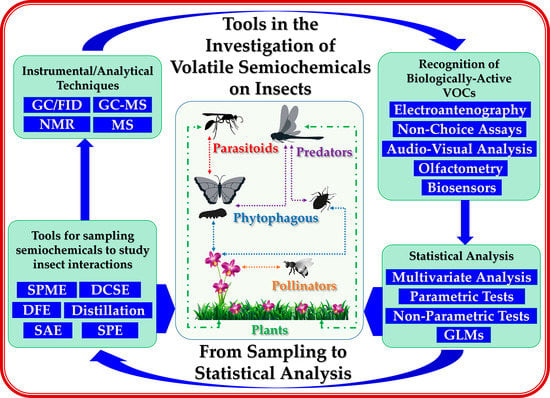
Tools in the Investigation of Volatile Semiochemicals on Insects: From Sampling to Statistical Analysis
Abstract
1. Introduction
2. Tools for Sampling Semiochemicals to Study Insect Interactions
2.1. Most Commonly Used Methods for Extracting Semiochemicals
2.1.1. Solvent-Assisted Extraction (SAE)
2.1.2. Distillation
2.2. Most Commonly Used Methods for Collecting (Trapping) Semiochemicals
2.2.1. Enclosure Techniques
Static (SHS) and Dynamic Headspace (DHS) Sampling
Sorbent-Based Trapping [Solid-Phase Extraction (SPE)]
Solid-Phase Micro-Extraction (SPME)
Headspace—Solid-Phase Micro-Extraction HS-SPME
Other Enclosure Technique Devices
2.2.2. Direct-Contact Sorptive Extraction (DCSE)
3. Instrumental Analytical Techniques
3.1. Gas Chromatography (GC)
3.1.1. Gas Chromatography Coupled to Flame Ionization Detectors (GC-FID)
3.1.2. Gas Chromatography Coupled to an Electroantennography Detector (GC-EAD)
3.1.3. Gas Chromatography Coupled to Mass Spectrometry (GC-MS)
3.2. Mass-Spectrometry not Coupled with GC
3.3. Nuclear Magnetic Resonance (NMR)
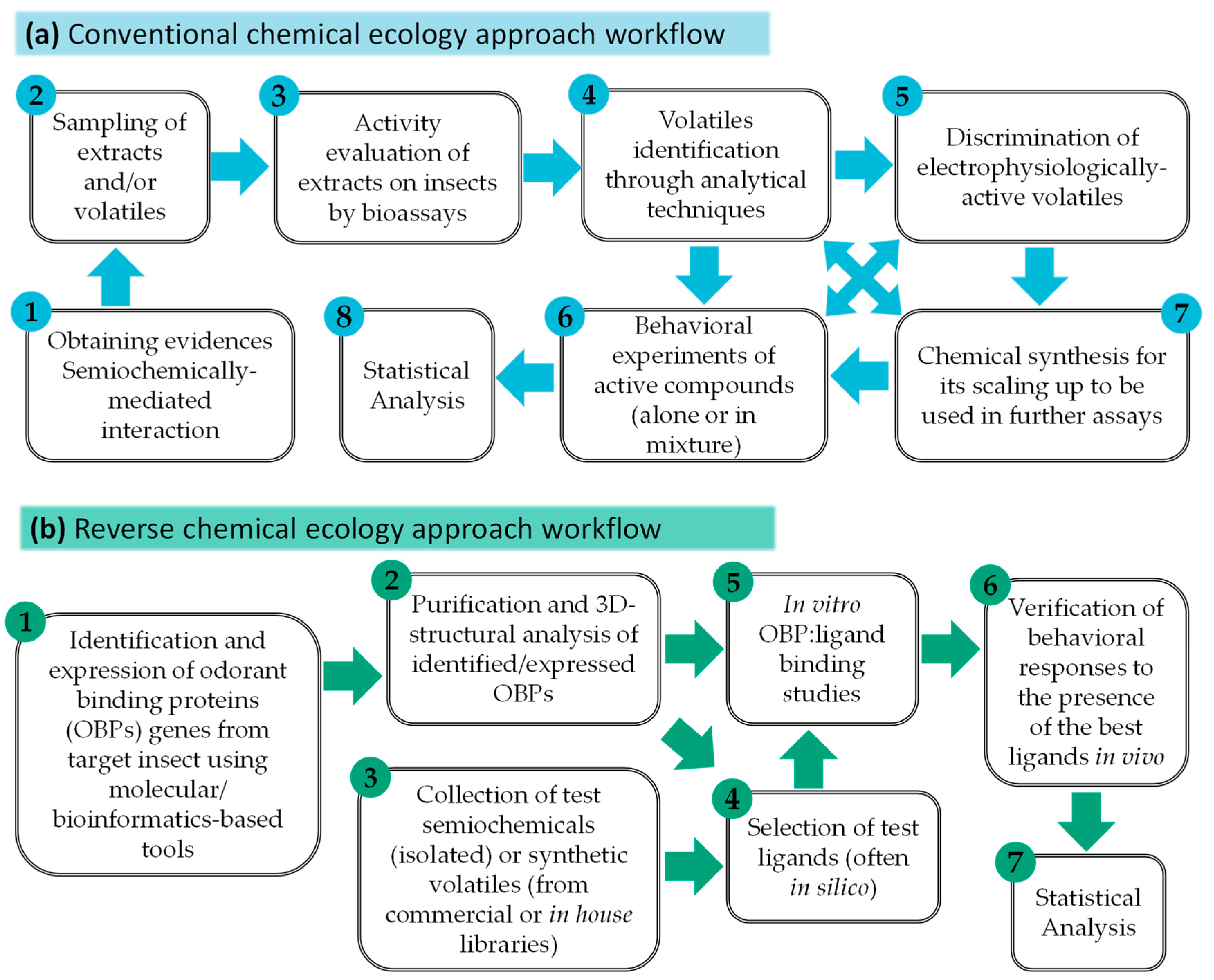
4. Chemical Synthesis
5. Recognition of Biologically Active Compounds
5.1. Electroantennography (EAG)
5.2. Single Sensillum Recording (SSR)
5.3. Behavioral Experiments
5.3.1. Linear Olfactometry
5.3.2. Two-Path Olfactometry
5.3.3. Four-Path (or More) Olfactometry
5.3.4. Wind-Tunnel Assay
5.4. Non-Choice Assays
5.5. Audiovisual Analysis
5.6. Sensor-Based Detection of Semiochemicals
6. Statistical Analysis
6.1. Statistics in Olfactometry and Behavior
6.1.1. Generalities
- (1)
- Experimental design and scope: The utilization of robust experimental designs provides better accuracy for making conclusions about the results of an experiment, but it should be taken into account that incorrect interpretations of data by the researcher are always possible [203].
- (2)
- (3)
