The Effect of Longwave Ultraviolet Light Radiation on Dendrolimus tabulaeformis Antioxidant and Detoxifying Enzymes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. UV Irradiation
2.3. Sample Preparation
2.4. Oxidative Parameters Assay
2.5. Antioxidant Enzyme Activity Assay
2.6. Detoxifying Enzyme Activity Assay
2.7. Data Analysis
3. Results
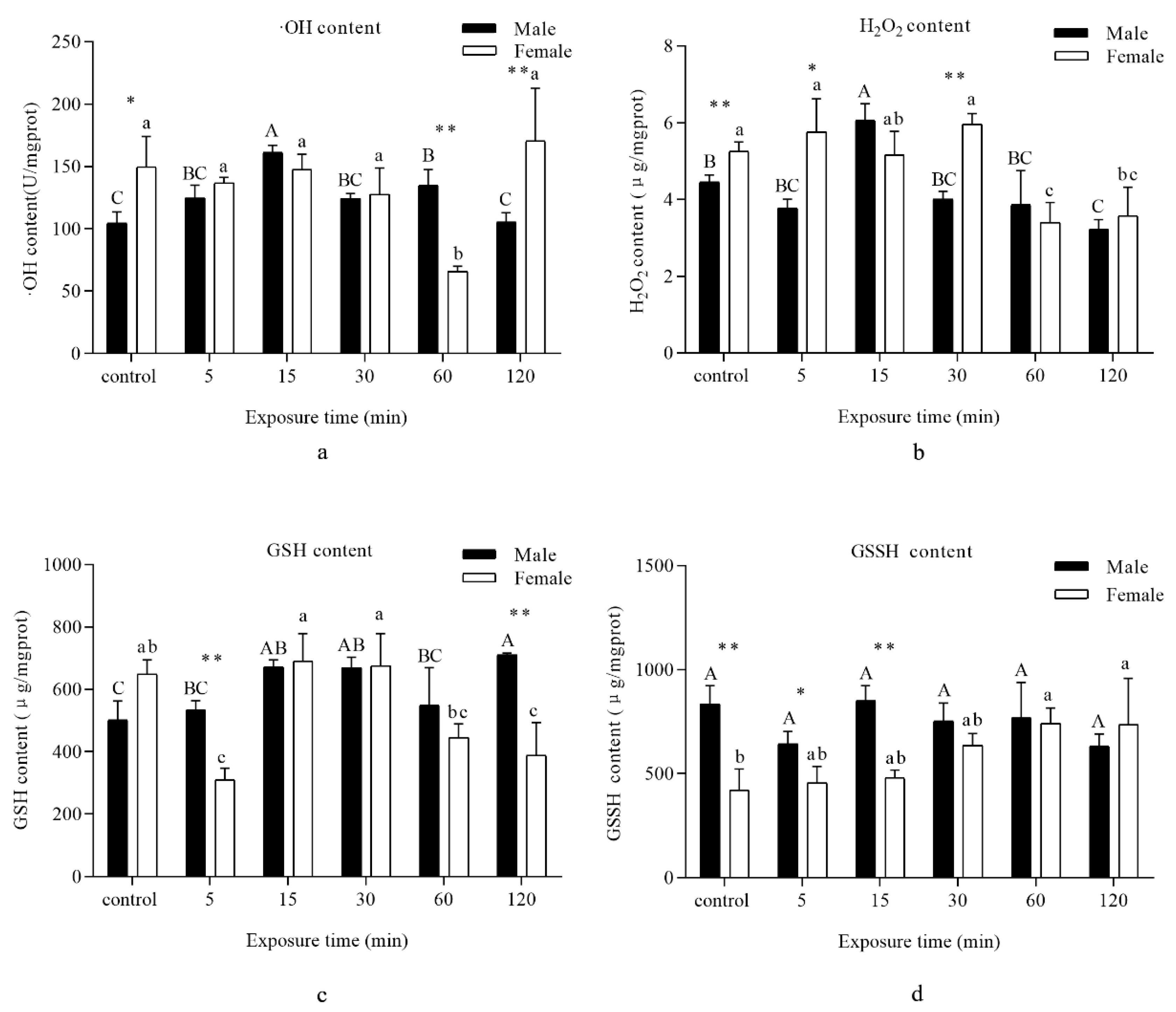
3.1. Oxidative Damage
3.2. Intermediates of Oxidation–Reduction Reactions
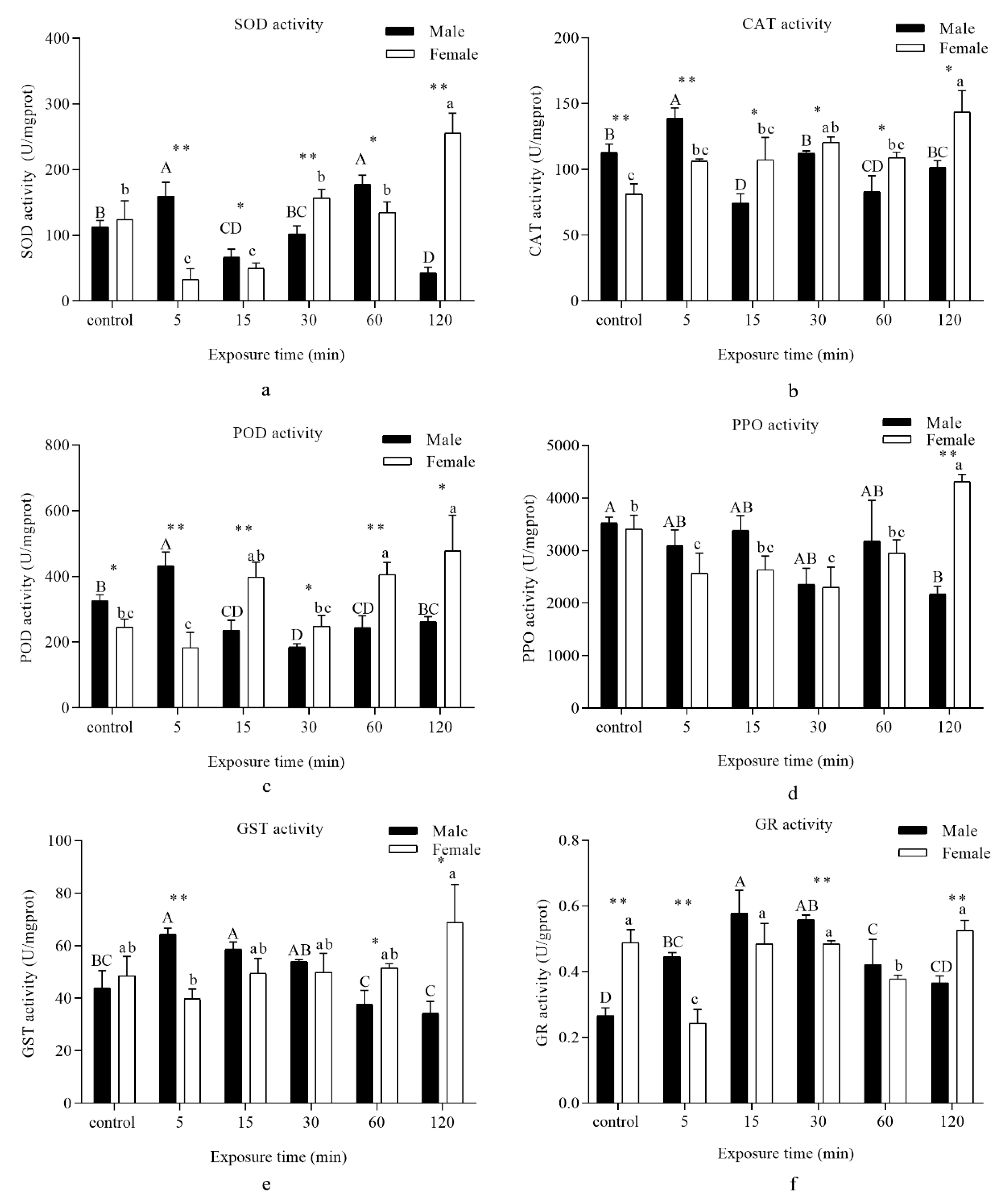
3.3. Antioxidant Enzymes
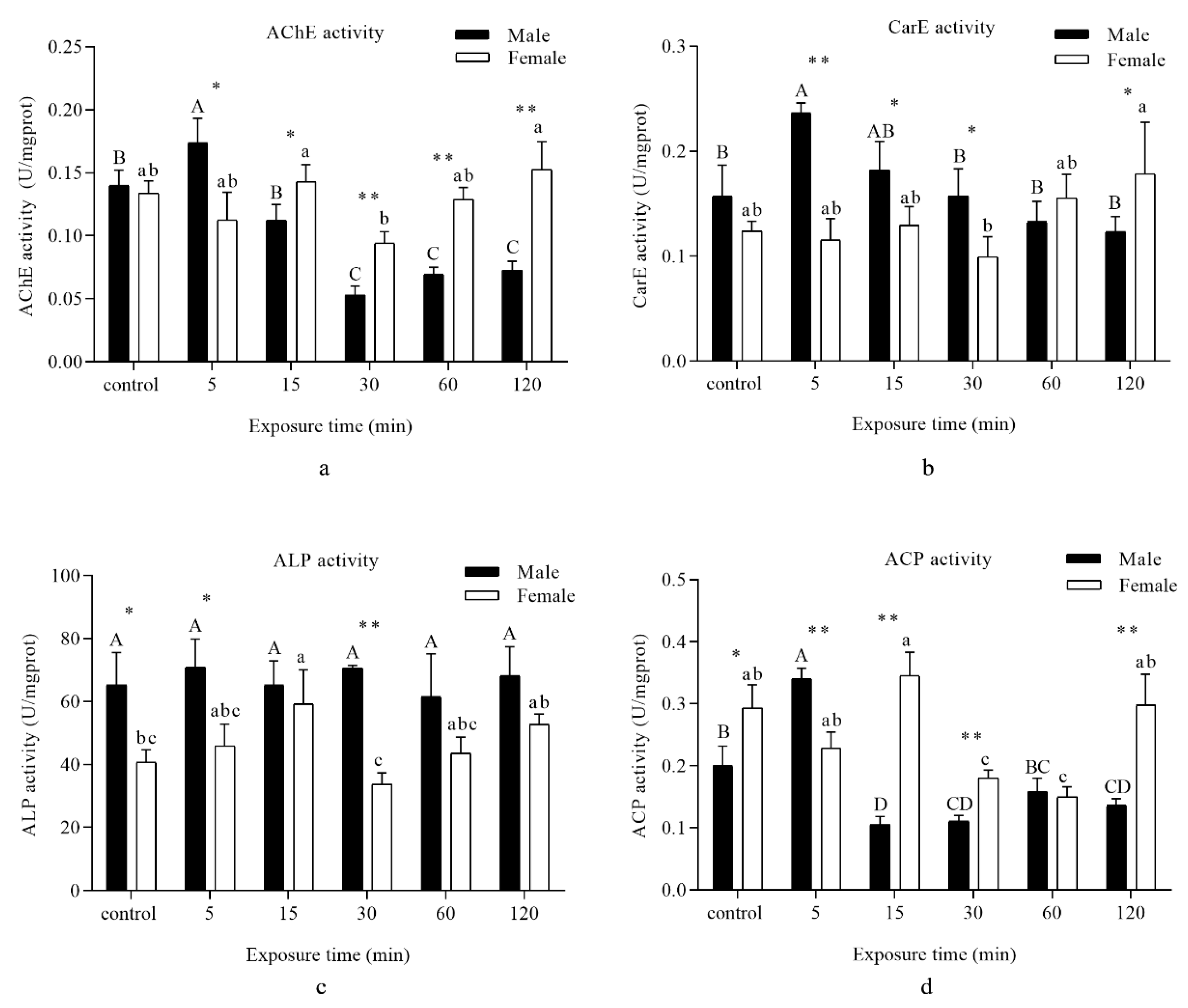
3.4. Detoxifying Enzyme
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.Q.; Wu, C.S. Fauna Sinica, Insecta, Lepidoptera, Lasiocampidae; Science Press: Beijing, China, 2006; Volume 47, pp. 47–48, 158–162. [Google Scholar]
- Hou, T.Q. The Pine Caterpillars in China; Science Press: Beijing, China, 1987; pp. 53–57. [Google Scholar]
- Wang, W.L.; Ren, L.L.; Zhang, L.S.; Ma, Y.B.; Luo, Y.Q. Effect and evaluation of trapping Dendrolimus tabulaeformis Tsai et Liu by different wavelength LED lights. Chin. J. Appl. Entomol. 2017, 54, 955–960. [Google Scholar] [CrossRef]
- Hölker, F.; Wolter, C.; Perkin, E.K.; Tockner, K. Light pollution as a biodiversity threat. Trends Ecol. Evol. 2010, 25, 681–682. [Google Scholar] [CrossRef]
- Cinzano, P.; Falchi, F.; Elvidge, C.D. The first world atlas of the artificial night sky brightness. Mon. Not. R. Astron. Soc. 2001, 328, 689–707. [Google Scholar] [CrossRef] [Green Version]
- Langevelde, F.V.; Ettema, J.A.; Donners, M.; Wallisdevries, M.F.; Groenendijk, D. Effect of spectral composition of artificial light on the attraction of moths. Biol. Conserv. 2011, 144, 2274–2281. [Google Scholar] [CrossRef]
