Size of Openings in Water-Holding Containers: Impact on Oviposition by Culex (Culex) Mosquitoes
Abstract
:1. Introduction
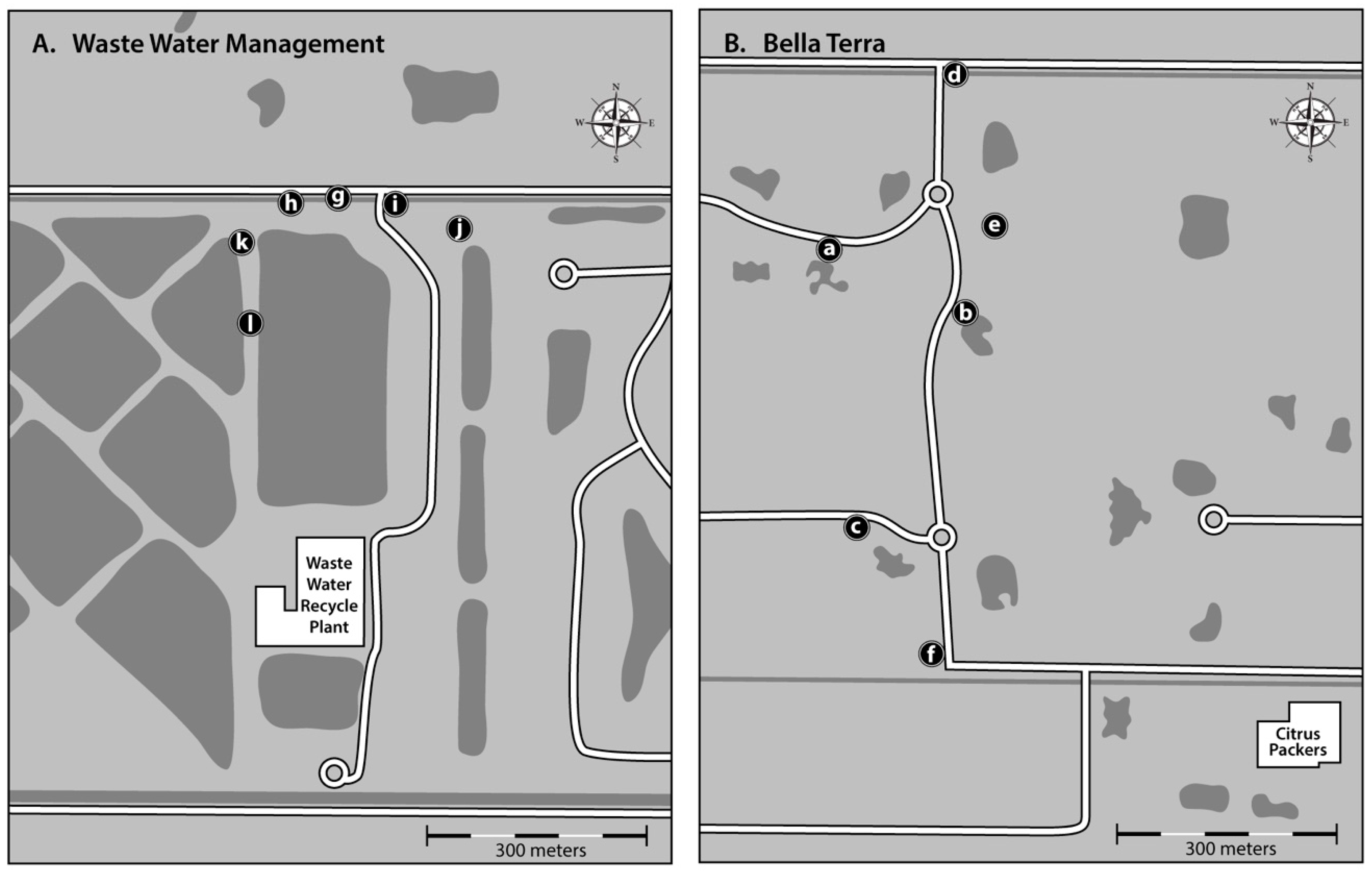
2. Materials and Methods
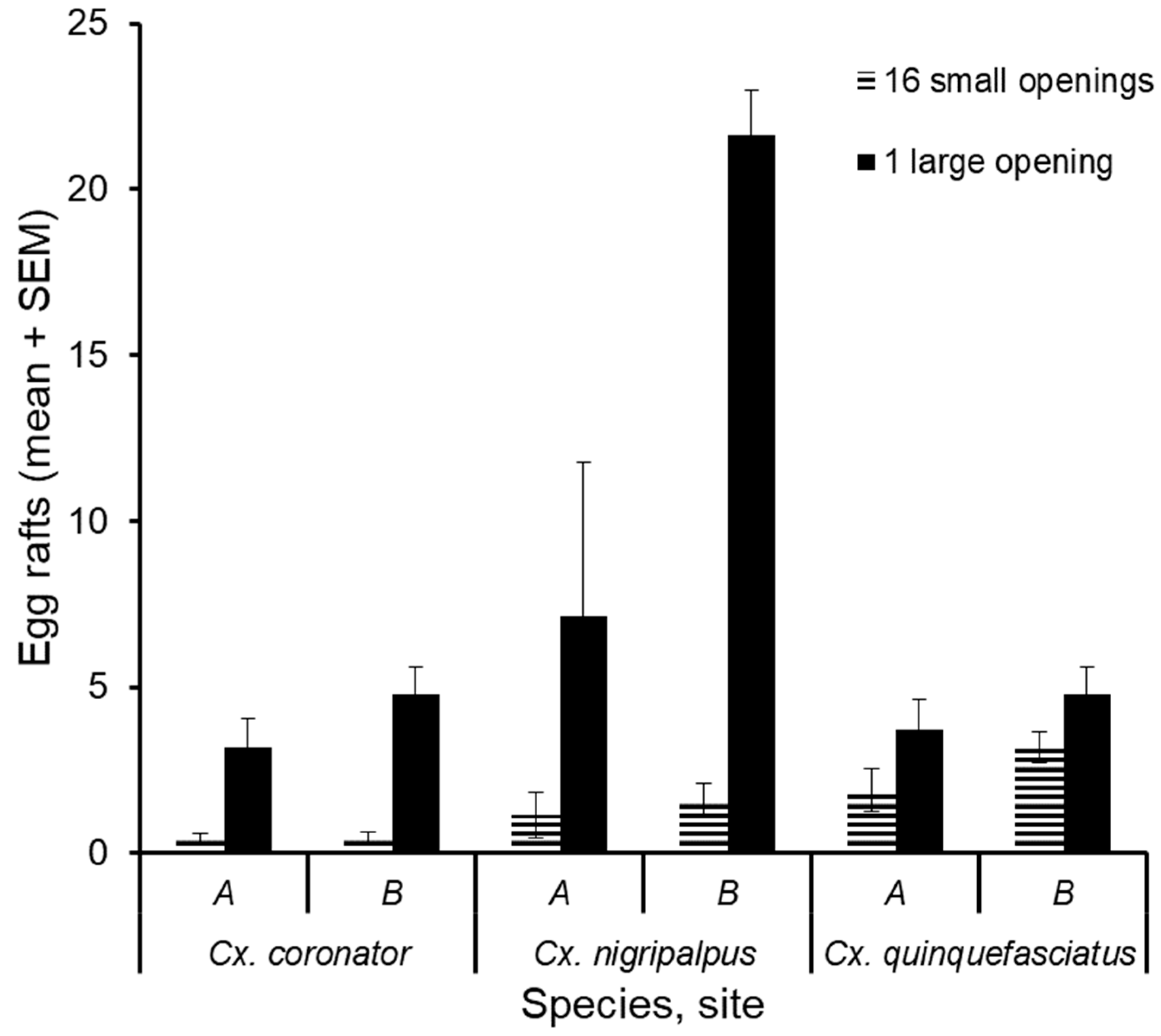
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Provost, M.W. The Natural History of Culex Nigripalpus. In St. Encephalitis in Florida; Florida State Board of Health Monograph Series: Jacksonville, FL, USA, 1969; Volume 12, pp. 46–62. [Google Scholar]
- Rey, J.R.; O’Meara, G.F.; O’Connell, S.M.; Cutwa-Francis, M.M. Mosquito production from four constructed treatment wetlands in peninsular Florida. J. Am. Mosq. Control Assoc. 2006, 22, 198–205. [Google Scholar] [CrossRef]
- O’Meara, G.F.; Evans, F.D.S. Seasonal Patterns of Abundance among three species of Culex mosquitoes in a south florida wastewater lagoon. Ann. Entomol. Soc. Am. 1983, 76, 130–133. [Google Scholar] [CrossRef]
- O’Meara, G.F.; Cutwa-Francis, M.; Rey, J.R. Seasonal variation in the abundance of Culex nigripalpus and Culex quinquefasciatus in wastewater ponds at two Florida dairies. J. Am. Mosq. Control Assoc. 2010, 26, 160–166. [Google Scholar] [CrossRef]
- O’Meara, G.F.; Cutwa, M.M.; Evans, L.F. Bromeliad-inhabiting mosquitoes in south Florida: Native and exotic plants differ in species composition. J. Vector Ecol. 2003, 28, 37–46. [Google Scholar]
- Leisnham, P.T.; LaDeau, S.L.; Juliano, S.A. Spatial and temporal habitat segregation of mosquitoes in urban Florida. PLoS ONE 2014, 9, e91655. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, G.F.; Vose, F.E.; Carlson, D.B. Environmental factors influencing oviposition by Culex (Culex) (Diptera: Culicidae) in two types of traps. J. Med. Entomol. 1989, 26, 528–534. [Google Scholar] [CrossRef]
- Reiter, P. A portable battery-powered trap for collecting gravid Culex mosquitoes. Mosq. News 1983, 43, 496–498. [Google Scholar]
