Can Isogroup Selection of Highly Zoophagous Lines of a Zoophytophagous Bug Improve Biocontrol of Spider Mites in Apple Orchards?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isogroup Line Foundation
2.2. Mass Rearing
2.3. Study Sites
2.4. Experimental Design
2.4.1. Damage on Apple Fruitlets
2.4.2. Spider Mite Predation
2.5. Statistical Analysis
3. Results
3.1. Damage on Apple Fruitlets
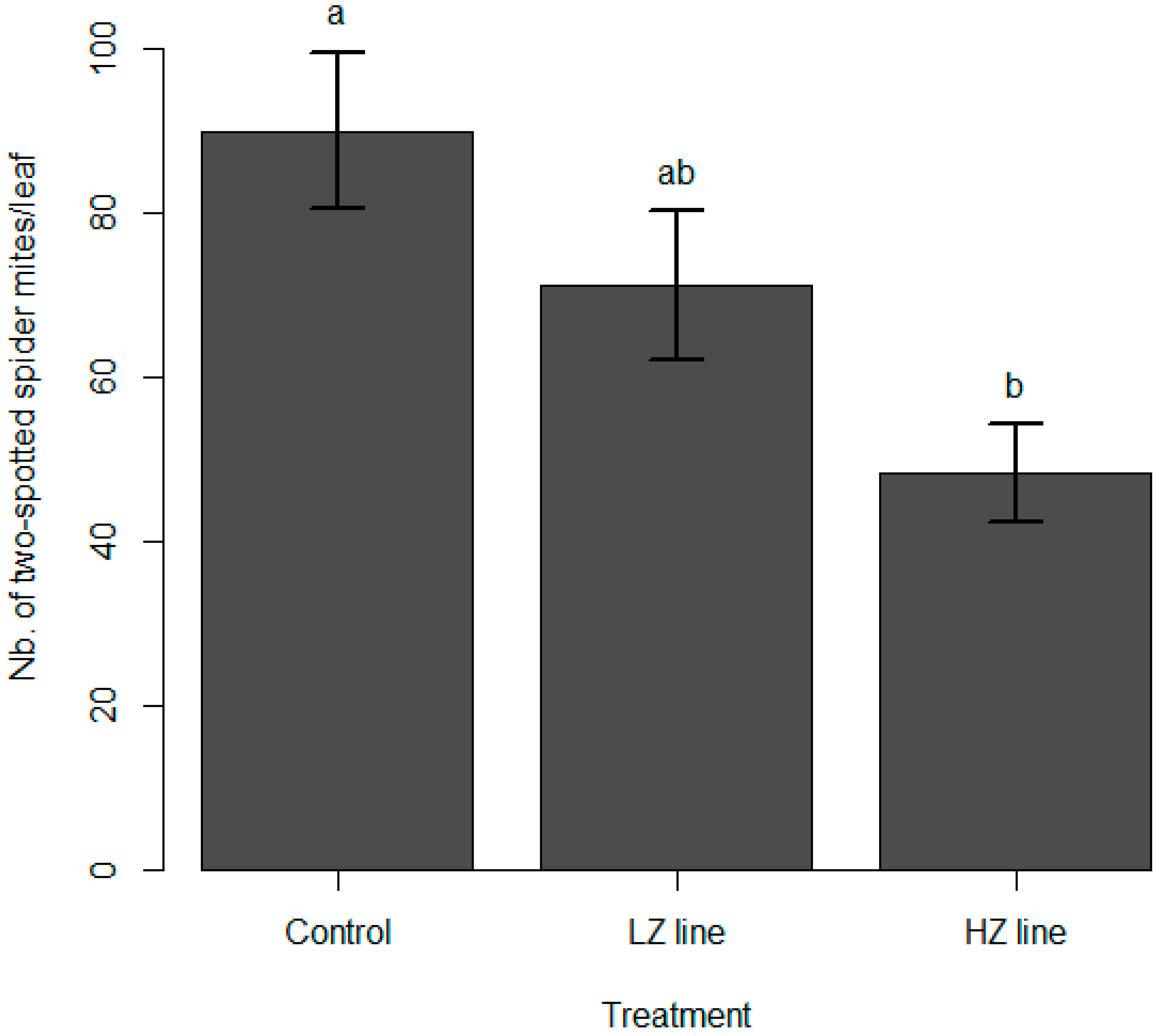
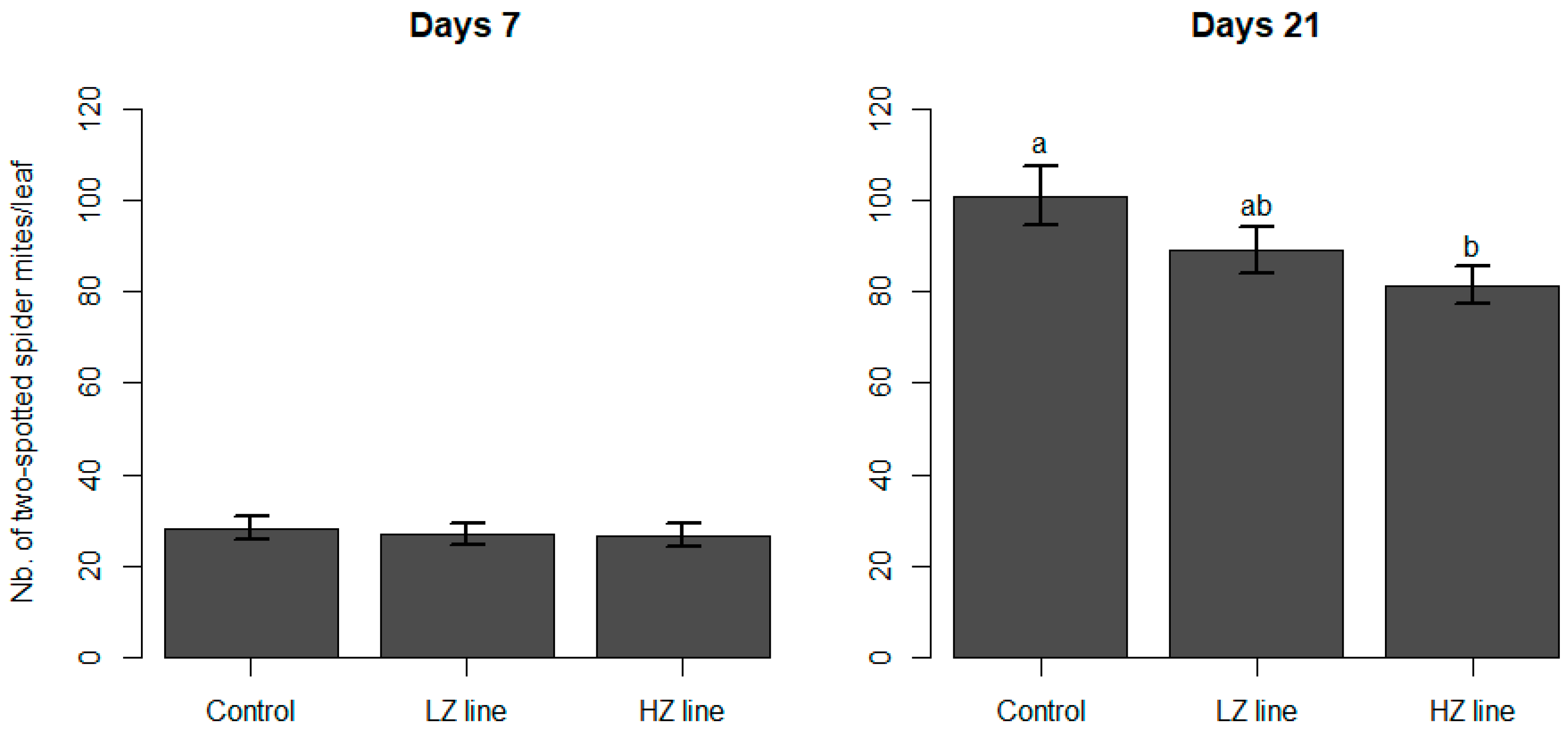
3.2. Spider Mite Predation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coll, M.; Guershon, M. Omnivory in terrestrial arthropods: Mixing plant and prey diets. Annu. Rev. Entomol. 2002, 47, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Fauvel, G. Diversity of Heteroptera in agroecosystems: Role of sustainability and bioindication. In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Elsevier: Amsterdam, The Netherlands, 1999; pp. 275–303. [Google Scholar]
- Urbaneja, A.; González-Cabrera, J.; Arno, J.; Gabarra, R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag. Sci. 2012, 68, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Eubanks, M.D.; Denno, R.F. The ecological consequences of variation in plants and prey for an omnivorous insect. Ecology 1999, 80, 1253–1266. [Google Scholar] [CrossRef]
- Calvo, J.; Bolckmans, K.; Stansly, P.A.; Urbaneja, A. Predation by Nesidiocoris tenuis on Bemisia tabaci and injury to tomato. BioControl 2009, 54, 237–246. [Google Scholar] [CrossRef]
