Oviposition Behavior and Distribution of Eucryptorrhynchus scrobiculatus and E. brandti (Coleoptera: Curculionidae) on Ailanthus altissima (Mill.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Observation of the Oviposition Behavior of E. scrobiculatus
2.3. Investigation of Oviposition Sites of E. scrobiculatus and E. brandti in the Field
2.4. Oviposition Preference of E. scrobiculatus and E. brandti in the Laboratory
2.5. Data Analyses
3. Results
3.1. Behavior of E. scrobiculatus Females during Oviposition
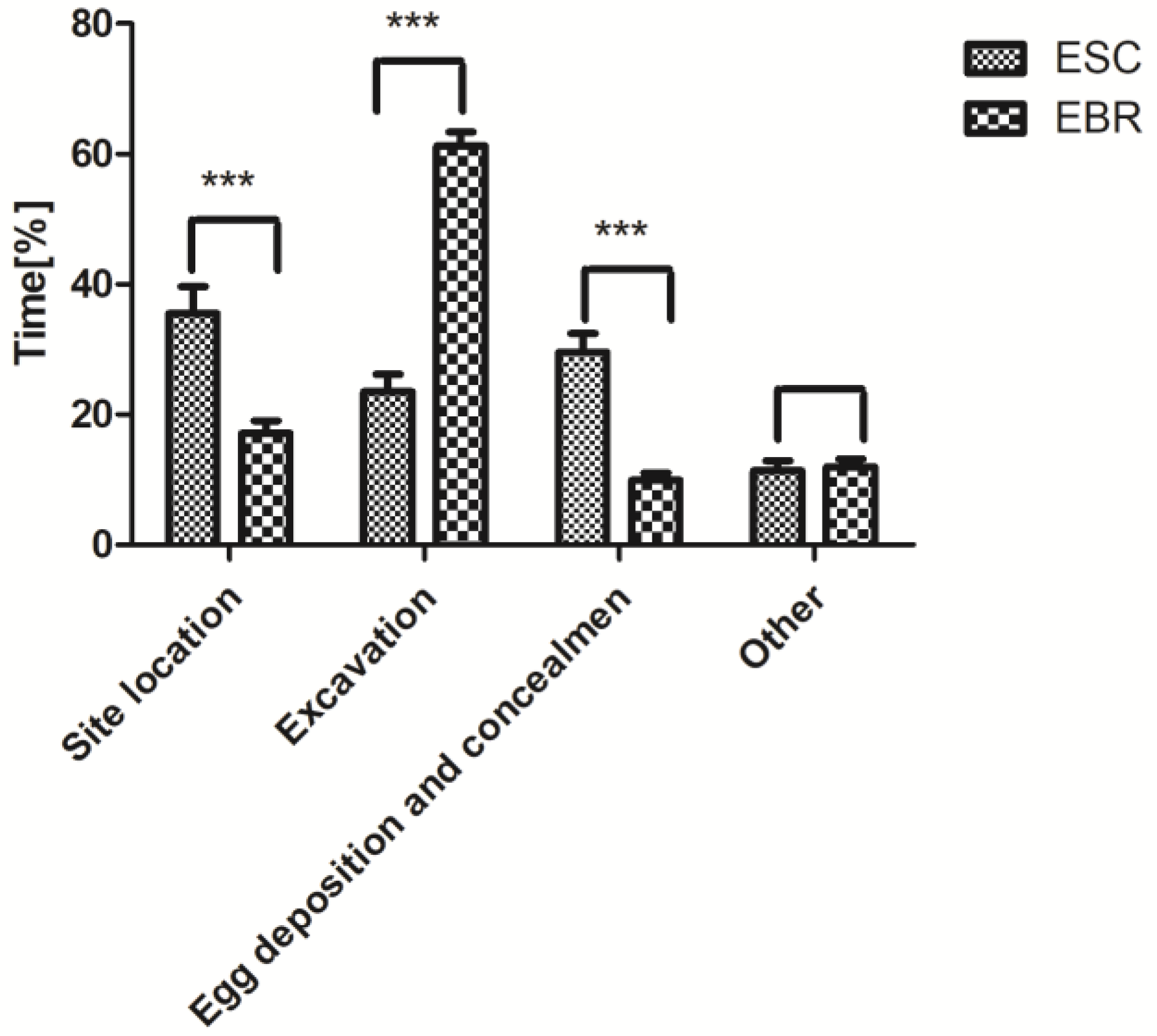
3.2. Comparison of the Oviposition Behavior of E. scrobiculatus and E. brandti
3.3. Eucryptorrhynchus scrobiculatus Lay Eggs in Compound Leaf Petioles
3.4. Oviposition Sites Distribution in the Field
3.5. Oviposition Preference of E. scrobiculatus and E. brandti in the Laboratory
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kowarik, I.; Säumel, I. Biological flora of central Europe: Ailanthus altissima (Mill.) swingle. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Herrick, N.J.; Salom, S.M.; Kok, L.T.; Mcavoy, T.J. Foliage feeding tests of Eucryptorrhynchus brandti (Harold) (Coleoptera: Curculionidae), a potential biological control agent of the tree-of-heaven, Ailanthus altissima. USDA Res. Forum Invasive Spec. 2009, 77, 13–16. [Google Scholar]
- Ni, T.L.; Li, F.S.; Li, Z.Y.; Wang, M. The harm of main diseases and insect pests of Ailanthus altissima and its comprehensive control. For. Sci. Technol. 2004, 29, 24–25. [Google Scholar]
- Davies, P.A. The history, distribution, and value of Ailanthusin North America. Trans. Ky. Acad. Sci. 1942, 9, 12–14. [Google Scholar]
- Feret, P.P. Ailanthus: Variation, cultivation, and frustration. J. Arboric. 1985, 11, 361–368. [Google Scholar]
- Tellman, B. Exotic pest plant introduction in the American Southwest. Desert Plants 1997, 13, 3–10. [Google Scholar]
- Herrick, N.J.; Mcavoy, T.J.; Snyder, A.L.; Salom, S.M.; Kok, L.T. Host-range testing of Eucryptorrhynchus brandti (Coleoptera: Curculionidae), a candidate for biological control of tree-of-heaven, Ailanthus altissima. Environ. Entomol. 2012, 41, 118–124. [Google Scholar] [CrossRef]
