Aedes albopictus Adult Medium Mass Rearing for SIT Program Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Rearing, Maintenance, and Blood Feeding
2.2. Experiment 1: Effect of Sex Ratio
2.3. Experiment 2: Effect of Adult Density
2.4. Experiment 3: Effect of Blood Delivery
2.5. Experiment 4: Effect of Blood Quantity/Plate Surface
2.6. Experiment 5: Effect of Continuous or Discontinuous Blood-Feeding Periods
2.7. Experiment 6: Effect of Substrate Surface for Egg Laying
2.8. Statistical Analysis
3. Results
3.1. Effect of Sex Ratio
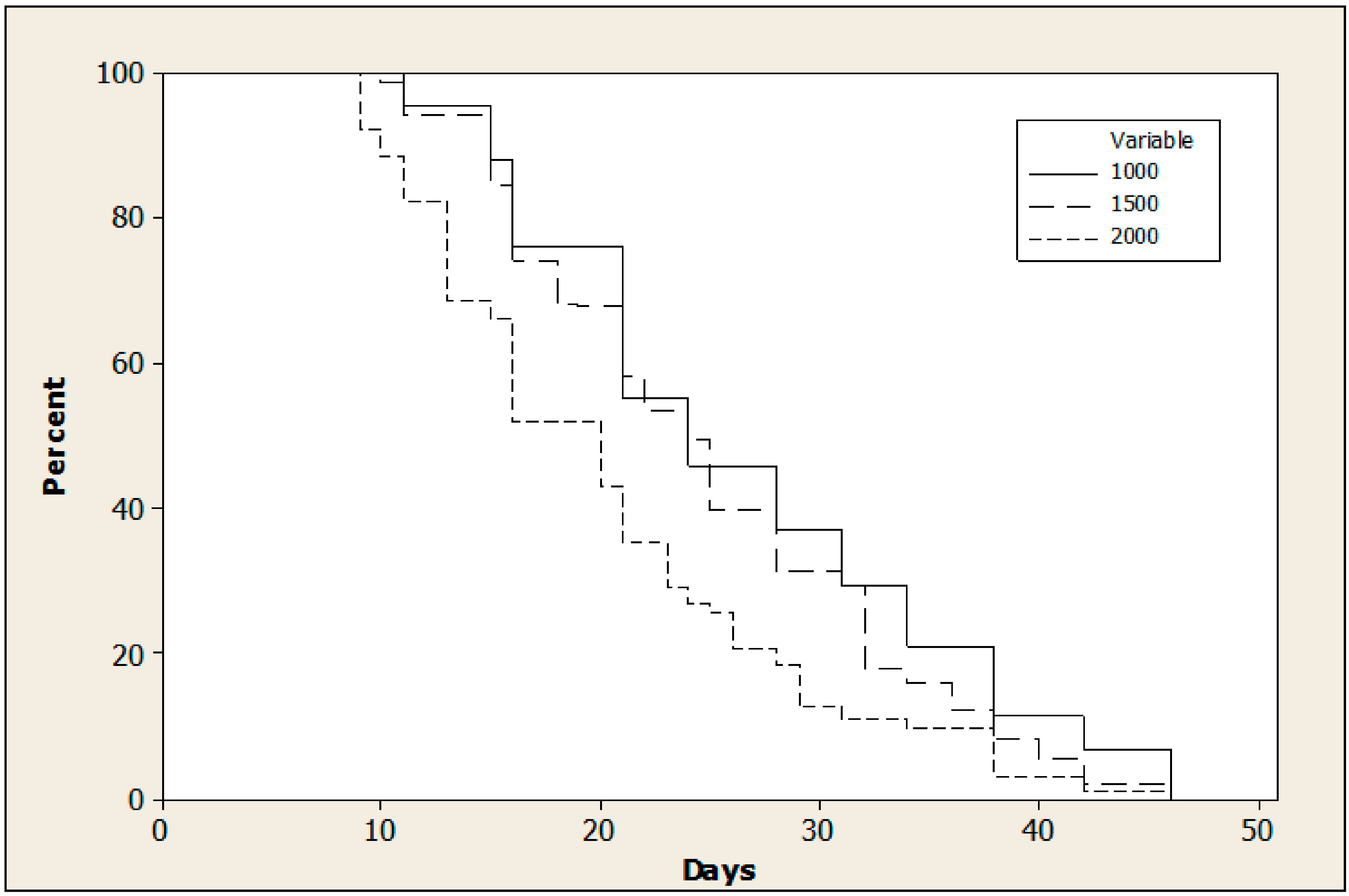
3.2. Effect of Adult Density
3.3. Effect of Blood-Feeding Membrane
3.4. Effect of Blood Quantity/Plate Surface
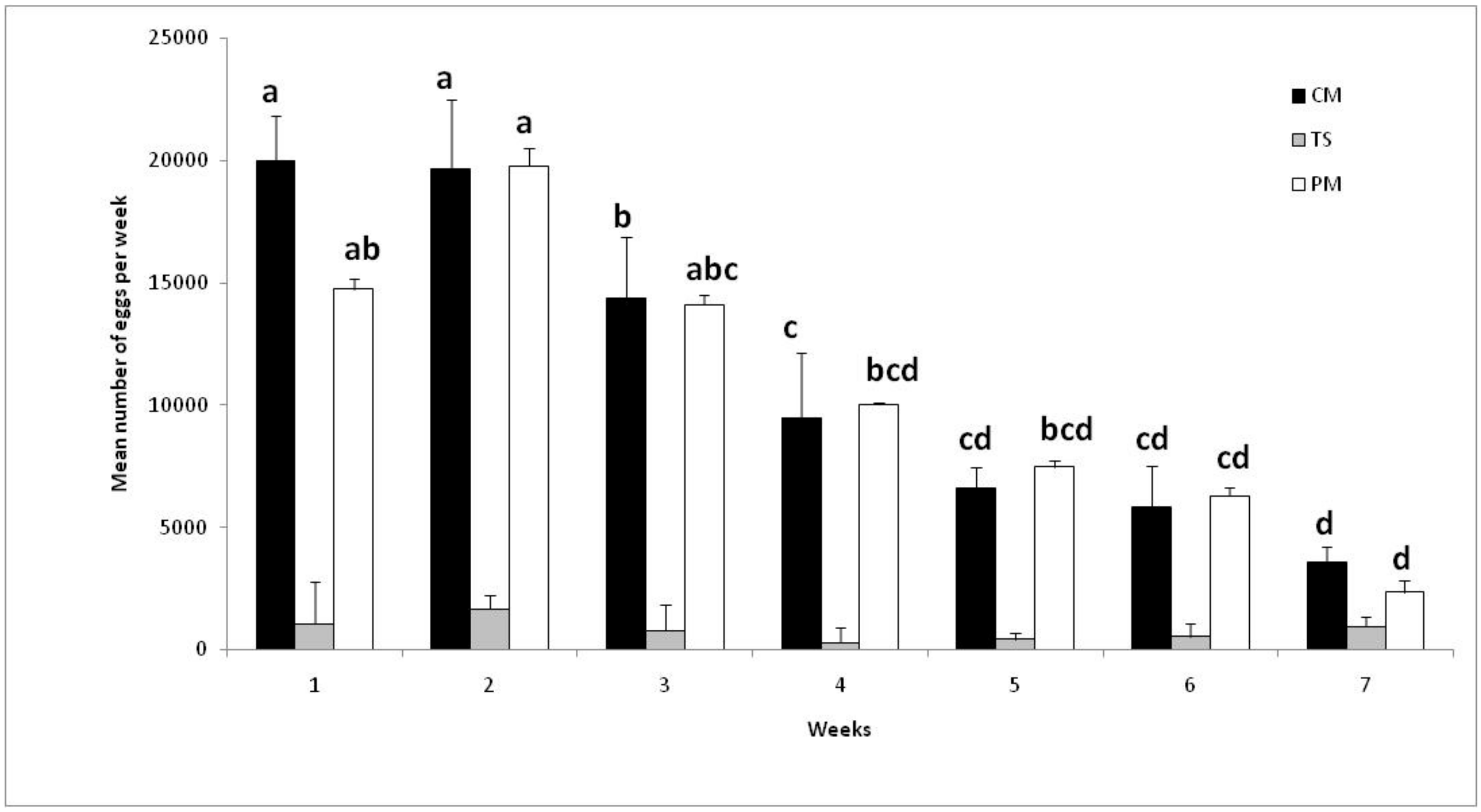
3.5. Effect of Continuous or Discontinuous Blood-Feeding Period
3.6. Effect of Substrate Surface Available for Egg Laying
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Knipling, E.F. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955, 48, 459–462. [Google Scholar] [CrossRef]
- Lowe, R.E.; Bailey, D.L.; Dame, D.A.; Savage, K.E.; Kaiser, P.E. Efficiency of techniques for the mass release of sterile male Anopheles albimanus Wiedemann in El Salvador. Am. J. Trop. Med. Hyg. 1980, 29, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Dame, D.; Lowe, R.; Williamson, D. Assessment of Released Sterile Anopheles albimanus and Glossina morsitans morsitans. In Cytogenetics and Genetics of Vectors; Pal, R., Kitzmiller, J., Kanda, T.A., Eds.; Elsevier Biomedical: Amsterdam, The Netherlands, 1981; pp. 231–248. [Google Scholar]
- Balestrino, F.; Benedict, M.Q.; Gilles, J.R. A new larval tray and rack system for improved mosquito mass rearing. J. Med. Entomol. 2012, 49, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, M.; Wu, Y.; Gilles, J.R.; Yamada, H.; Wu, Z.; Xi, Z.; Zheng, X. Establishment of a medium-scale mosquito facility: Optimization of the larval mass-rearing unit for Aedes albopictus (Diptera: Culicidae). Parasit. Vectors 2017, 10, 569. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, F.; Puggioli, A.; Bellini, R.; Petric, D.; Gilles, J.R.L. Mass production cage for Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.; Sun, Q.; Zheng, X.; Gilles, J.R.; Yamada, H.; Wu, Z.; Xi, Z.; Wu, Y. Establishment of a medium-scale mosquito facility: Tests on mass production cages for Aedes albopictus (Diptera: Culicidae). Parasit. Vectors 2018, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.L.; Zhang, D.J.; Damiens, D.D.; Yamada, H.; Gilles, J.R. Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes aegypti and Aedes albopictus (Diptera: Culicidae)-I-Egg quantification. Parasit. Vectors 2015, 8, 42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, M.L.; Zhang, D.J.; Damiens, D.D.; Lees, R.S.; Gilles, J.R. Standard operating procedures for standardized mass rearing of the dengue and chikungunya vectors Aedes aegypti and Aedes albopictus (Diptera: Culicidae)-II-Egg storage and hatching. Parasit. Vectors 2015, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Puggioli, A.; Balestrino, F.; Damiens, D.; Lees, R.S.; Soliban, S.M.; Madakacherry, O.; Dindo, M.L.; Bellini, R.; Gilles, J.R.L. Efficiency of three diets for larval development in mass rearing Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 819–825. [Google Scholar] [CrossRef]
- Quinlan, M.M.; Mumford, J.D.; Knight, J.D.; Stonehouse, J.M. Model Business Plan for a Sterile Insect Production Facility; (INT/5/145); Insect Pest Control Using the Sterile Insect Technique, IAEA: Vienna, Austria, 2002; pp. 1–3. [Google Scholar]
- Fay, R.W.; Eliason, D.A. A preferred oviposition site as a surveillance method for Aedes aegypti. Mosq. News 1966, 26, 531–535. [Google Scholar]
- Mogi, M.; Khamboonruang, C.; Choochote, W.; Suwanpanit, P. Ovitrap surveys of dengue vector mosquitoes in Chiang Mai, northern Thailand: Seasonal shifts in relative abundance of Aedes albopictus and Ae. aegypti. Med. Vet. Entomol. 1988, 2, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, R.; Delatte, H.; Hue, T.; Reiter, P. Dispersal and survival of male and female Aedes albopictus (Diptera: Culicidae) on Reunion Island. J. Med. Entomol. 2009, 46, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Cianci, D.; Van den Broek, J.; Caputo, B.; Marini, F.; Della Torre, A.; Heesterbeek, H.; Hartemink, N. Estimating mosquito population size from mark-release-recapture data. J. Med. Entomol. 2013, 50, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Gouagna, L.C.; Dehecq, J.S.; Fontenille, D.; Dumont, Y.; Boyer, S. Seasonal variation in size estimates of Aedes albopictus population based on standard mark-release-recapture experiments in an urban area on Reunion Island. Acta Tropica 2015, 143, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bellini, R.; Calvitti, M.; Medici, A.; Carrieri, M.; Celli, G.; Maini, S. Use of the Sterile Insect Technique Against Aedes albopictus in Italy: First Results of a Pilot Trial. In Area-Wide Control of Insect Pests; Springer: Dordrecht, The Netherlands, 2007; pp. 505–515. [Google Scholar]