- Calvo, F.J.; Lorente, M.J.; Stansly, P.A.; Belda, J.E. Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol. Exp. Appl. 2012, 143, 111–119. [Google Scholar] [CrossRef]
- Mollá, O.; González-Cabrera, J.; Urbaneja, A. The combined use of Bacillus thuringiensis and Nesidiocoris tenuis against the tomato borer Tuta absoluta. BioControl 2011, 56, 883–891. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.-H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; Stewart, R.K. Identification and evaluation of damage to McIntosh apples by phytophagous mirids (Hemiptera: Miridae) in southwestern Quebec. Can. Entomol. 1982, 114, 1037–1045. [Google Scholar] [CrossRef]
- Aubry, O.; Cormier, D.; Chouinard, G.; Lucas, E. Phytophagy by the Mullein Bug (Hemiptera: Miridae) on Apples: Feeding Behavior and Fruit Damage. J. Econ. Entomol. 2016, 109, 2463–2471. [Google Scholar] [CrossRef]
- McMullen, R.D.; Jong, C. The biology and influence of pesticides on Campylomma verbasci (Heteroptera: Miridae). Can. Entomol. 1970, 102, 1390–1394. [Google Scholar] [CrossRef]
- Thistlewood, H.M.A.; Borden, J.H.; McMullen, R.D. Seasonal abundance of the mullein bug, Campylomma verbasci (Meyer) (Heteroptera: Miridae), on apple and mullein in the Okanagan Valley. Can. Entomol. 1990, 122, 1045–1058. [Google Scholar] [CrossRef]
- Beers, E.H.; Hull, L.A.; Grimm, J.W. Relationships between leaf: Fruit ratio and varying levels of European red mite stress on fruit size and return bloom of apple. J. Am. Soc. Hortic. Sci. 1987, 112, 608–612. [Google Scholar]
