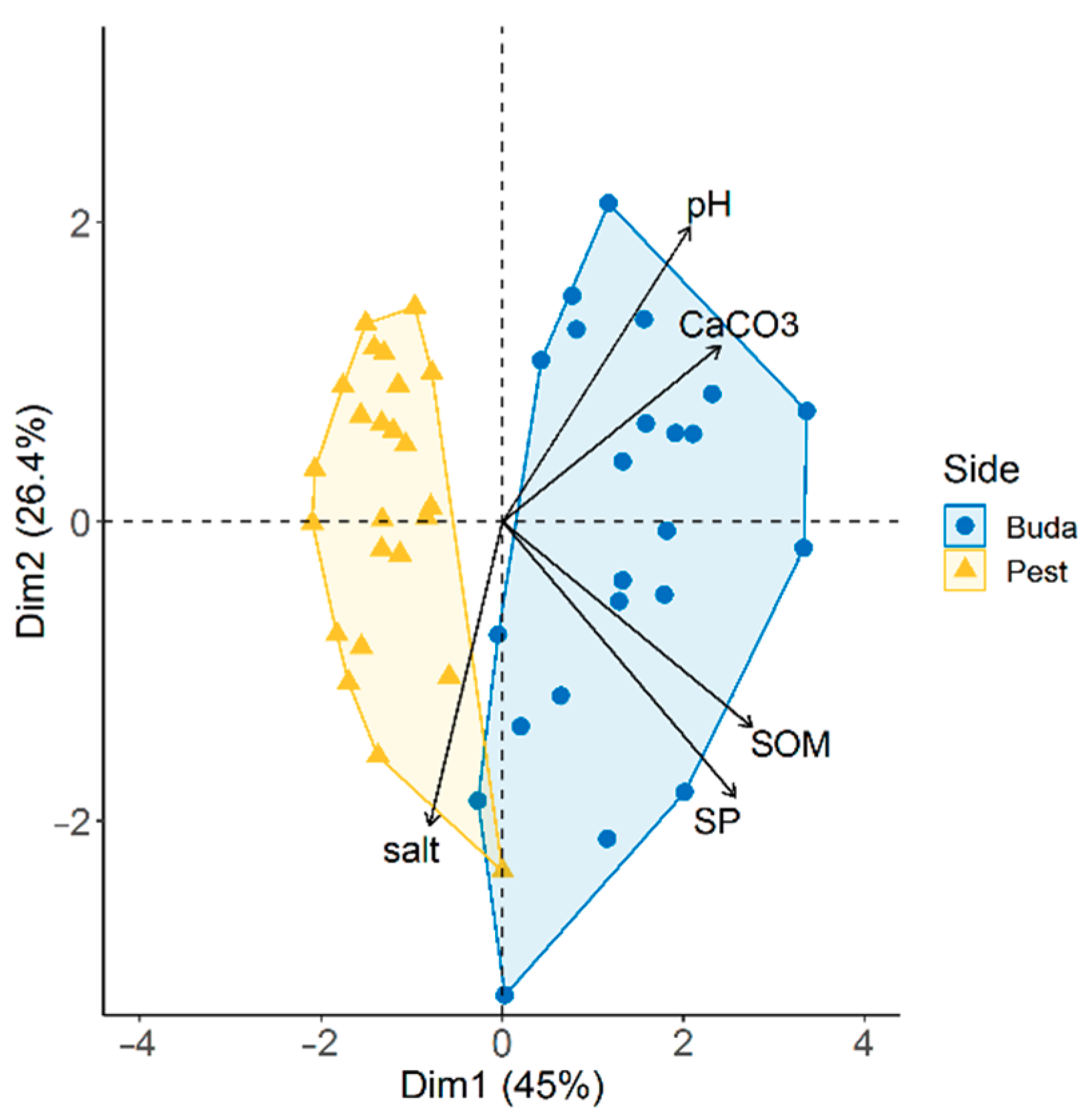
3.3. Diplopoda Assemblages and Soil Properties
4. Discussion
4.1. Diplopoda Fauna of Budapest
4.2. Urban Effects on Taxonomic/Functional Diversity and Composition of Millipedes
4.3. Diplopoda Assemblages and Soil Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2018 Revision (ST/ESA/SER.A/420); United Nations: New York, NY, USA, 2019. [Google Scholar]
- McKinney, M.L. Urbanization, biodiversity, and conservation. Bioscience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Russell-Anelli, J.; Yesilonis, I.D.; Groffman, P.M. Soil carbon in urban forest ecosystems. In The Potential of U.S. Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect; Kimble, J.M., Heath, L.S., Birdsey, R.A., Lal, R., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 347–362. [Google Scholar]
- Schwartz, M.W.; Thorne, J.H.; Viers, J.H. Biotic homogenization of the California flora in urban and urbanizing regions. Biol. Conserv. 2006, 127, 282–291. [Google Scholar] [CrossRef]
- Epp Schmidt, D.J.; Pouyat, R.; Szlavecz, K.; Setälä, H.; Kotze, D.J.; Yesilonis, I.; Cilliers, S.; Hornung, E.; Dombos, M.; Yarwood, S.A. Urbanization erodes ectomycorrhizal fungal diversity and may cause microbial communities to converge. Nat. Ecol. Evol. 2017, 1, 0123. [Google Scholar] [CrossRef]
- Knop, E. Biotic homogenization of three insect groups due to urbanization. Glob. Chang. Biol. 2016, 22, 228–236. [Google Scholar] [CrossRef]
- Lizée, M.H.; Mauffrey, J.F.; Tatoni, T.; Deschamps-Cottin, M. Monitoring urban environments on the basis of biological traits. Ecol. Indic. 2011, 11, 353–361. [Google Scholar] [CrossRef]
- van Rensburg, B.J.; Peacock, D.S.; Robertson, M.P. Biotic homogenization and alien bird species along an urban gradient in South Africa. Landsc. Urban Plan. 2009, 92, 233–241. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Mirotchnick, N.; Jain, M.; Palmer, M.I.; Naeem, S. Functional and phylogenetic diversity as predictors of biodiversity–ecosystem-function relationships. Ecology 2011, 92, 1573–1581. [Google Scholar] [CrossRef]