- Antignus, Y. Manipulation of wavelength-dependent behaviour of insects: An IPM tool to impede insects and restrict epidemics of insect-borne viruses. Virus Res. 2000, 71, 213–220. [Google Scholar] [CrossRef]
- Kojima, Y.; Aoyagi, K.; Yasue, T. Effect of lithium ion addition on afterglow time of green-emitting Ce3+ and Pr3+ codoped CaS phosphor by black light irradiation. J. Lumin. 2005, 115, 13–18. [Google Scholar] [CrossRef]
- Van Grunsven, R.H.A.; Donners, M.; Boekee, K.; Tichelaar, I.; Van Geffen, K.G.; Groenendijk, D.; Berendse, F.; Veenendaal, E.M. Spectral composition of light sources and insect phototaxis, with an evaluation of existing spectral response models. J. Insect Conserv. 2014, 18, 225–231. [Google Scholar] [CrossRef]
- Dahms, H.U.; Lee, J.S. UV radiation in marine ectotherms: Molecular effects and responses. Aquat. Toxicol. 2010, 97, 3–14. [Google Scholar] [CrossRef]
- Wang, Y.; Oberley, L.W.; Murhammer, D.W. Antioxidant defense systems of two lepidopteran insect cell lines. Free Radic. Biol. Med. 2001, 30, 1254–1262. [Google Scholar] [CrossRef]
- Wharton, D.R.A. Ultraviolet repellent and lethal action on the American cockroach. J. Econ. Entomol. 1971, 64, 252–255. [Google Scholar] [CrossRef]
- Beard, R.L. Lethal action of UV irradiation on insects. J. Econ. Entomol. 1972, 65, 650–654. [Google Scholar] [CrossRef]
- Cohen, S.H.; Sousa, J.A.; Roach, J.F. Effects of UV irradiation on nymphs of five species of cockroaches. J. Econ. Entomol. 1973, 66, 859–862. [Google Scholar] [CrossRef]
- Cohen, S.H.; Sousa, J.A.; Roach, J.F.; Gingrich, J.B. Effects of UV irradiation on nymphs of Blattella germanica and Periplaneta americana. J. Econ. Entomol. 1975, 68, 687–693. [Google Scholar] [CrossRef]