- Parent, B.J. Natural population densities of the European red mite on apple in Quebec. Environ. Entomol. 1973, 2, 1064–1068. [Google Scholar] [CrossRef]
- Arnoldi, D.; Stewart, R.K.; Boivin, G. Predatory mirids of the green apple aphid Aphis pomi, the two-spotted spider mite Tetranychus urticae and the European red mite Panonychus ulmi in apple orchards in Québec. Entomophaga 1992, 37, 283–292. [Google Scholar] [CrossRef]
- Murdoch, W.W.; Chesson, J.; Chesson, P.L. Biological control in theory and practice. Am. Nat. 1985, 125, 344–366. [Google Scholar] [CrossRef]
- Landis, D.A.; Van der Werf, W. Early-season predation impacts the establishment of aphids and spread of beet yellows virus in sugar beet. Entomophaga 1997, 42, 499–516. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef]
- Kain, D.P.; Agnello, A.M. Relationship between plant phenology and Campylomma verbasci (Hemiptera: Miridae) damage to apple fruit. Environ. Entomol. 2013, 42, 307–313. [Google Scholar] [CrossRef]
- Aubry, O.; Cormier, D.; Chouinard, G.; Lucas, E. Influence of plant, animal and mixed resources on development of the zoophytophagous plant bug Campylomma verbasci (Hemiptera: Miridae). Biocontrol Sci. Technol. 2015, 25, 1426–1442. [Google Scholar] [CrossRef]
- Reding, M.E.; Beers, E.H.; Brunner, J.F.; Dunley, J.E. Influence of timing and prey availability on fruit damage to apple by Campylomma verbasci (Hemiptera: Miridae). J. Econ. Entomol. 2001, 94, 33–38. [Google Scholar] [CrossRef]