- McGill, B.J.; Dornelas, M.; Gotelli, N.J.; Magurran, A.E. Fifteen forms of biodiversity trend in the Anthropocene. Trends Ecol. Evol. 2015, 30, 104–113. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Hedde, M.; van Oort, F.; Lamy, I. Functional traits of soil invertebrates as indicators for exposure to soil disturbance. Environ. Pollut. 2012, 164, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Pavao-Zuckerman, M.A. Urbanization, soils and ecosystem services. In Soil Ecology and Ecosystem Services; Wall, D.H., Bardgett, R.D., Behan-Pelletier, V., Herrick, J.E., Jones, T.H., Ritz, K., Six, J., Strong, D.R., van der Putten, W.H., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 270–281. [Google Scholar]
- Lavelle, P.; Spain, A.V. Soil Ecology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Edwards, C.A. Macroarthropods. In Biology of Plant Litter Decomposition; Dickinson, C.H., Pugh, G.J.F., Eds.; Academic Press: London, UK, 1974; Volume 2, pp. 533–555. [Google Scholar]
- Paoletti, M.G.; Osler, G.H.R.; Kinnear, A.; Black, D.G.; Thomson, L.J.; Tsitsilas, A.; Sharley, D.; Judd, S.; Neville, P.; D’Inca, A. Detritivores as indicators of landscape stress and soil degradation. Aust. J. Exp. Agric. 2007, 47, 412–423. [Google Scholar] [CrossRef]
- Hopkin, S.P.; Read, H.J. The Biology of Millipedes; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Kime, R.D.; Wauthy, G. Aspects of relationships between millipedes, soil texture and temperature in deciduousnforests. Pedobiologia 1984, 26, 387–402. [Google Scholar]
- Hungarian Central Statistical Office. Available online: https://www.ksh.hu/docs/hun/xstadat/xstadat_eves/i_wdsd003c.html (accessed on 15 October 2019).