- Faruki, S.I.; Das, D.R.; Khan, A.R.; Khatun, M. Effects of ultraviolet (254 nm) irradiation on egg hatching and adult emergence of the flour beetles, Tribolium castaneum, T. confusum and the almond moth, Cadra cautella. J. Insect Sci. 2007, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, I.; Shamma, M. Effect of non-ionizing radiation (UVC) on the development of Trogoderma granarium Everts. J. Stored Prod. Res. 2007, 43, 362–366. [Google Scholar] [CrossRef]
- Tariq, K.; Noor, M.; Saeed, S.; Zhang, H.Y. The effect of ultraviolet—A radiation exposure on the reproductive ability, longevity, and development of the Dialeurodes citri (Homoptera: Aleyrodidae) F1 generation. Environ. Entomol. 2015, 44, 1614–1618. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant systems in insects. Arch. Insect Biochem. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Urbach, F. The biological effects of increased ultraviolet radiation: An update. Photochem. Photobiol. 1989, 50, 439–441. [Google Scholar] [CrossRef]
- Schauen, M.; Hornig-Do, H.T.; Schomberg, S.; Herrmann, G.; Wiesner, R.J. Mitochondrial electron transport chain activity is not involved in ultraviolet A (UVA)-induced cell death. Free Radic. Biol. Med. 2007, 42, 499–509. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. Superoxide dismutase: A photochemical augmentation assay. Arch. Biochem. Biophys. 1977, 181, 308–312. [Google Scholar] [CrossRef]
- Fridovich, I. Oxygen is toxic! Bioscience 1977, 27, 462–466. [Google Scholar] [CrossRef]
- Rael, L.T.; Thomas, G.W.; Craun, M.L.; Curtis, C.G.; Bar-Or, R.; Bar-Or, D. Lipid peroxidation and the thiobarbituric acid assay: Standardization of the assay when using saturated and unsaturated fatty acids. J. Biochem. Mol. Biol. 2004, 37, 749–752. [Google Scholar] [CrossRef]
- Rio, D.D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Meng, J.Y.; Zhang, C.Y.; Zhu, F.; Wang, X.P.; Lei, C.L. Ultraviolet light-induced oxidative stress: Effects on antioxidant response of Helicoverpa armigera adults. J. Insect Physiol. 2009, 55, 588–592. [Google Scholar] [CrossRef]