- Dumont, F.; Lucas, E.; Réale, D. Evidence of genetic basis of zoophagy and nymphal developmental time in isogroup lines of the zoophytophagous mullein bug, Campylomma verbasci. BioControl 2016, 61, 425–435. [Google Scholar] [CrossRef]
- Dumont, F.; Lucas, E.; Réale, D. Coexistence of zoophytophagous and phytozoophagous strategies linked to genotypic diet specialization in plant bug. PLoS ONE 2017, 12, e0176369. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.; Réale, D.; Lucas, E. Isogroup Selection to Optimize Biocontrol Increases Cannibalism in Omnivorous (Zoophytophagous) Bugs. Insects 2017, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Dumont, F.; Aubry, O.; Lucas, E. From evolutionary aspects of zoophytophagy to biological control. Front. Ecol. Evol. 2018, 6, 221. [Google Scholar] [CrossRef]
- David, J.R.; Gibert, P.; Legout, H.; Pétavy, G.; Capy, P.; Moreteau, B. Isofemale lines in Drosophila: An empirical approach to quantitative trait analysis in natural populations. Heredity 2005, 94, 3. [Google Scholar] [CrossRef] [PubMed]
- Moreteau, B.; Capy, P.; Alonso-Moraga, A.; Munoz-Serrano, A.; Stockel, J.; David, J.R. Genetic characterization of geographic populations using morphometrical traits in Drosophila melanogaster: Isogroups versus isofemale lines. Genetica 1995, 96, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 978-0-387-87458-6. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; ISBN 3-900051-07-0. Available online: https://www.R-project.Org (accessed on 17 September 2019).