- Tóth-Ronkay, M.; Bajor, Z.; Bárány, A.; Földvári, G.; Görföl, T.; Halpern, B.; Leél-Őssy, S.; Mészáros, R.; Péntek, A.L.; Tóth, B.; et al. Vertebrates and Invertebrates of European Cities: Selected Non-Avian Fauna; Kelcey, J.G., Ed.; Springer: Berlin, Germany, 2016; pp. 27–73. [Google Scholar]
- Varga, Z. Animals. In National Atlas of Hungary: Natural Environment; Kocsis, K., Ed.; MTA CSFK Geographical Institute: Budapest, Hungary, 2018; pp. 104–111. [Google Scholar]
- Snyder, B.A.; Draney, M.L.; Sierwald, P. Development of an optimal sampling protocol for millipedes (Diplopoda). J. Insect Conserv. 2006, 10, 277–288. [Google Scholar] [CrossRef]
- Schubart, O. Tausendfüssler oder Myriapoda I: Diplopoda. In Die Tierwelt Deutschlands und der angränzenden Meeresteile, Teil 28; Dahl, F., Ed.; Gustav Fischer Verlag: Jena, Germany, 1934; p. 318. [Google Scholar]
- Korsós, Z. Magyarország Ikerszelvényesei. Illusztrációtáblák és Adatlapok a Fajok Meghatározásához (Diplopods of Hungary. Illustrations and Data Sheets to Species Identification); Hungarian Natural History Museum: Budapest, Hungary, 2015. [Google Scholar]
- Baselga, A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 2012, 21, 1223–1232. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Laliberte, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Liker, A.; Papp, Z.; Bókony, V.; Lendvai, Á.Z. Lean birds in the city: Body size and condition of house sparrows along the urbanization gradient. J. Anim. Ecol. 2008, 77, 789–795. [Google Scholar] [CrossRef] [PubMed]
- ASTM D2974-14. Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Westerman, R.L. Soil Testing and Plant Analysis; Soil Science Society of America: Madison, WI, USA, 1990. [Google Scholar]
- ISO 10693. Soil Quality—Determination of Carbonate Content—Volumetric Method; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wang, Y.; Naumann, U.; Wright, S.; Warton, D.I. mvabund: An R package for model-based analysis of multivariate data. Methods Ecol. Evol. 2012, 3, 471–474. [Google Scholar] [CrossRef]
- Konietschke, F.; Placzek, M.; Schaarschmidt, F.; Hothorn, L.A. nparcomp: An R Software Package for Nonparametric Multiple Comparisons and Simultaneous Confidence Intervals. J. Stat. Softw. 2015, 64, 1–17. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.5-2. Available online: https://CRAN.R-project.org/package=vegan (accessed on 28 December 2019).
- Korsós, Z.; Lazányi, E. Millipede fauna of Hungary: Present status. In Proceedings of the 18th International Congress of Myriapodology, Program and Abstracts, Budapest, Hungary, 25–31 August 2019; p. 42. [Google Scholar]
- Kime, R.D.; Enghoff, H. Orders Polyxenida, Glomerida, Platydesmida, Siphonocryptida, Polyzoniida, Callipodida, Polydesmida. In Atlas of European Millipedes (Class Diplopoda); Pensoft Publishers: Sofia, Bulgaria, 2011; Volume 1. [Google Scholar]
- Kime, R.D.; Enghoff, H. Atlas of European millipedes 2: Order Julida (Class Diplopoda). Eur. J. Taxon. 2017, 346. [Google Scholar] [CrossRef]
- Bogyó, D.; Magura, T.; Simon, E.; Tóthmérész, B. Millipede (Diplopoda) assemblages alter drastically by urbanization. Landsc. Urban Plan. 2015, 133, 118–126. [Google Scholar] [CrossRef]
- Enghoff, H. Diplopoda and Chilopoda from suburban localities around Copenhagen. Vidensk. Meddel. Dansk Naturhist. Foren. Kjøbenhavn. 1973, 136, 43–48. [Google Scholar]
