Influence of Age, Host Plant and Mating Status in Pheromone Production and New Insights on Perception Plasticity in Tuta Absoluta
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. Chemicals
2.3. Pheromone Extracts
2.4. Chemical Analysis
2.5. Electroantennogram (EAG) Assays
2.6. Statistical Analysis
3. Results
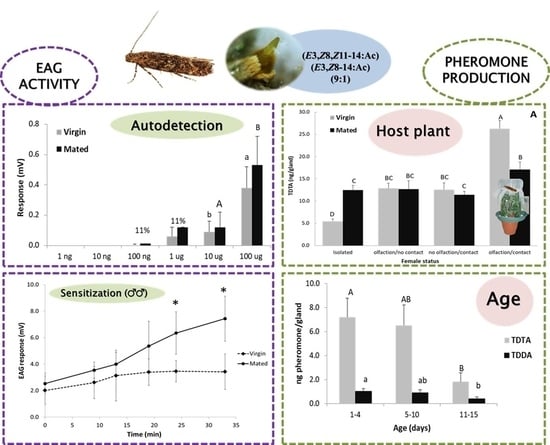
3.1. Pheromone Composition and Effect of Time into Scotophase and Female Age on Pheromone Production
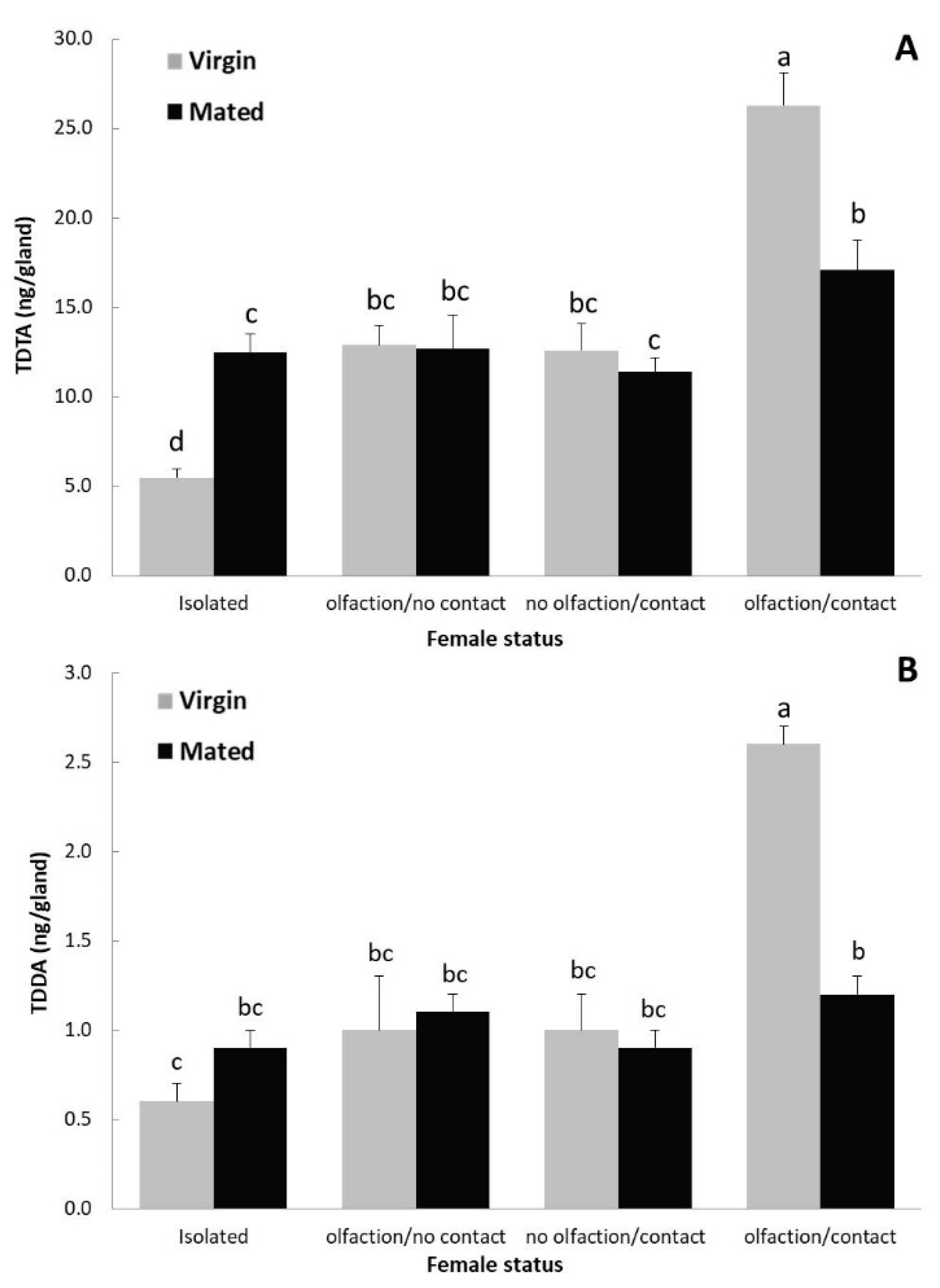
3.2. Effect of Mating Status and the Presence/Absence of the Host Plant in Pheromone Production
3.3. Electrophysiological Activity of Both Pheromone Components on Males
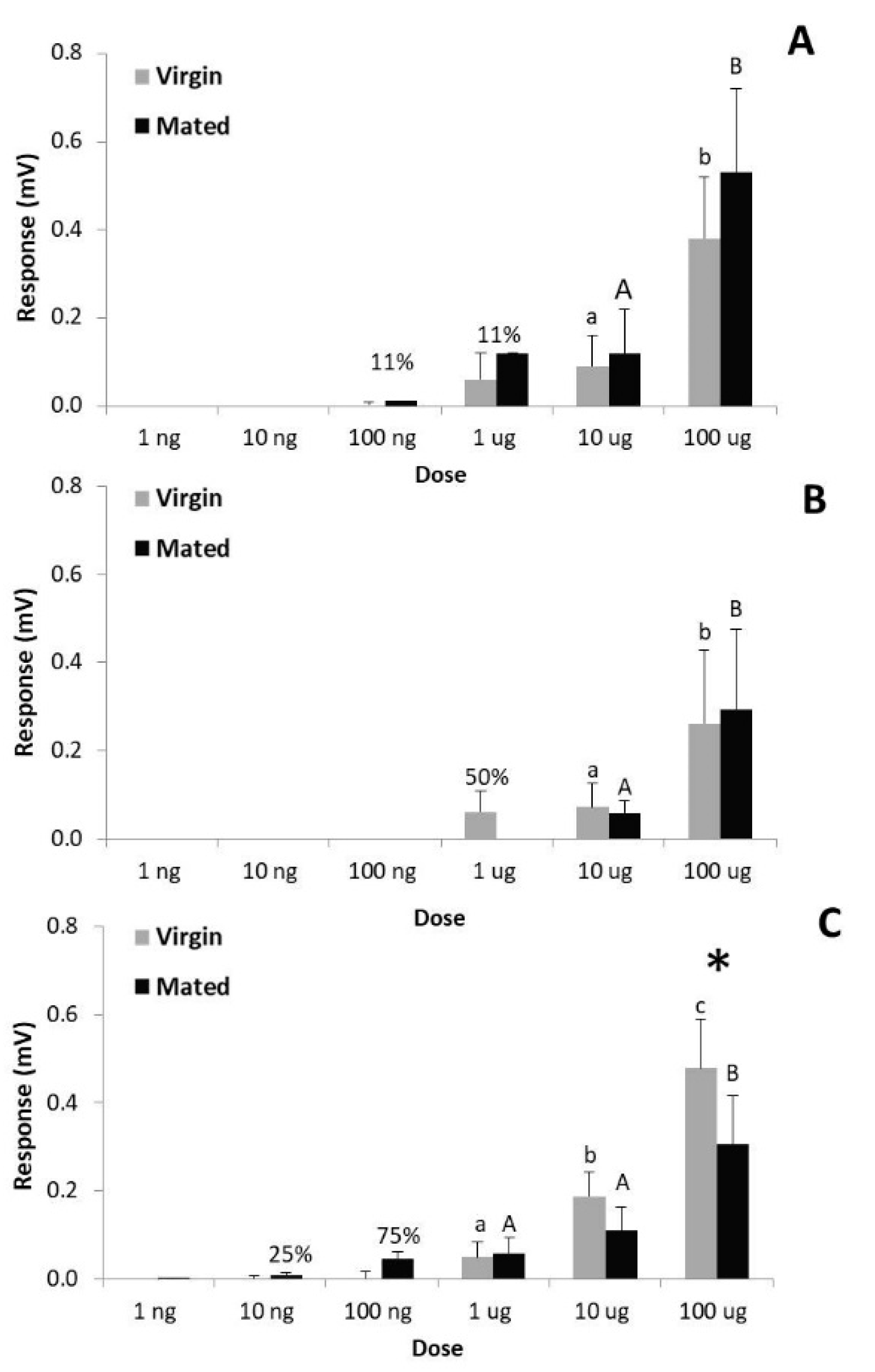
3.4. Electrophysiological Activity of Both Pheromone Components on Females (Pheromone Autodetection)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biondi, A.; Guedes, R.N.; Wan, F.-H.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.R.; Biondi, A.; Adiga, A.; Guedes, R.N.C.; Desneux, N. From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest Sci. 2017, 90, 787–796. [Google Scholar] [CrossRef]
- Giorgini, M.; Guerrieri, E.; Cascone, P.; Gontijo, L. Current strategies and future outlook for managing the Neotropical tomato pest Tuta absoluta (Meyrick) in the Mediterranean Basin. Neotrop. Entomol. 2019, 48, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Urbaneja, A.; Vercher, R.; Navarro, V.; García Marí, F.; Porcuna, J. La polilla del tomate, Tuta absoluta. Phytoma España 2008, 194, 16–23. [Google Scholar]
- Mansour, R.; Brevault, T.; Chailleux, A.; Cherif, A.; Grissa-Lebdi, K.; Haddi, K.; Mohamed, S.A.; Nofemela, R.S.; Oke, A.; Sylla, S.; et al. Occurrence, biology, natural enemies and management of Tuta absoluta in Africa. Entomol. Gen. 2018, 38, 83–112. [Google Scholar] [CrossRef]
- Han, P.; Bayram, Y.; Shaltiel-Harpaz, L.; Sohrabi, F.; Saji, A.; Esenali, U.T.; Jalilov, A.; Ali, A.; Shashank, P.R.; Ismoilov, K.; et al. Tuta absoluta continues to disperse in Asia: Damage, ongoing management and future challenges. J. Pest Sci. 2019, 92, 1317–1327. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Roditakis, E.; Campos, M.R.; Haddi, K.; Bielza, P.; Siqueira, H.A.A.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. J. Pest Sci. 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Verheggen, F.; Fontus, R.B. First record of Tuta absoluta in Haiti. Entomol. Gen. 2019, 38, 349–353. [Google Scholar] [CrossRef]
- Tabuloc, C.A.; Lewald, K.M.; Conner, W.R.; Lee, Y.; Lee, E.K.; Cain, A.B.; Godfrey, K.E.; Arnó, J.; Agustí, N.; Perini, C.R.; et al. Sequencing of Tuta absoluta genome to develop SNP genotyping assays for species identification. J. Pest Sci. 2019, 92, 1397–1407. [Google Scholar] [CrossRef]