- Pseudoreplication: Pseudoreplication is one of the most extensive problems in olfactometry and, subsequently, in associated reports [14,203,205,206]. It is strongly advisable to avoid this when possible. This consists of the use of non-independent experimental units during assays, generating a result bias and incorrect statistical conclusions. Often this is derived from incorrect experimental unit definitions (border effects, lack of randomization, poor variable control, etc.) or management (reutilization of the VOC source, insects, materials, and more), producing a “residual” effect amongst a unit and its replicates [205,206].
- (4)
- Unbalanced data: This refers to data deficits due to unforeseen experimental events, such as difficulty measuring some variable, atypical or out-of-range values, etc., which produce an incomplete data matrix for analysis. In some cases, this can be corrected through two strategies: data deletion when experimental data are sufficient and insignificant information is lost or data filling for situations in which the data behavior is well known. Unfortunately, these approaches are not always feasible [206].
6.1.2. Statistical Tools for Olfactometry Data Treatment
Parametric Tests
Non-Parametric Tests
Circular Statistics
General Linear Models (GLMs)
6.2. Multivariate Analysis
- (1)
- Conditioning: Multivariate analysis is strongly scale dependent, and because of this, it is common that the data matrix be conditioned to ensure a convenient scale, misinforming variations are systematically reduced, noise is reduced [213], information among samples is aligned, remaining values are introduced, and data is filtered. For this purpose, it is possible to use several normalization methods [202].
- (2)
- (3)
- Treatment: This consists of performing the analysis to detect and identify general discrimination patterns among samples or groups, and the application of probabilistic models that determine the response, with statistical significance, to a factor under investigation [212]. For this work, descriptive models and classification models can be employed [213].
- (4)
- Validation: The final step, validation, consists of the analytical characterization of a marker, i.e., a VOC that can be associated with a group defined by characteristics or properties in absolute terms to find the corresponding differences among sample groups and controls and corroborate the marker in question [212].