- Popko, D.A.; Walton, W.E. Large-volume gravid traps enhance collection of culex vectors. J. Am. Mosq. Control Assoc. 2016, 32, 91–102. [Google Scholar] [CrossRef]
- Derraik, J.G.; Slaney, D. Container aperture size and nutrient preferences of mosquitoes (Diptera: Culicidae) in the Auckland region, New Zealand. J. Vector Ecol. 2005, 30, 73–82. [Google Scholar]
- Rey, J.R.; O’Meara, G.F.; O’Connell, S.M.; Cutwa-Francis, M.M. Factors affecting mosquito production from stormwater drains and catch basins in two Florida cities. J. Vector Ecol. 2006, 31, 334–343. [Google Scholar] [CrossRef]
- Haeger, J.S.; O’Meara, G.F. Separation of 1st-instar larvae of 4 Florida Culex (Culex). Mosq. News 1983, 43, 76–77. [Google Scholar]
- Darsie, R.F.; Ward, R.A. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico; University of Florida Press: Gainesville, FL, USA, 2005; p. 383. [Google Scholar]
- Smith, J.P.; Walsh, J.D.; Cope, E.H.; Tennant, R.A.; Kozak, J.A.; Darsie, R.F. Culex coronator dyar and knab: A new Florida species record. J. Am. Mosq. Control Assoc. 2006, 22, 330–332. [Google Scholar] [CrossRef]
- Connelly, C.R.; Alto, B.W.; O’Meara, G.F. The spread of Culex coronator (Diptera: Culicidae) throughout Florida. J. Vector Ecol. 2016, 41, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; O’Meara, G.F.; Civana, A.; Shroyer, D.A.; Miqueli, E. Culex interrogator (Diptera: Culicidae), a mosquito species new to Florida. J. Vector Ecol. 2016, 41, 316–319. [Google Scholar] [CrossRef]
- Day, J.F.; Curtis, G.A. When It Rains, They Soar—and That Makes Culex Nigripalpus a Dangerous Mosquito. Am. Entomol. 1994, 40, 162–167. [Google Scholar] [CrossRef]
- Allan, S.A.; Day, J.F.; Edman, J.D. Visual ecology of biting flies. Annu. Rev. Entomol 1987, 32, 297–316. [Google Scholar] [CrossRef]