- Han, P.; Zhang, Y.N.; Lu, Z.Z.; Wang, S.; Ma, D.Y.; Biondi, A. Are we ready for the invasion of Tuta absoluta? Unanswered key questions for elaborating an Integrated Pest Management package in Xinjiang, China. Entomol. Gen. 2018, 38, 113–125. [Google Scholar] [CrossRef]
- Sankarganesh, E.; Firake, D.M.; Sharma, B.; Verma, V.K.; Behere, G.T. Invasion of the South American tomato pinworm, Tuta absoluta, in northeastern India: A new challenge and biosecurity concerns. Entomol. Gen. 2017, 36, 335–345. [Google Scholar] [CrossRef]
- Roda, A.L.; Brambila, J.; Barria, J.; Euceda, X.; Korytkowski, C. Efficiency of trapping systems for detecting Tuta absoluta (Lepidoptera: Gelechiidae). J. Econ. Entomol. 2015, 108, 2648–2654. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Brostaux, Y.; Haubruge, E.; Verheggen, F.J. Propensity of the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae), to develop on four potato plant varieties. Am. J. Potato Res. 2013, 90, 255–260. [Google Scholar] [CrossRef]
- Pereyra, P.C.; Sánchez, N.E. Effect of two solanaceous plants on developmental and population parameters of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2006, 35, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Unlu, L. Potato: A new host plant of Tuta absoluta Povolny (Lepidoptera: Gelechiidae) in Turkey. Pak. J. Zool. 2012, 44, 1183–1184. [Google Scholar]
- Campolo, O.; Cherif, A.; Ricupero, M.; Siscaro, G.; Grissa-Lebdi, K.; Russo, A.; Cucci, L.M.; Di Pietro, P.; Satriano, C.; Desneux, N.; et al. Citrus peel essential oil nanoformulations to control the tomato borer, Tuta absoluta: Chemical properties and biological activity. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.A.; Campos, M.R.; Passos, L.C.; Carvalho, G.A.; Haro, M.M.; Lavoir, A.-V.; Biondi, A.; Zappalà, L.; Desneux, N. Botanical insecticide and natural enemies: A potential combination for pest management against Tuta absoluta. J. Pest Sci. 2019, 92, 1433–1443. [Google Scholar] [CrossRef]
- Blazheyski, S.; Kalaitzaki, A.P.; Tsagkarakis, A.E. Impact of nitrogen and potassium fertilization regimes on the biology of the tomato leaf miner Tuta absoluta. Entomol. Gen. 2018, 37, 157–174. [Google Scholar] [CrossRef]
- Han, P.; Desneux, N.; Becker, C.; Larbat, R.; Le Bot, J.; Adamowicz, S.; Zhang, J.; Lavoir, A.-V. Bottom-up effects of irrigation, fertilization and plant resistance on Tuta absoluta: Implications for Integrated Pest Management. J. Pest Sci. 2018, 92, 1359–1370. [Google Scholar] [CrossRef]