6.2.1. Descriptive, Exploratory or Discriminating Models
6.2.2. Classification Models
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Foti, M.C.; Rostás, M.; Peri, E.; Park, K.C.; Slimani, T.; Wratten, S.D.; Colazza, S. Chemical ecology meets conservation biological control: Identifying plant volatiles as predictors of floral resource suitability for an egg parasitoid of stink bugs. J. Pest Sci. 2016, 90, 299–310. [Google Scholar] [CrossRef]
- Haggarty, J.; Burgess, K.E. Recent advances in liquid and gas chromatography methodology for extending coverage of the metabolome. Curr. Opin. Biotechnol. 2017, 43, 77–85. [Google Scholar] [CrossRef]
- Ormeño, E.; Goldstein, A.; Niinemets, Ü. Extracting and trapping biogenic volatile organic compounds stored in plant species. Trends Anal. Chem. 2011, 30, 978–989. [Google Scholar] [CrossRef]
- Tholl, D.; Boland, W.; Hansel, A.; Loreto, F.; Röse, U.S.R.; Schnitzler, J.-P. Practical approaches to plant volatile analysis. Plant J. 2006, 45, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Hern, A.; Dorn, S. Statistical modelling of insect behavioural responses in relation to the chemical composition of test extracts. Physiol. Entomol. 2001, 26, 381–390. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef] [PubMed]
- Stökl, J.; Steiger, S. Evolutionary origin of insect pheromones. Curr. Opin. Insect Sci. 2017, 24, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. Communication Systems. In Physiological Systems in Insects; Elsevier: Amsterdam, The Netherlands, 2008; pp. 597–642. [Google Scholar]
- Yuan, J.S.; Himanen, S.J.; Holopainen, J.K.; Chen, F.; Stewart, C.N., Jr. Smelling global climate change: Mitigation of function for plant volatile organic compounds. Trends Ecol. Evol. 2009, 24, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Tenenboim, H.; Brotman, Y. Omic relief for the biotically stressed: Metabolomics of plant biotic interactions. Trends Plant Sci. 2016, 21, 781–791. [Google Scholar] [CrossRef]
- Delory, B.M.; Delaplace, P.; Fauconnier, M.-L.; du Jardin, P. Root-emitted volatile organic compounds: Can they mediate belowground plant-plant interactions? Plant Soil 2016, 402, 1–26. [Google Scholar] [CrossRef]
- Johnson, S.N.; Benefer, C.M.; Frew, A.; Griffiths, B.S.; Hartley, S.E.; Karley, A.J.; Rasmann, S.; Schumann, M.; Sonnemann, I.; Robert, C.A.M. New frontiers in belowground ecology for plant protection from root-feeding insects. Appl. Soil Ecol. 2016, 108, 96–107. [Google Scholar] [CrossRef]
- Yew, J.Y.; Chung, H. Insect pheromones: An overview of function, form, and discovery. Prog. Lipid Res. 2015, 59, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Knolhoff, L.M.; Heckel, D.G. Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Entomol. 2014, 59, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Blight, M.M. Techniques for isolation and characterization of volatile semiochemicals of phytophagous insects. In Chromatography and Isolation of Insect Hormones and Pheromones; McCaffery, A.R., Wilson, I.D., Eds.; Springer US: New York, NY, USA, 1990; pp. 281–288. ISBN 978-1-4684-8062-7. [Google Scholar]
- Vander Meer, R. Ant interactions with soil organisms and associated semiochemicals. J. Chem. Ecol. 2012, 38, 728–745. [Google Scholar] [CrossRef] [PubMed]
- Rork, A.M.; Renner, T. Carabidae semiochemistry: Current and future directions. J. Chem. Ecol. 2018, 44, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Steiger, S.; Stökl, J. Pheromones involved in insect parental care and family life. Curr. Opin. Insect Sci. 2017, 24, 89–95. [Google Scholar] [CrossRef]
- Burkle, L.A.; Runyon, J.B. The smell of environmental change: Using floral scent to explain shifts in pollinator attraction. Appl. Plant Sci. 2017, 5, 1600123. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.-M.; Xu, P.; Hwang, J.K.; Zeng, F.; Tan, K.; Bhagavathy, G.; Chauhan, K.R.; Leal, W.S. Reverse chemical ecology approach for the identification of an oviposition attractant for Culex quinquefasciatus. Proc. Natl. Acad. Sci. USA 2018, 115, 714 LP–719. [Google Scholar] [CrossRef]