- Enghoff, H.; Pedersen, J.; Thomsen, P.F.; Iversen, L. Tusindben, skolopendre og mejere fra Rødbyhavn og omegn–med fem nye arter for den danske fauna (Diplopoda, Chilopoda, Opiliones). Entomol. Medd. 2011, 79, 3–12. [Google Scholar]
- Kocourek, P. Mnohonožky (Myriapoda: Diplopoda) Prahy [Millipedes (Myriapoda: Diplopoda) of Prague (Central Bohemia)]. Nat. Prag. 2013, 21, 3–146. [Google Scholar]
- Korsós, Z. Millipedes from anthropogenic habitats in Hungary. Ber. Naturwiss. Med. Ver. Innsb. 1992, 10, 237–241. [Google Scholar]
- Korsós, Z.; Hornung, E.; Szlávecz, K.; Kontschán, J. Isopoda and Diplopoda of urban habitats: New data to the fauna of Budapest. Ann. Hist. Nat. Musei Natl. Hung. 2002, 94, 193–208. [Google Scholar]
- Riedel, P.; Navrátil, M.; Tuf, I.H.; Tufová, J. Terrestrial isopods (Isopoda: Oniscidea) and millipedes (Diplopoda) of the City of Olomouc (Czech Republic). In Contributions to Soil Zoology in Central Europe III, Proceedings of the 9th Central European Workshop on Soil Zoology, České Budějovice, Czech Republic, 17–20 April 2007; Pižl, V., Ed.; Institute of Soil Biology, Biology Centre, Academy of Sciences of the Czech Republic: České Budějovice, Czech Republic, 2009; pp. 125–132. [Google Scholar]
- Smith, J.; Chapman, A.; Eggleton, P. Baseline biodiversity surveys of the soil macrofauna of London’s green spaces. Urban Ecosyst. 2006, 9, 337–349. [Google Scholar] [CrossRef]
- Stoev, P. Myriapoda (Chilopoda, Diplopoda) in urban environments in the City of Sofia. In Ecology of the City of Sofia. Species and Communities in Urban Environment; Penev, L., Niemelä, J., Kotze, D.J., Chipev, N., Eds.; Pensoft: Sofia, Bulgaria, 2004; pp. 299–306. [Google Scholar]
- Tischler, W. Asseln (Isopoda) und Tausenfüßer (Myriopoda) eines Stadtparks im Vergleich mit der Umgebung der Stadt: Zum Problem der Urbanbiologie. Drosera 1980, 1, 41–52. [Google Scholar]
- Vilisics, F.; Bogyó, D.; Sattler, T.; Moretti, M. Occurrence and assemblage composition of millipedes (Myriapoda, Diplopoda) and terrestrial isopods (Crustacea, Isopoda, Oniscidea) in urban areas of Switzerland. Zookeys 2012, 176, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Bachvarova, D.; Doichinov, A.; Abdulova, R. Seasonal activity of Leptoiulus trilineatus (C.L. Koch, 1847) and Megaphyllum trassylvanicum (Verhoeff, 1897) (Diplopoda: Julida: Julidae). Acta Sci. Nat. 2018, 5, 86–95. [Google Scholar] [CrossRef]
- Mwabvu, T. Spirostreptid millipedes (Diplopoda, Spirostreptida) of urban and peri-urban habitats in Zimbabwe. Afr. J. Ecol. 2007, 45, 311–314. [Google Scholar] [CrossRef]
- Nagy, D.D.; Magura, T.; Horváth, R.; Debnár, Z.; Tóthmérész, B. Arthropod assemblages and functional responses along an urbanization gradient: A trait-based multi-taxa approach. Urban For. Urban Green. 2018, 30, 157–168. [Google Scholar] [CrossRef]
- Fournier, B.; Gillet, F.; Le Bayon, R.C.; Mitchell, E.A.D.; Moretti, M. Functional responses of multitaxa communities to disturbance and stress gradients in a restored floodplain. J. Appl. Ecol. 2015, 52, 1364–1373. [Google Scholar] [CrossRef]
- Van Dyck, H.; Baguette, M. Dispersal behaviour in fragmented landscapes: Routine or special movements? Basic Appl. Ecol. 2005, 6, 535–545. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Pywell, R.F. Effects of vegetation structure and floristic diversity on detritivore, herbivore and predatory invertebrates within calcareous grasslands. Biodivers. Conserv. 2009, 19, 81–95. [Google Scholar] [CrossRef]