- Roditakis, E.; Skarmoutsou, C.; Staurakaki, M. Toxicity of insecticides to populations of tomato borer Tuta absoluta (Meyrick) from Greece. Pest Manag. Sci. 2013, 69, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Silvério, F.O.; de Alvarenga, E.S.; Moreno, S.C.; Picanço, M.C. Synthesis and insecticidal activity of new pyrethroids. Pest Manag. Sci. 2009, 65, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Picanço, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, H.A.A.; Guedes, R.N.C.; Fragoso, D.B.; Magalhaes, L.C. Abamectin resistance and synergism in Brazilian populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Int. J. Pest Manag. 2001, 47, 247–251. [Google Scholar] [CrossRef]
- Siqueira, H.Á.A.; Guedes, R.N.C.; Picanço, M.C. Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric. For. Entomol. 2000, 2, 147–153. [Google Scholar] [CrossRef]
- Silva, W.M.; Berger, M.; Bass, C.; Williamson, M.; Moura, D.M.N.; Ribeiro, L.M.S.; Siqueira, H.A.A. Mutation (G275E) of the nicotinic acetylcholine receptor α6 subunit is associated with high levels of resistance to spinosyns in Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Pest. Biochem. Physiol. 2016, 131, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.R.; Rodrigues, A.R.S.; Silva, W.M.; Silva, T.B.M.; Silva, V.R.F.; Guedes, R.N.C.; Siqueira, H.A.A. Spinosad and the tomato borer Tuta absoluta: A bioinsecticide, an invasive pest threat, and high insecticide resistance. PLoS ONE 2014, 9, e103235. [Google Scholar] [CrossRef]
- Haddi, K.; Berger, M.; Bielza, P.; Cifuentes, D.; Field, L.M.; Gorman, K.; Rapisarda, C.; Williamson, M.S.; Bass, C. Identification of mutations associated with pyrethroid resistance in the voltage-gated sodium channel of the tomato leaf miner (Tuta absoluta). Insect Biochem. Mol. Biol. 2012, 42, 506–513. [Google Scholar] [CrossRef]
- Roditakis, E.; Vasakis, E.; Grispou, M.; Stavrakaki, M.; Nauen, R.; Gravouil, M.; Bassi, A. First report of Tuta absoluta resistance to diamide insecticides. J. Pest Sci. 2015, 88, 9–16. [Google Scholar] [CrossRef]
- Roditakis, E.; Mavridis, K.; Riga, M.; Vasakis, E.; Morou, E.; Rison, J.L.; Vontas, J. Identification and detection of indoxacarb resistance mutations in the para sodium channel of the tomato leafminer, Tuta absoluta. Pest Manag. Sci. 2017, 73, 1679–1688. [Google Scholar] [CrossRef]
- Roditakis, E.; Vasakis, E.; García-Vidal, L.; del Rosario Martínez-Aguirre, M.; Rison, J.L.; Haxaire-Lutun, M.O.; Nauen, R.; Tsagkarakou, A.; Bielza, P. A four-year survey on insecticide resistance and likelihood of chemical control failure for tomato leaf miner Tuta absoluta in the European/Asian region. J. Pest Sci. 2018, 91, 421–435. [Google Scholar] [CrossRef]
- Passos, L.C.; Soares, M.A.; Collares, L.J.; Malagoli, I.; Desneux, N.; Carvalho, G.A. Lethal, sublethal and transgenerational effects of insecticides on Macrolophus basicornis, predator of Tuta absoluta. Entomol. Gen. 2018, 38, 127–143. [Google Scholar] [CrossRef]
- Biondi, A.; Zappalà, L.; Stark, J.D.; Desneux, N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 2013, 8, e76548. [Google Scholar] [CrossRef] [PubMed]