- Xu, H.; Bohman, B.; Wong, D.C.J.; Rodriguez-Delgado, C.; Scaffidi, A.; Flematti, G.R.; Phillips, R.D.; Pichersky, E.; Peakall, R. Complex sexual deception in an orchid is achieved by co-opting two independent biosynthetic pathways for pollinator attraction. Curr. Biol. 2017, 27, 1867–1877. [Google Scholar] [CrossRef]
- Ortiz-Rojas, L.Y.; Chaves-Bedoya, G. Composición fitoquímica del extracto de raíz de Ichthyothere terminalis de dos regiones geográficas diferentes de Colombia. Rev. Colomb. Química 2017, 46, 11–16. [Google Scholar] [CrossRef]
- Jaramillo, C.B.E.; Duarte, R.E.; Delgado, W. Bioactividad del aceite esencial de Chenopodium ambrosioides colombiano. Rev. Cuba. Plantas Med. 2012, 17, 54–64. [Google Scholar]
- Lundborg, L.; Fedderwitz, F.; Björklund, N.; Nordlander, G.; Borg-Karlson, A.-K. Induced defenses change the chemical composition of pine seedlings and influence meal properties of the pine weevil Hylobius abietis. Phytochemistry 2016, 130, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, S.; Tadic, V.; Skala, D.; Zizovic, I. Separation of phytochemicals from Helichrysum italicum: An analysis of different isolation techniques and biological activity of prepared extracts. Phytochemistry 2017, 138, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Röder, G.; Mota, M.; Turlings, T.C.J. Host plant location by chemotaxis in an aquatic beetle. Aquat. Sci. 2017, 79, 309–318. [Google Scholar] [CrossRef]
- Roux, O.; Martin, J.-M.; Ghomsi, N.T.; Dejean, A. A non-lethal water-based removal-reapplication technique for behavioral analysis of cuticular compounds of ants. J. Chem. Ecol. 2009, 35, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Cañas-Hoyos, N.; Lobo-Echeverri, T.; Saldamando-Benjumea, C.I. Chemical composition of female sexual flands of Spodoptera frugiperda corn and rice strains from Tolima, Colombia. Southwest. Entomol. 2017, 42, 375–394. [Google Scholar] [CrossRef]
- Hassemer, M.J.; Santana, J.; de Oliveira, M.W.M.; Borges, M.; Laumann, R.A.; Caumo, M.; Blassioli-Moraes, M.C. Chemical composition of Alphitobius diaperinus (Coleoptera: Tenebrionidae) abdominal glands and the influence of 1,4-benzoquinones on its behavior. J. Econ. Entomol. 2015, 108, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Kiatbenjakul, P.; Intarapichet, K.-O.; Cadwallader, K.R. Identification of potent sulfur-containing odorants in scent glands of edible male giant water bug, Lethocerus indicus (Lep. and Serv.). Flavour Fragr. J. 2014, 29, 107–113. [Google Scholar] [CrossRef]
- Waseem, R.; Low, K.H. Advanced analytical techniques for the extraction and characterization of plant-derived essential oils by gas chromatography with mass spectrometry. J. Sep. Sci. 2015, 38, 483–501. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Pino-Benitez, N.; Pajaro-Castro, N.; Stashenko, E.; Olivero-Verbel, J. Plants cultivated in Choco, Colombia, as source of repellents against Tribolium castaneum (Herbst). J. Asia. Pac. Entomol. 2014, 17, 753–759. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R.; Cárdenas-Vargas, S.; Saavedra-Barrera, R.; Durán, D.C. GC-MS study of compounds isolated from Coffea arabica flowers by different extraction techniques. J. Sep. Sci. 2013, 36, 2901–2914. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.; Helmig, D.; Daly, R.W.; Tanner, D.M.; Guenther, A.B.; Herrick, J.D. Approaches for quantifying reactive and low-volatility biogenic organic compound emissions by vegetation enclosure techniques—Part B: Applications. Chemosphere 2008, 72, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Martínez, J.R. In vivo sampling of flavor components. In Comprehensive Sampling and Sample Preparation; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, pp. 147–158. [Google Scholar]
- Liberto, E.; Cagliero, C.; Cordero, C.; Rubiolo, P.; Bicchi, C.; Sgorbini, B. Fractionated dynamic headspace sampling in the analysis of matrices of vegetable origin in the food field. J. Chromatogr. A 2017, 1489, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Fürstenau, B.; Adler, C.; Schulz, H.; Hilker, M. Host habitat volatiles enhance the olfactory response of the larval parasitoid Holepyris sylvanidis to specifically host-associated cues. Chem. Senses 2016, 41, 611–621. [Google Scholar] [PubMed]