- Jokimäki, J.; Kaisanlahti-Jokimäki, M.-L. Spatial similarity of urban bird communities: A multiscale approach. J. Biogeogr. 2003, 30, 1183–1193. [Google Scholar] [CrossRef]
- Magura, T.; Lövei, G.L.; Tóthmérész, B. Does urbanization decrease diversity in ground beetle (Carabidae) assemblages? Glob. Ecol. Biogeogr. 2010, 19, 16–26. [Google Scholar] [CrossRef]
- Mori, A.S.; Ota, A.T.; Fujii, S.; Kabeya, T.S.D.; Okamoto, T.; Ito, M.T.; Kaneko, N.; Hasegawa, M. Biotic homogenization and differentiation of soil faunal communities in the production forest landscape: Taxonomic and functional perspectives. Oecologia 2015, 177, 533–544. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- David, J.F.; Handa, I.T. The ecology of saprophagous macroarthropods (millipedes, woodlice) in the context of global change. Biol. Rev. 2010, 85, 881–895. [Google Scholar] [CrossRef]
- Golovatch, S.I.; Kime, R.D. Millipede (Diplopoda) distributions: A review. Soil Org. 2009, 81, 565–597. [Google Scholar]
- Magura, T.; Horvath, R.; Tóthmérész, B. Effects of urbanization on ground-dwelling spiders in forest patches, in Hungary. Landsc. Ecol. 2010, 25, 621–629. [Google Scholar] [CrossRef]
- Hornung, E.; Kásler, A.; Tóth, Z. The role of urban forest patches in maintaining isopod diversity (Oniscidea). ZooKeys 2018, 801, 371–388. [Google Scholar] [CrossRef]
- Magura, T.; Hornung, E.; Tóthmérész, B. Abundance patterns of terrestrial isopods along an urbanization gradient. Community Ecol. 2008, 9, 115–120. [Google Scholar] [CrossRef]
- Magura, T.; Tóthmérész, B.; Lövei, G.L. Body size inequality of carabids along an urbanisation gradient. Basic Appl. Ecol. 2006, 7, 472–482. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Szlavecz, K.; Yesilonis, I.D.; Groffman, P.M.; Schwarz, K. Chemical, Physical, and Biological Characteristics of Urban Soils. In Urban Ecosystem Ecology; Aitkenhead-Peterson, J., Volder, A., Eds.; Agronomy Monograph 55; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, Wisconsin, WI, USA, 2010; pp. 119–152. [Google Scholar] [CrossRef]
- Alexandrovskaya, E.I.; Alexandrovskiy, A.L. History of the cultural layer in Moscow and accumulation of anthropogenic substances in it. Catena 2000, 41, 249–259. [Google Scholar] [CrossRef]
- Craul, P.J.; Klein, C.J. Characterization of streetside soils of Syracuse, New York. Metria 1980, 3, 88–101. [Google Scholar]
- Enghoff, H. The size of a millipede. Ber. Naturwiss. Med. Ver. Innsb. 1992, 10, 47–56. [Google Scholar]
- Keddy, P.A. Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 1992, 3, 157–164. [Google Scholar] [CrossRef]
- Kula, E.; Lazorík, M. Centipedes, millipedes, terrestrial isopods and their relationships to physical and chemical properties of forest soils. Entomol. Fennica 2016, 27, 33–51. [Google Scholar] [CrossRef][Green Version]
- Branquart, É.; Kime, R.D.; Dufrêne, M.; Tavernier, J.; Wauthy, G. Macroarthropod-habitat relationships in oak forests in South Belgium. 1. Environments and communities. Pedobiologia 1995, 39, 243–263. [Google Scholar]
- Owojori, O.J.; Reinecke, A.J.; Voua-Otomo, P.; Reinecke, S.A. Comparative study of the effects of salinity on life-cycle parameters of four soil dwelling species (Folsomia candida, Enchytraeus doerjesi, Eisenia fetida and Aporrectodea caliginosa). Pedobiologia 2009, 52, 351–360. [Google Scholar] [CrossRef]