- Mcavoy, T.J.; Salom, S.M.; Yu, B.; Ji, H.L.; Du, Y.Z.; Johnson, N.; Reardon, R.; Kok, L.T. Occurrence and development of Eucryptorrhynchus brandti and E. chinensis (Coleoptera: Curculionidae) on Ailanthus altissima trees subjected to different levels of mechanical damage. Biocontrol Sci. Technol. 2014, 24, 65–79. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, Y. Economic insect fauna of China. Fasc. 20. Coleoptera: Curculionidae, 1st ed.; Wanhai Books: Hong Kong, China, 2015; pp. 173–184. [Google Scholar]
- Tong, Z. The discovery and control of Eucryptorrhynchus brandti (Harold) in Shenyang area. In Proceedings of the Summary of the tenth Academic Symposium of the National Garden Plant Protection 2001, Urumqi, China, August 2001. [Google Scholar]
- Xu, L. Control on Eucryptorrhynchus brandti (Harold). New Agric. 2008, 9, 48. [Google Scholar]
- Zhou, L. Investigation on the harm of Eucryptorrhynchus scrobiculatus Motschulsky to Qiantouchun and suggestions for prevention and treatment in Akesu. Agric. Technol. 2007, 27, 61–62. [Google Scholar]
- Su, H.; Chen, P. The harm of Eucryptorrhynchus brandti (Harold) in Ningxia area and its control methods. Xiandai Hortic. 2013, 11, 109–110. [Google Scholar]
- Shi, Y.; Wang, A.; Sun, X.; Gao, X. The occurrence and control measures of Eucryptorrhynchus scrobiculatus. Plant Prot. Technol. Ext. 1994, 1, 15–16. [Google Scholar]
- Yang, G.; Yong, H.; Wang, X. The biological characters and behavior of Eucryptorrhynchus chinesis. Chin. Bull. Entomol. 2008, 45, 65–69. [Google Scholar]
- Zhang, Y. Study on Pest-Control Related Key Biological Characteristics of Eucryptorrhynchus scrobiculatus Motschulsky. Master’s Thesis, Beijing Forestry University, Beijing, China, 2015. [Google Scholar]
- Ding, J.; Wu, Y.; Zheng, H.; Fu, W.; Reardon, R.; Liu, M. Assessing potential biological control of the invasive plant, tree-of-heaven, Ailanthus altissima. Biocontrol Sci. Technol. 2006, 16, 547–566. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Ji, Y.C.; Wen, X.J.; Li, Q.; Ren, Y.; Wen, J.B. Oviposition behavior of Eucryptorrhynchus brandti (Coleoptera: Curculionidae: Cryptorrhychinae) on Ailanthus altissima (Mill.) Swingle (Sapindales: Simaroubaceae). Biocontrol Sci. Technol. 2017, 27, 1153–1167. [Google Scholar] [CrossRef]
- Kok, L.T.; Salom, S.M.; Yan, S.; Herrick, N.J.; McAvoy, T.J. Quarantine evaluation of Eucryptorrhynchus brandti (Harold) (Coleoptera: Curculionidae), a potential biological control agent of tree of heaven, Ailanthus altissima. In Proceedings of the XII International Symposium on Biological Control of Weeds, CAB International, Wallingford, UK; 2008; pp. 292–300. [Google Scholar]