- Attygalle, A.B.; Jham, G.N.; Svatos, A.; Frighetto, R.T.S.; Meinwald, J.; Vilela, E.F.; Ferrara, F.A.; Uchoa Fernandes, M.A. Microscale, random reduction: Application to the characterization of (3E,8Z,11Z)-3,8,11-tetradecatrienyl acetate, a new lepidopteran sex pheromone. Tetrahedron Lett. 1995, 36, 5471–5474. [Google Scholar] [CrossRef]
- Svatos, A.; Attygalle, A.B.; Jham, G.N.; Frighetto, R.T.S.; Vilela, E.F.; Saman, D.; Meinwald, J. Sex pheromone of tomato pest Scrobipalpuloides absoluta (Lepidoptera: Gelechiidae). J. Chem. Ecol. 1996, 22, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Filho, M.M.; Vilela, E.F.; Attygalle, A.; Meinwald, J.; Svatos, A.; Jham, G.N. Field trapping of tomato moth, Tuta absoluta with pheromone traps. J. Chem. Ecol. 2000, 26, 875–881. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Haubruge, E.; Verheggen, F.J. Pheromone-based management strategies to control the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). A review. Biotechnol. Agron. Soc. Environ. 2013, 17, 475–482. [Google Scholar]
- Cocco, A.; Deliperi, S.; Delrio, G. Control of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in greenhouse tomato crops using the mating disruption technique. J. Appl. Entomol. 2013, 137, 16–28. [Google Scholar] [CrossRef]
- Vacas, S.; Alfaro, C.; Primo, J.; Navarro-LLopis, V. Studies on the development of a mating disruption system to control the tomato leafminer, Tuta absoluta Povolny (Lepidoptera: Gelechiidae). Pest Manag. Sci. 2011, 67, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Uchôa Fernandes, M.A.; Della Lucia, T.M.C.; Vilela, E.F. Mating, oviposition and pupation of Scrobipalpula absoluta (Meyrick) (Lepidoptera: Gelechiidae). An. Soc. Entomol. Brasil 1995, 24, 159–164. [Google Scholar]
- Lee, M.; Albajes, R.; Eizaguirre, M. Mating behaviour of female Tuta absoluta (Lepidoptera: Gelechiidae): Polyandry increases reproductive output. J. Pest Sci. 2014, 87, 427–439. [Google Scholar] [CrossRef]
- Abbes, K.; Chermiti, B. Propensity of three Tunisian populations of the tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae) for deuterotokous parthenogenetic reproduction. Afr. Entomol. 2014, 22, 538–544. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Haubruge, E.; Verheggen, F.J. First evidence of deuterotokous parthenogenesis in the tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Pest Sci. 2012, 87, 429–439. [Google Scholar] [CrossRef]
- Burkholder, W.E. Practical use of pheromones and other attractants for stored-product insects. In Behavior-Modifying Chemicals for Insect Management; Ridgway, R., Silverstein, R., Inscoe, M., Eds.; Marcel Dekker: New York, NY, USA, 1990; pp. 531–537. [Google Scholar]