- Cosgrove, J.B.; Wood, R.J.; Petric, D.; Evans, D.T.; Abbot, R.H. A convenient mosquito membrane feeding system. J. Am. Mosq. Control Assoc. 1994, 10, 434–436. [Google Scholar] [PubMed]
- Minitab. Minitab 16 Statistical Software (Computer Software); Minitab, Inc.: State College, PA, USA, 2010. [Google Scholar]
- Zhang, D.; Zheng, X.; Xi, Z.; Bourtzis, K.; Gilles, J.R.L. Combining the sterile insect technique with the incompatible insect technique: I-impact of Wolbachia infection on the fitness of triple- and double-infected strains of Aedes albopictus. PLoS ONE 2015, 10, e0121126. [Google Scholar] [CrossRef]
- Imam, H.; Nigar, Z.; Sofi, G.; Seikh, A. The basic rules and methods of mosquito rearing (Aedes aegypti). Trop. Parasitol. 2014, 4, 53–55. [Google Scholar] [CrossRef]
- Carvalho, D.O.; Nimmo, D.; Naish, N.; McKemey, A.R.; Gray, P.; Wilke, A.B.; Marrelli, M.T.; Virginio, J.F.; Alphey, L.; Capurro, M.L. Mass production of genetically modified Aedes aegypti for field releases in Brazil. J. Vis. Exp. 2014, 83, e3579. [Google Scholar] [CrossRef]
- Oliva, C.F.; Damiens, D.; Vreysen, M.J.; Lemperière, G.; Gilles, J. Reproductive strategies of Aedes albopictus (Diptera: Culicidae) and implications for the sterile insect technique. PLoS ONE 2013, 8, e78884. [Google Scholar] [CrossRef]
- Peters, T.; Barbosa, P. Influence of population density on size, fecundity, and developmental rate of insects in culture. Annu. Rev. Entomol. 1977, 22, 431–450. [Google Scholar] [CrossRef]
- Armbruster, P.; Conn, J.E. Geographic variation of larval growth in North American Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 2006, 99, 1234–1243. [Google Scholar] [CrossRef]
- Lounibos, L.P.; Escher, R.L.; Lourenço-de-Oliveira, R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 2003, 96, 512–518. [Google Scholar] [CrossRef]
- Leisnham, P.T.; Sala, L.M.; Juliano, S.A. Geographic variation in adult survival and reproductive tactics of the mosquito Aedes albopictus. J. Med. Entomol. 2008, 45, 210–221. [Google Scholar] [CrossRef]
- O’Donnell, D.; Armbruster, P. Evolutionary differentiation of fitness traits across multiple geographic scales in Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 2009, 102, 1135–1144. [Google Scholar] [CrossRef]