- Hutchinson, G.E. Concluding Remarks. Cold Spring Harbor. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Homage to Santa Rosalia or Why Are There So Many Kinds of Animals? Am. Nat. 1959, 93, 145–159. [Google Scholar] [CrossRef]
- Townsend, C.R.; Begon, M.; Harper, J.L. Fundamentos em ecologia. Artmed. Editor. 2009, 9, 592. [Google Scholar]
- Wang, J.; Lang, X.; Sun, P.; Lei, Y.; Zhang, X.; Wu, S. Study on the biological habit of Eucryptorrhynchus scrobiculatus and Eucryptorrhynchus brandti in the Yellow River irrigated area of Ningxia. Ningxia J. Agric. For. Sci. Technol. 2011, 52, 33–34. [Google Scholar]
- Liu, Z.K. Molecular Phylogeny and Species Differentiation of the Eucryptorrhynchus scrobiculatus and E. brandti. Doctoral Thesis, Beijing Forestry University, Beijing, China, 2016. [Google Scholar]
- Ji, Y.C.; Gao, P.; Zhang, G.Y.; Wen, C.; Wen, J.B. Micro-habitat niche differentiation contributing to coexistence of Eucryptorrhynchus scrobiculatus Motschulsky and Eucryptorrhynchus brandti Harold. Biocontrol Sci. Technol. 2017, 27, 1180–1194. [Google Scholar] [CrossRef]
- Yu, Q. Preliminary Study on Biology and Artificial Rearing of Eucryptorrhynchus chinensis (Olivier) (Coleptera: Curculionidae). Master’s Thesis, Beijing Forestry University, Beijing, China, 2013. [Google Scholar]
- Yang, P.A. Preliminary Study of the Artificial Raising and Prevention of Eucryptorrhynchus chinensis. Master’s Thesis, Beijing Forestry University, Beijing, China, 2015. [Google Scholar]
- Wen, X.J.; Yang, K.L.; Wen, J.B. Host-independent artificial rearing of Eucryptorrhynchus brandti (Coleoptera: curculionidae). Biocontrol Sci. Technol. 2016, 26, 1–19. [Google Scholar] [CrossRef]
- Ellis, A.M. Linking movement and oviposition behavior to spatial population distribution in the tree hole mosquito Ochlerotatus triseriatus. J. Anim. Ecol. 2010, 77, 156–166. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, C.; Chen, X. Progress in the oviposition behavioral ecology of herbivorous insects. For. Res. 2010, 23, 770–777. [Google Scholar]
- Howden, A.T. Structures related to oviposition in Curculionoidea. Mem. Ent. Soc. Wash. 1995, 14, 53–100. [Google Scholar]
- Fisher, H. Lust, Attraction, Attachment: Biology and Evolution of the Three Primary Emotion Systems for Mating, Reproduction, and Parenting. J. Sex Educ. Ther. 2000, 25, 96–104. [Google Scholar] [CrossRef]