- Karthi, S.; Sankari, R.; Shivakumar, M.S. Ultraviolet-B light induced oxidative stress: Effects on antioxidant response of Spodoptera litura. J. Photochem. Photobiol. B 2014, 135, 1–6. [Google Scholar] [CrossRef]
- Kaplowitz, N.; Aw, T.Y.; Ookhtens, M. The regulation of hepatic glutathione. Annu. Rev. Pharmacol. 1985, 25, 715–744. [Google Scholar] [CrossRef]
- Carmel-Harel, O.; Storz, G. Roles of the glutathione and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae, responses to oxidative stress. Annu. Rev. Microbiol. 2000, 54, 439–461. [Google Scholar] [CrossRef]
- Lopez-Martinez, G.; Elnitsky, M.A.; Benoit, J.B.; Lee, R.E., Jr.; Denlinger, D.L. High resistance to oxidative damage in the Antarctic midge Belgica antarctica, and developmentally linked expression of genes encoding superoxide dismutase, catalase and heat shock proteins. Insect Biochem. Mol. Biol. 2008, 38, 796–804. [Google Scholar] [CrossRef]
- John, S.; Kale, M.; Rathore, N.; Bhatnagar, D. Protective effect of vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J. Nutr. Biochem. 2001, 12, 500–504. [Google Scholar] [CrossRef]
- Williams, G.C. Natural selection, the costs of reproduction, and a refinement of lack’s principle. Am. Nat. 1966, 100, 687–690. [Google Scholar] [CrossRef] [Green Version]
- Wootton, R.J. The evolution of life histories: Theory and analysis. Rev. Fish Biol. Fisher. 1993, 3, 384–385. [Google Scholar] [CrossRef]
- Ali, A.; Rashid, M.A.; Huang, Q.Y.; Lei, C.L. Effect of UV-A radiation as an environmental stress on the development, longevity, and reproduction of the oriental armyworm, Mythimna separate (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2016, 23, 17002–17007. [Google Scholar] [CrossRef]