- Smart, L.E.; Blight, M.M.; Pickett, J.A.; Pye, B.J. Development of field strategies incorporating semiochemicals for the control of the pea and bean weevil, Sitona lineatus L. Crop Prot. 1994, 13, 127–135. [Google Scholar] [CrossRef]
- Gadenne, C.; Renou, M.; Sreng, L. Hormonal control of sex pheromone responsiveness in the male black cutworm, Agrotis ipsilon. Experientia 1993, 49, 721–724. [Google Scholar] [CrossRef]
- Turgeon, J.J.; McNeil, J.N.; Roelofs, W.L. Responsiveness of Pseudaletia unipuncta males to the female sex pheromone. Physiol. Entomol. 1983, 8, 339–344. [Google Scholar] [CrossRef]
- Spurgeon, D.W. Age dependence of pheromone production by the boll weevil (Coleoptera: Curculionidae). Environ. Entomol. 2003, 32, 31–38. [Google Scholar] [CrossRef]
- Hock, V.; Chouinard, G.; Lucas, É.; Cormier, D.; Leskey, T.; Wright, S.; Zhang, A.; Pichette, A. Establishing abiotic and biotic factors necessary for reliable male pheromone production and attraction to pheromones by female plum curculios Conotrachelus nenuphar (Coleoptera: Curculionidae). Can. Entomol. 2014, 146, 528–547. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Fukami, H.; Ishii, S.; Matsumura, F.; Burkholder, W.E. Studies on the isolation and bioassay of the sex pheromone of the drugstore beetle, Stegobium paniceum (Coleoptera: Anobiidae). J. Chem. Ecol. 1975, 1, 413–422. [Google Scholar] [CrossRef]
- Hardee, D.D.; McKibben, G.H.; Rummel, D.R.; Huddleston, P.M.; Coppedge, J.R. Response of boll weevils to component ratios and doses of the pheromone. Environ. Entomol. 1974, 3, 135–138. [Google Scholar] [CrossRef]
- Linn, C.E.; Campbell, M.G.; Roelofs, W.L. Photoperiod cues and the modulatory action of octopamine and 5-hydroxytryptamine on locomotor and pheromone in male gypsy moths, Lymantria dispar. Arch. Insect Biochem. Physiol. 1992, 20, 265–284. [Google Scholar] [CrossRef]
- Linn, C.E.; Roelofs, W.L. Role of photoperiod cues in regulating the modulatory action of octopamine on pheromone-response thesholds in the cabbage looper moth. Arch. Insect Biochem. Physiol. 1992, 20, 285–302. [Google Scholar] [CrossRef]
- Yamane, T.; Yasuda, T. The effects of mating status and time since mating on female sex pheromone levels in the rice leaf bug, Trigonotylus caelestialium. Naturwissenschaften 2014, 101, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Genc, H. The tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae): Pupal key characters for sexing individuals. Turk. J. Zool. 2016, 40, 801–805. [Google Scholar] [CrossRef]
- Puigmartí, M.; Bosch, M.P.; Guerrero, A. An improved and convenient new synthesis of the pheromone components of the tomato leafminer Tuta absoluta. Synthesis 2015, 47, 961–968. [Google Scholar]