| Trait | Type | Units/levels | |
|---|---|---|---|
| Morphological traits | length | quantitative | mm |
| width | quantitative | mm | |
| Ecological preferences | habitat affinity | ordinal | 1 open habitat, 2 generalist, 3 forest specialist |
| humidity preference | ordinal | 1 xerotolerant, 2 mesophilic, 3 hygrophilous | |
| disturbance sensitivity | ordinal | 1 insensitive, 2 moderately sensitive, 3 sensitive |
| Family | Species | Abbreviations | Frequency (%) | |
|---|---|---|---|---|
| Buda | Pest | |||
| Blaniulidae | Blaniulidae sp. | Blsp | 4.3 | 12.5 |
| Proteroiulus fuscus (Am Stein, 1857) | Prfu | 0 | 4.2 | |
| Chordeumatidae | Chordeuma sylvestre C. L. Koch, 1847 | Chsy | 4.3 | 0 |
| Dorypetalidae | Dorypetalum degenerans (Latzel, 1884) | Dode | 13 | 0 |
| Glomeridae | Glomeris hexasticha Brandt, 1833 | Glhe | 13.0 | 8.3 |
| Julidae | Brachyiulus bagnalli Brölemann, 1924 | Brba | 0 | 29.2 |
| Cylindroiulus boleti (C. L. Koch, 1847) | Cybo | 78.3 | 58.3 | |
| Cylindroiulus caeruleocinctus (Wood, 1864) | Cyca | 0 | 4.2 | |
| Cylindroiulus sp. | Cysp | 0 | 8.3 | |
| Julus scandinavius (Latzel, 1884) | Jusc | 8.7 | 0 | |
| Kryphioiulus occultus (Koch, C. L., 1847) | Kroc | 0 | 33.3 | |
| Leptoiulus trilineatus (Koch, C. L., 1847) | Letrili | 43.5 | 0 | |
| Leptoiulus trilobatus Verhoeff, 1894 | Letrilo | 0 | 4.2 | |
| Megaphyllum projectum Verhoeff, 1894 | Mepr | 8.7 | 20.8 | |
| Megaphyllum unilineatum (Koch, 1838) | Meun | 4.3 | 58.3 | |
| Ommatoiulus sabulosus (Linnaeus, 1758) | Omsa | 39.1 | 25.0 | |
| Ophyiulus pilosus (Newport, 1843) | Oppi | 82.6 | 37.5 | |
| Mastigophorophyllidae | Mastigona bosniensis(Verhoeff, 1897) | Mabo | 21.7 | 25.0 |
| Mastigona sp. | Masp | 0 | 4.2 | |
| Polydesmidae | Brachydesmus sp. | Brsp | 17.4 | 20.8 |
| Eubrachydesmus superus (Latzel, 1884) | Eusu | 0 | 12.5 | |
| Polydesmus complanatus (Linnaeus, 1761) | Poco | 0 | 20.8 | |
| Polydesmus sp. | Posp | 0 | 16.7 | |
| Polyxenidae | Polyxenus lagurus (Linnaeus, 1758) | Pola | 30.4 | 4.2 |
| Traits/Ecological Preferences | Levels | Edaphic Factors | t | p |
|---|---|---|---|---|
| Habitat affinity | forest specialist | CaCO3 | 2.7 | 0.01 |
| Humidity preference | xerotolerant | SOM | 2.0 | 0.05 |
| mesophilic | SOM | −2.7 | 0.01 | |
| salt | 2.6 | 0.02 | ||
| Disturbance sensitivity | insensitive | SOM | −3.8 | <0.001 |
| moderately sensitive | SOM | 3.8 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, Z.; Hornung, E. Taxonomic and Functional Response of Millipedes (Diplopoda) to Urban Soil Disturbance in a Metropolitan Area. Insects 2020, 11, 25. https://doi.org/10.3390/insects11010025
Tóth Z, Hornung E. Taxonomic and Functional Response of Millipedes (Diplopoda) to Urban Soil Disturbance in a Metropolitan Area. Insects. 2020; 11(1):25. https://doi.org/10.3390/insects11010025
Chicago/Turabian StyleTóth, Zsolt, and Elisabeth Hornung. 2020. "Taxonomic and Functional Response of Millipedes (Diplopoda) to Urban Soil Disturbance in a Metropolitan Area" Insects 11, no. 1: 25. https://doi.org/10.3390/insects11010025
APA StyleTóth, Z., & Hornung, E. (2020). Taxonomic and Functional Response of Millipedes (Diplopoda) to Urban Soil Disturbance in a Metropolitan Area. Insects, 11(1), 25. https://doi.org/10.3390/insects11010025