- Su, Y.; Feng, X. Study on biological characteristics and sustainable control techniques of Eucryptorrhynchus scrobiculatus. J. Agric. Sci. Technol. 2008, 5, 13–14. [Google Scholar]
- Anderson, R.S. An evolutionary perspective on diversity in Curculionoidea. Mem. Ent. Soc. Wash. 1995, 14, 103–114. [Google Scholar]
- Hughes, J.; Vogler, A.P. Ecomorphological adaptation of acorn weevils to their oviposition site. Evolution 2010, 58, 1971–1983. [Google Scholar] [CrossRef]
- Toju, H.; Sota, T. Adaptive divergence of scaling relationships mediates the arms race between a weevil and its host plant. Biol. Lett. 2006, 2, 539–542. [Google Scholar] [CrossRef][Green Version]
- Toju, H.; Sota, T. Imbalance of predator and prey armament: geographic clines in phenotypic interface and natural selection. Am. Naturalist 2005, 167, 105–117. [Google Scholar] [CrossRef]
- Toju, H.; Sota, T. Do arms races punctuate evolutionary stasis? Unified insights from phylogeny, phylogeography and microevolutionary processes. J. Mol. Ecol. 2009, 18, 3940–3954. [Google Scholar] [CrossRef]
- Mumm, R.; Hilker, M. The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem. Senses 2005, 30, 337–343. [Google Scholar] [CrossRef][Green Version]
- Renwick, J.A.A.; Chew, F.S. Oviposition behavior in Lepidoptera. Annu. Rev. Entomol. 1994, 39, 377–400. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.-Y.; Ji, Y.-C.; Gao, P.; Wen, J.-B. Oviposition Behavior and Distribution of Eucryptorrhynchus scrobiculatus and E. brandti (Coleoptera: Curculionidae) on Ailanthus altissima (Mill.). Insects 2019, 10, 284. https://doi.org/10.3390/insects10090284
Zhang G-Y, Ji Y-C, Gao P, Wen J-B. Oviposition Behavior and Distribution of Eucryptorrhynchus scrobiculatus and E. brandti (Coleoptera: Curculionidae) on Ailanthus altissima (Mill.). Insects. 2019; 10(9):284. https://doi.org/10.3390/insects10090284
Chicago/Turabian StyleZhang, Gan-Yu, Ying-Chao Ji, Peng Gao, and Jun-Bao Wen. 2019. "Oviposition Behavior and Distribution of Eucryptorrhynchus scrobiculatus and E. brandti (Coleoptera: Curculionidae) on Ailanthus altissima (Mill.)" Insects 10, no. 9: 284. https://doi.org/10.3390/insects10090284
APA StyleZhang, G.-Y., Ji, Y.-C., Gao, P., & Wen, J.-B. (2019). Oviposition Behavior and Distribution of Eucryptorrhynchus scrobiculatus and E. brandti (Coleoptera: Curculionidae) on Ailanthus altissima (Mill.). Insects, 10(9), 284. https://doi.org/10.3390/insects10090284