- Tropea Garzia, G.; Siscaro, G.; Biondi, A.; Zappalà, L. Tuta absoluta, a South American pest of tomato now in the EPPO region: Biology, distribution and damage. EPPO Bull. 2012, 42, 205–210. [Google Scholar] [CrossRef]
- Acín, P.; Rosell, G.; Guerrero, A.; Quero, C. Sex pheromone of the Spanish population of the beet armyworm Spodoptera exigua. J. Chem. Ecol. 2010, 36, 778–786. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Guerrero, A.; Bleda, M.J.; Quero, C. Short-term peripheral sensitization by brief exposure to pheromone components in Spodoptera littoralis. J. Comp. Physiol. A 2017, 203, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Corp, S. Stata Statistical Software: Release 12; Stata Co.: College Station, TX, USA, 2011. [Google Scholar]
- Babilis, N.A.; Mazomenos, B.E. Pheromone production in Sesamia nonagrioides: Diel periodicity and effect of age and mating. J. Insect Physiol. 1992, 38, 561–564. [Google Scholar] [CrossRef]
- Mistrot Pope, M.; Gaston, L.K.; Baker, T.C. Composition, quantification, and periodicity of sex pheromone gland volatiles from individual Heliothis virescens females. J. Chem. Ecol. 1982, 8, 1043–1055. [Google Scholar] [CrossRef]
- Mistrot Pope, M.; Gaston, L.K.; Baker, T.C. Composition, quantification, and periodicity of sex pheromone volatiles from individual Heliothis zea females. J. Insect Physiol. 1984, 30, 943–945. [Google Scholar] [CrossRef]
- Brent, C.S. Reproduction of the western tarnished plant bug, Lygus hesperus, in relation to age, gonadal activity and mating status. J. Insect Physiol. 2010, 56, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.V.P.; Guerrero, A. Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Phillips, T.W. Host plant influences on sex pheromone behavior of phytophagous insects. Annu. Rev. Entomol. 1997, 42, 371–391. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.; Delisle, J. Host plant pollen influences calling behavior and ovarian development of the sunflower moth, Homoeosoma electellum. Oecologia 1989, 80, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Beran, F.; Mewis, I.; Ramasamy, S.; Svoboda, J.; Vial, C.; Mosimann, H.; Boland, W.; Büttner, C.; Ulrichs, C.; Hansson, B.; et al. Male Phyllotreta striolata (F.) produce an aggregation pheromone: Identification of male-specific compounds and interaction with host plant volatiles. J. Chem. Ecol. 2011, 37, 85–97. [Google Scholar] [CrossRef]
- Proffit, M.; Birgersson, G.; Bengtsson, M.; Reiss, R., Jr.; Witzgall, P.; Lima, E. Attraction and oviposition of Tuta absoluta females in response to tomato leaf volatiles. J. Chem. Ecol. 2011, 37, 565–574. [Google Scholar] [CrossRef]
- Anastasaki, E.; Drizou, F.; Milonas, P.G. Electrophysiological and oviposition responses of Tuta absoluta females to herbivore-induced volatiles in tomato plants. J. Chem. Ecol. 2018, 44, 288–298. [Google Scholar] [CrossRef]