- Heck, D.E.; Vetrano, A.M.; Mariano, T.M.; Laskin, J.D. UVB light stimulates production of reactive oxygen species: Unexpected role for catalase. J. Biol. Chem. 2003, 278, 22432–22436. [Google Scholar] [CrossRef] [Green Version]
- Polte, T.; Tyrrell, R.M. Involvement of lipid peroxidation and organic peroxides in UVA-induced matrix metalloproteinase-1 expression. Free Radic. Biol. Med. 2004, 36, 1566–1574. [Google Scholar] [CrossRef]
- Orr, W.C.; Sohal, R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 1994, 263, 1128–1130. [Google Scholar] [CrossRef]
- Mathews, M.C.; Summers, C.B.; Felton, G.W. Ascorbate peroxidase: A novel antioxidant enzyme in insects. Arch. Insect Biochem. 1997, 34, 57–68. [Google Scholar] [CrossRef]
- Tabatabaie, T.; Floyd, R.A. Susceptibility of glutathione peroxidase and glutathione reductase to oxidative damage and the protective effect of spin trapping agents. Arch. Biochem. Biophys. 1994, 314, 112–119. [Google Scholar] [CrossRef]
- Ahmad, S.; Duval, D.L.; Weinhold, L.C.; Pardini, R.S. Cabbage looper antioxidant enzymes: Tissue specificity. Insect Biochem. 1991, 21, 563–572. [Google Scholar] [CrossRef]
- Ganguli, D.; Kumar, C.; Bachhawat, A.K. The alternative pathway of glutathione degradation is mediated by a novel protein complex involving three new genes in Saccharomyces cerevisiae. Genetics 2006, 175, 1137–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marty, L.; Siala, W.; Schwarzländer, M.; Fricker, M.D.; Wirtz, M.; Sweetlove, L.J.; Meyer, Y.; Meyer, A.J.; Reichheld, J.P.; Hell, R. The NADPH-dependent thioredoxin system constitutes a functional backup for cytosolic glutathione reductase in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 9109–9114. [Google Scholar] [CrossRef] [Green Version]