- Black, W.C., IV; Hawley, W.A.; Rai, K.S.; Craig, G.B. Breeding structure of a colonizing species: Aedes albopictus (Skuse) in peninsular Malaysia and Borneo. Heredity 1988, 61, 439–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Black, W.C., IV; Karamjit, S.R.; Turco, B.J.; Arroyo, D.C. Laboratory study of competition between United States strains of Aedes albopictus and Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1989, 26, 260–271. [Google Scholar] [CrossRef]
- Romano, D.; Stefanini, C.; Canale, A.; Benelli, G. Artificial blood feeders for mosquito and ticks—Where from, where to? Acta. Tropica 2018, 183, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.; Torr, S.J. Visual and olfactory responses of hematophagous Diptera to host stimuli. Med. Vet. Entomol. 1999, 13, 2–23. [Google Scholar] [CrossRef]
- Charlwood, J.D.; Tomás, E.V.; Kelly-Hope, L.; Briët, O.J. Evidence of an ‘invitation’ effect in feeding sylvatic Stegomyia albopicta from Cambodia. Parasit. Vectors 2014, 7, 324. [Google Scholar] [CrossRef]
- Davis, T.J.; Kaufman, P.E.; Hogsette, J.A.; Kline, D.L. The effects of larval habitat quality on Aedes albopictus skip oviposition. J. Am. Mosq. Control Assoc. 2015, 31, 321–328. [Google Scholar] [CrossRef]
- Davis, T.J.; Kline, D.L.; Kaufman, P.E. Assessment of Aedes albopictus (Skuse) (Diptera: Culicidae) clutch size in wild and laboratory populations. J. Vector Ecol. 2016, 41, 11–17. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiens, D.; Marquereau, L.; Lebon, C.; Le Goff, G.; Gaudillat, B.; Habchi-Hanriot, N.; Gouagna, L.-C. Aedes albopictus Adult Medium Mass Rearing for SIT Program Development. Insects 2019, 10, 246. https://doi.org/10.3390/insects10080246
Damiens D, Marquereau L, Lebon C, Le Goff G, Gaudillat B, Habchi-Hanriot N, Gouagna L-C. Aedes albopictus Adult Medium Mass Rearing for SIT Program Development. Insects. 2019; 10(8):246. https://doi.org/10.3390/insects10080246
Chicago/Turabian StyleDamiens, David, Lucie Marquereau, Cyrille Lebon, Gilbert Le Goff, Benjamin Gaudillat, Nausicaa Habchi-Hanriot, and Louis-Clément Gouagna. 2019. "Aedes albopictus Adult Medium Mass Rearing for SIT Program Development" Insects 10, no. 8: 246. https://doi.org/10.3390/insects10080246
APA StyleDamiens, D., Marquereau, L., Lebon, C., Le Goff, G., Gaudillat, B., Habchi-Hanriot, N., & Gouagna, L.-C. (2019). Aedes albopictus Adult Medium Mass Rearing for SIT Program Development. Insects, 10(8), 246. https://doi.org/10.3390/insects10080246