- Cohen, A.C. Plant feeding by predatory Heteroptera: Evolutionary and adaptational aspects of trophic switching. In Zoophytophagous Heteroptera: Implications for Life History and Integrated Pest Management; Entomological Society of Amer: Annapolis, MD, USA, 1996; pp. 1–17. [Google Scholar]
- Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 2011, 59, 22–29. [Google Scholar] [CrossRef]
- Sanchez, J.A. Zoophytophagy in the plantbug Nesidiocoris tenuis. Agric. For. Entomol. 2008, 10, 75–80. [Google Scholar] [CrossRef]
- Arnó, J.; Castañé, C.; Riudavets, J.; Gabarra, R. Risk of damage to tomato crops by the generalist zoophytophagous predator Nesidiocoris tenuis (Reuter) (Hemiptera: Miridae). Bull. Entomol. Res. 2010, 100, 105–115. [Google Scholar] [CrossRef]
- Carriere, Y.; Deland, J.-P.; Roff, D.A.; Vincent, C. Life-history costs associated with the evolution of insecticide resistance. Proc. R. Soc. London Ser. B Biol. Sci. 1994, 258, 35–40. [Google Scholar]
- Thrall, P.H.; Oakeshott, J.G.; Fitt, G.; Southerton, S.; Burdon, J.J.; Sheppard, A.; Russell, R.J.; Zalucki, M.; Heino, M.; Ford Denison, R. Evolution in agriculture: The application of evolutionary approaches to the management of biotic interactions in agro-ecosystems. Evol. Appl. 2011, 4, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Lankau, R.A. Rapid evolutionary change and the coexistence of species. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 335–354. [Google Scholar] [CrossRef]
- Palkovacs, E.P.; Kinnison, M.T.; Correa, C.; Dalton, C.M.; Hendry, A.P. Fates beyond traits: Ecological consequences of human-induced trait change. Evol. Appl. 2012, 5, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Ferrari, M.C.; Harris, D.J. Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 2011, 4, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Sih, A. Understanding variation in behavioural responses to human-induced rapid environmental change: A conceptual overview. Anim. Behav. 2013, 85, 1077–1088. [Google Scholar] [CrossRef]
- Ashley, M.V.; Willson, M.F.; Pergams, O.R.; O’Dowd, D.J.; Gende, S.M.; Brown, J.S. Evolutionarily enlightened management. Biol. Conserv. 2003, 111, 115–123. [Google Scholar] [CrossRef]
- Stockwell, C.A.; Hendry, A.P.; Kinnison, M.T. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 2003, 18, 94–101. [Google Scholar] [CrossRef]
- Lankau, R.; Jørgensen, P.S.; Harris, D.J.; Sih, A. Incorporating evolutionary principles into environmental management and policy. Evol. Appl. 2011, 4, 315–325. [Google Scholar] [CrossRef]
- Lommen, S.T.; de Jong, P.W.; Pannebakker, B.A. It is time to bridge the gap between exploring and exploiting: Prospects for utilizing intraspecific genetic variation to optimize arthropods for augmentative pest control—A review. Entomol. Exp. Appl. 2017, 162, 108–123. [Google Scholar] [CrossRef]
- Nachappa, P.; Margolies, D.C.; Nechols, J.R.; Campbell, J.F. Variation in predator foraging behaviour changes predator–prey spatio-temporal dynamics. Funct. Ecol. 2011, 25, 1309–1317. [Google Scholar] [CrossRef]
- Hoy, M.A.; McKelvey, J.J., Jr. Genetics in Relation to Insect Management; Rockefeller Foundation: New York, NY, USA, 1979. [Google Scholar]
- Roush, R.T. Genetic variation in natural enemies: Critical issues for colonization in biological control. Crit. Issues Biol. Control 1990, 263–288. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumont, F.; Réale, D.; Lucas, É. Can Isogroup Selection of Highly Zoophagous Lines of a Zoophytophagous Bug Improve Biocontrol of Spider Mites in Apple Orchards? Insects 2019, 10, 303. https://doi.org/10.3390/insects10090303
Dumont F, Réale D, Lucas É. Can Isogroup Selection of Highly Zoophagous Lines of a Zoophytophagous Bug Improve Biocontrol of Spider Mites in Apple Orchards? Insects. 2019; 10(9):303. https://doi.org/10.3390/insects10090303
Chicago/Turabian StyleDumont, François, Denis Réale, and Éric Lucas. 2019. "Can Isogroup Selection of Highly Zoophagous Lines of a Zoophytophagous Bug Improve Biocontrol of Spider Mites in Apple Orchards?" Insects 10, no. 9: 303. https://doi.org/10.3390/insects10090303
APA StyleDumont, F., Réale, D., & Lucas, É. (2019). Can Isogroup Selection of Highly Zoophagous Lines of a Zoophytophagous Bug Improve Biocontrol of Spider Mites in Apple Orchards? Insects, 10(9), 303. https://doi.org/10.3390/insects10090303