- Bawin, T.; Collard, F.; De Backer, L.; Yarou, B.B.; Compère, P.; Francis, F.; Verheggen, F.J. Structure and distribution of the sensilla on the antennae of Tuta absoluta (Lepidoptera: Gelechiidae). Micron 2017, 96, 16–28. [Google Scholar] [CrossRef]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant defense against herbivorous pests: Exploiting resistance and tolerance traits for sustainable crop protection. Front. Plant Sci. 2016, 7, 420. [Google Scholar] [CrossRef]
- Sridhar, V.; Sadashiva, A.T.; Keshava Rao, V.; Swathi, P.; Gadad, H.S. Trichome and biochemical basis of resistance against Tuta absoluta in tomato genotypes. Plant Genet. Resour. 2019, 17, 301–305. [Google Scholar]
- Figueredo, A.J.; Baker, T.C. Reduction of the response to sex pheromone in the Oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae) following successive pheromonal exposures. J. Insect Behav. 1992, 5, 347–363. [Google Scholar] [CrossRef]
- Judd, G.J.R.; Gardiner, M.G.T.; DeLury, N.C.; Karg, G. Reduced antennal sensitivity, behavioural response, and attraction of male codling moths, Cydia pomonella, to their pheromone (E,E)-8,10-dodecadien-1-ol following various pre-exposure regimes. Entomol. Exp. Appl. 2005, 114, 65–78. [Google Scholar] [CrossRef]
- Anderson, P.; Hansson, B.; Nilsson, U.; Han, Q.; Sjoholm, M. Increased behavioral and neuronal sensitivity to sex pheromone after brief odor experience in a moth. Chem. Senses 2007, 32, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Sadek, M.M.; Hansson, B.S. Pre-exposure modulates attraction to sex pheromone in a moth. Chem. Senses 2003, 28, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Quero, C.; Vidal, B.; Guerrero, A. EAG responses increase of Spodoptera littoralis antennae after a single pheromone pulse. Nat. Prod. Commun. 2014, 9, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Gut, L.J. Mating disruption for the 21st century: Matching technology with mechanism. Environ. Entomol. 2015, 44, 427–453. [Google Scholar] [CrossRef] [PubMed]
- Evenden, M.L.; Silk, P.J. The influence of Canadian research on semiochemical-based management of forest insect pests in Canada. Can. Entomol. 2015, 148, S170–S209. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Holdcraft, R.; Rodriguez-Saona, C. Female moth calling and flight behavior are altered hours following pheromone autodetection: Possible implications for practical management with mating disruption. Insects 2014, 5, 459–473. [Google Scholar] [CrossRef]
- Holdcraft, R.; Rodriguez-Saona, C.; Stelinski, L.L. Pheromone autodetection: Evidence and implications. Insects 2016, 7, 17. [Google Scholar] [CrossRef]