- Bentley, M.D.; Day, J.F. Chemical ecology and behavioral aspects of mosquito oviposition. Annu. Rev. Entomol. 1989, 34, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Beehler, J.W.; Millar, J.G.; Mulla, M.S. Synergism between chemical attractants and visual cues influencing oviposition of the mosquito, Culex quinquefasciatus (Diptera: Culicidae). J. Chem. Ecol. 1993, 19, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Day, J.F. Mosquito Oviposition Behavior and Vector Control. Insects 2016, 7, 65. [Google Scholar] [CrossRef]
- Day, J.F.; Curtis, G.A.; Edman, J.D. Rainfall-directed oviposition behavior of Culex nigripalpus (Diptera: Culicidae) and its influence on St. Louis encephalitis virus transmission in Indian River County, Florida. J. Med. Entomol. 1990, 27, 43–50. [Google Scholar] [CrossRef]
- Richards, S.L.; Mores, C.N.; Lord, C.C.; Tabachnick, W.J. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne Zoonotic Dis. 2007, 7, 629–636. [Google Scholar] [CrossRef]
- Richards, S.L.; Lord, C.C.; Pesko, K.; Tabachnick, W.J. Environmental and biological factors influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) vector competence for Saint Louis encephalitis virus. Am. J. Trop Med. Hyg. 2009, 81, 264–272. [Google Scholar] [CrossRef]
- Day, J.F.; Tabachnick, W.J.; Smartt, C.T. Factors That Influence the Transmission of West Nile Virus in Florida. J. Med. Entomol. 2015, 52, 743–754. [Google Scholar] [CrossRef]
- Alto, B.W.; Connelly, C.R.; O’Meara, G.F.; Hickman, D.; Karr, N. Reproductive biology and susceptibility of Florida Culex coronator to infection with West Nile virus. Vector Borne Zoonotic Dis. 2014, 14, 606–614. [Google Scholar] [CrossRef]
- Reisen, W.K.; Meyer, R.P. Attractiveness of selected oviposition substrates for gravid Culex tarsalis and Culex quinquefasciatus in California. J. Am. Mosq. Control Assoc. 1990, 6, 244–250. [Google Scholar]
- Isoe, J.; Beehler, J.W.; Millar, J.G.; Mulla, M.S. Oviposition responses of Culex tarsalis and Culex quinquefasciatus to aged Bermuda grass infusions. J. Am. Mosq. Control Assoc. 1995, 11, 39–44. [Google Scholar]