- Meister, A. Glutathione metabolism and its selective modification. J. Biol Chem. 1988, 263, 17205–17208. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Miao, L.; Dong, F.; Wang, J. Advances in the research on invertebrate acetylcholinesterase. J. Environ. Entomol. 2019, 41, 508–519. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.H. Which acetylcholinesterase functions as the main catalytic enzyme in the class insecta? Insect Biochem. Mol. Biol. 2013, 43, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gupta, G.P.; Rajam, M.V. Silencing of acetylcholinesterase gene of Helicoverpa armigera by siRNA affects larval growth and its life cycle. J. Insect Physiol. 2009, 55, 273–278. [Google Scholar] [CrossRef]
- Vincent, J.B.; Crowder, M.W.; Averill, B.A. Hydrolysis of phosphate monoesters: A biological problem with multiple chemical solutions. Trends Biochem. Sci. 1992, 17, 105–110. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Gao, C.; Ren, L.; Luo, Y. The Effect of Longwave Ultraviolet Light Radiation on Dendrolimus tabulaeformis Antioxidant and Detoxifying Enzymes. Insects 2020, 11, 1. https://doi.org/10.3390/insects11010001
Wang W, Gao C, Ren L, Luo Y. The Effect of Longwave Ultraviolet Light Radiation on Dendrolimus tabulaeformis Antioxidant and Detoxifying Enzymes. Insects. 2020; 11(1):1. https://doi.org/10.3390/insects11010001
Chicago/Turabian StyleWang, Wenlong, Chenglong Gao, Lili Ren, and Youqing Luo. 2020. "The Effect of Longwave Ultraviolet Light Radiation on Dendrolimus tabulaeformis Antioxidant and Detoxifying Enzymes" Insects 11, no. 1: 1. https://doi.org/10.3390/insects11010001
APA StyleWang, W., Gao, C., Ren, L., & Luo, Y. (2020). The Effect of Longwave Ultraviolet Light Radiation on Dendrolimus tabulaeformis Antioxidant and Detoxifying Enzymes. Insects, 11(1), 1. https://doi.org/10.3390/insects11010001