- Kuhns, E.H.; Pelz-Stelinski, K.; Stelinski, L.L. Reduced mating success of female tortricid moths following intense pheromone auto-exposure varies with sophistication of mating system. J. Chem. Ecol. 2012, 38, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Palaniswamy, P.; Seabrook, W.D. The alteration of calling behavior by female Choristoneura fumiferana when exposed to synthetic sex pheromone. Entomol. Exp. Appl. 1985, 37, 13–16. [Google Scholar] [CrossRef]
- Gökçe, A.; Stelinski, L.L.; Gut, J.L.; Whalon, E.M. Comparative behavioral and EAG responses of female obliquebanded and redbanded leafroller moths (Lepidoptera: Tortricidae) to their sex pheromone components. Eur. J. Entomol. 2007, 104, 187–194. [Google Scholar] [CrossRef]
- Birch, M.C. Response of both sexes of Trichoplusia ni (Lepidoptera: Noctuidae) to virgin females and to synthetic pheromone. Ecol. Entomol. 1977, 2, 99–104. [Google Scholar] [CrossRef]
- Den Otter, C.J.; De Cristofaro, A.; Voskamp, K.E.; Rotundo, G. Electrophysiological and behavioural responses of chestnut moths, Cydia fagiglandana and C. splendana (Lep., Tortricidae), to sex attractants and odours of host plants. J. Appl. Entomol. 1996, 120, 413–421. [Google Scholar] [CrossRef]


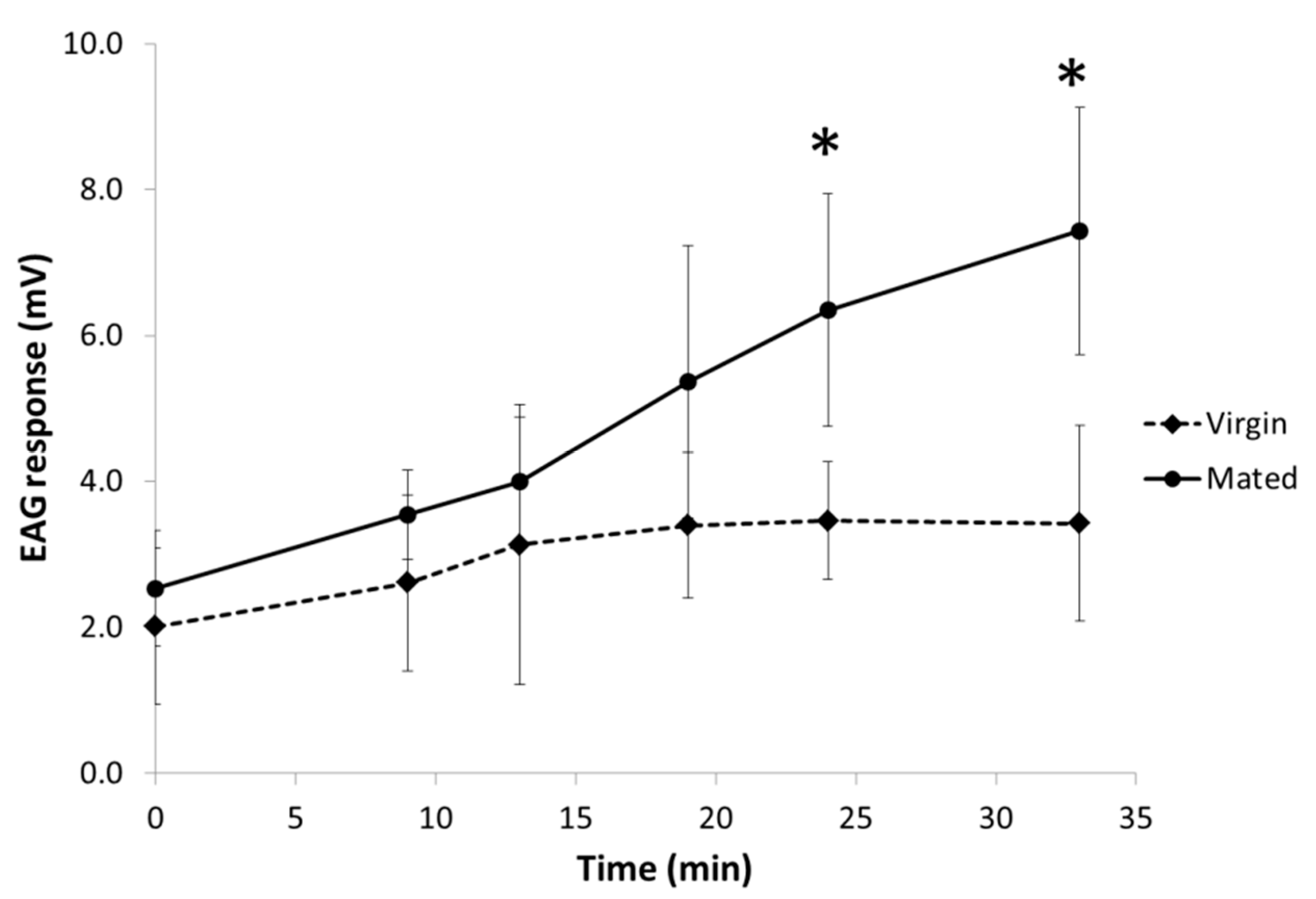
| Status | Time (min) | Increase of Response (mV) a | Percentage of Increase (%) | CI b |
|---|---|---|---|---|
| Mated | 9 | 0.82 | 33 | (−0.02; 1.67) |
| 13 | 1.47 * | 58 | (0.65; 2.29) | |
| 19 | 2.55 * | 101 | (1.72; 3.39) | |
| 24 | 3.46 * | 137 | (2.64; 4.28) | |
| 33 | 4.10 * | 163 | (3.29; 4.92) | |
| Virgin | 9 | 0.87 * | 47 | (0.03; 1.70) |
| 13 | 1.29 * | 70 | (0.45; 2.12) | |
| 19 | 1.67 * | 91 | (0.80; 2.53) | |
| 24 | 1.62 * | 88 | (0.78; 2.45) | |
| 33 | 1.47 * | 80 | (0.63; 2.30) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez, A.; López, S.; Bernabé, A.; Guerrero, Á.; Quero, C. Influence of Age, Host Plant and Mating Status in Pheromone Production and New Insights on Perception Plasticity in Tuta Absoluta. Insects 2019, 10, 256. https://doi.org/10.3390/insects10080256
Domínguez A, López S, Bernabé A, Guerrero Á, Quero C. Influence of Age, Host Plant and Mating Status in Pheromone Production and New Insights on Perception Plasticity in Tuta Absoluta. Insects. 2019; 10(8):256. https://doi.org/10.3390/insects10080256
Chicago/Turabian StyleDomínguez, Aroa, Sergio López, Ana Bernabé, Ángel Guerrero, and Carmen Quero. 2019. "Influence of Age, Host Plant and Mating Status in Pheromone Production and New Insights on Perception Plasticity in Tuta Absoluta" Insects 10, no. 8: 256. https://doi.org/10.3390/insects10080256
APA StyleDomínguez, A., López, S., Bernabé, A., Guerrero, Á., & Quero, C. (2019). Influence of Age, Host Plant and Mating Status in Pheromone Production and New Insights on Perception Plasticity in Tuta Absoluta. Insects, 10(8), 256. https://doi.org/10.3390/insects10080256