- Allan, S.A.; Kline, D. Evaluation of various attributes of gravid female traps for collection of Culex in Florida. J. Vector Ecol. 2004, 29, 285–294. [Google Scholar]
- Burkett-Cadena, N.D.; Mullen, G.R. Field comparison of Bermuda-hay infusion to infusions of emergent aquatic vegetation for collecting female mosquitoes. J. Am. Mosq. Control Assoc. 2007, 23, 117–123. [Google Scholar] [CrossRef]
- Kesavaraju, B.; Kiyoguchi, D.; Dickson, S. Efficacy of gravid traps in trapping Culex pipiens. J. Am. Mosq. Control Assoc. 2011, 27, 320–322. [Google Scholar] [CrossRef]

| Source | Species | Sum of Squares | Total Number | Prob > F |
|---|---|---|---|---|
| Opening Type | Cx. nigripalpus | 9491.3529 | 28.3308 | <0.0001 |
| Cx. coronator | 646.04126 | 33.1189 | <0.0001 | |
| Cx. quinquefasciatus | 138.2409 | 6.9672 | 0.0089 | |
| Site | Cx. nigripalpus | 7783.3218 | 2.1120 | 0.0209 |
| Cx. coronator | 575.73890 | 2.6832 | 0.0031 | |
| Cx. quinquefasciatus | 1724.6655 | 7.9019 | <0.0001 | |
| Time | Cx. nigripalpus | 713.7504 | 2.1305 | 0.1459 |
| Cx. coronator | 10.85875 | 0.5567 | 0.4565 | |
| Cx. quinquefasciatus | 393.1281 | 19.8132 | <0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, D.; O’Meara, G.F.; Civana, A. Size of Openings in Water-Holding Containers: Impact on Oviposition by Culex (Culex) Mosquitoes. Insects 2019, 10, 257. https://doi.org/10.3390/insects10090257
Shin D, O’Meara GF, Civana A. Size of Openings in Water-Holding Containers: Impact on Oviposition by Culex (Culex) Mosquitoes. Insects. 2019; 10(9):257. https://doi.org/10.3390/insects10090257
Chicago/Turabian StyleShin, Dongyoung, George F. O’Meara, and Ayse Civana. 2019. "Size of Openings in Water-Holding Containers: Impact on Oviposition by Culex (Culex) Mosquitoes" Insects 10, no. 9: 257. https://doi.org/10.3390/insects10090257
APA StyleShin, D., O’Meara, G. F., & Civana, A. (2019). Size of Openings in Water-Holding Containers: Impact on Oviposition by Culex (Culex) Mosquitoes. Insects, 10(9), 257. https://doi.org/10.3390/insects10090257