- Ponzio, C.; Cascone, P.; Cusumano, A.; Weldegergis, B.T.; Fatouros, N.E.; Guerrieri, E.; Dicke, M.; Gols, R. Volatile-mediated foraging behaviour of three parasitoid species under conditions of dual insect herbivore attack. Anim. Behav. 2016, 111, 197–206. [Google Scholar] [CrossRef]
- Azandémè-Hounmalon, G.Y.; Torto, B.; Fiaboe, K.K.M.; Subramanian, S.; Kreiter, S.; Martin, T. Visual, vibratory, and olfactory cues affect interactions between the red spider mite Tetranychus evansi and its predator Phytoseiulus longipes. J. Pest Sci. (2004) 2016, 89, 137–152. [Google Scholar] [CrossRef]
- Twidle, A.M.; Suckling, D.M.; Seal, A.G.; Fedrizzi, B.; Pilkington, L.I.; Barker, D. Identification of in situ flower volatiles from kiwifruit (Actinidia chinensis var. deliciosa) cultivars and their male pollenisers in a New Zealand orchard. Phytochemistry 2017, 141, 61–69. [Google Scholar] [CrossRef]
- Shuttleworth, A.; Johnson, S.D.; Jürgens, A. Entering through the narrow gate: A morphological filter explains specialized pollination of a carrion-scented stapeliad. Flora 2017, 232, 92–103. [Google Scholar] [CrossRef]
- Diaz-Santiz, E.; Rojas, J.C.; Cruz-López, L.; Hernández, E.; Malo, E.A. Olfactory response of Anastrepha striata (Diptera: Tephritidae) to guava and sweet orange volatiles. Insect Sci. 2016, 23, 720–727. [Google Scholar] [CrossRef]
- Rostás, M.; Cripps, M.G.; Silcock, P. Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect. Oecologia 2015, 177, 487–497. [Google Scholar] [CrossRef]
- Li, Y.; Mathews, R.A. In vivo real-time monitoring of aphrodisiac pheromone release of small white cabbage butterflies (Pieris rapae). J. Insect Physiol. 2016, 91, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kuenen, L.P.S.; Hicks, M.N. Gas chromatography column as an ambient-temperature volatile trap. Entomol. Exp. Appl. 2015, 154, 35–44. [Google Scholar] [CrossRef]
- De Alfonso, I.; Hernandez, E.; Velazquez, Y.; Navarro, I.; Primo, J. Identification of the sex pheromone of the mealybug Dysmicoccus grassii Leonardi. J. Agric. Food Chem. 2012, 60, 11959–11964. [Google Scholar] [CrossRef] [PubMed]
- Woolfenden, E. Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air: Part 1: Sorbent-based air monitoring options. J. Chromatogr. A 2010, 1217, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Harper, M. Sorbent trapping of volatile organic compounds from air. J. Chromatogr. A 2000, 885, 129–151. [Google Scholar] [CrossRef]
- Dettmer, K.; Engewald, W. Adsorbent materials commonly used in air analysis for adsorptive enrichment and thermal desorption of volatile organic compounds. Anal. Bioanal. Chem. 2002, 373, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Camel, V.; Caude, M. Trace enrichment methods for the determination of organic pollutants in ambient air. J. Chromatogr. A 1995, 710, 3–19. [Google Scholar] [CrossRef]
- Rodriguez, S.A.; Paliza, M.L.; Nazareno, M.A. Influence of adsorbent nature on the dynamic headspace study of insect semiochemicals. Aust. J. Chem. 2017, 70, 902. [Google Scholar] [CrossRef]
- Tholl, D.; Röse, U.S.R. Detection and identification of floral scent compounds. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; {CRC} Press: Boca Raton, FL, USA, 2006; pp. 3–26. [Google Scholar]
- Woolfenden, E. Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air. Part 2. Sorbent selection and other aspects of optimizing air monitoring methods. J. Chromatogr. A 2010, 1217, 2685–2694. [Google Scholar] [CrossRef]
- Sghaier, L.; Vial, J.; Sassiat, P.; Thiebaut, D.; Watiez, M.; Breton, S.; Rutledge, D.N.; Cordella, C.B.Y. An overview of recent developments in volatile compounds analysis from edible oils: technique-oriented perspectives. Eur. J. Lipid Sci. Technol. 2016, 118, 1853–1879. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Alam, M.N.; Pawliszyn, J. Review of geometries and coating materials in solid phase microextraction: Opportunities, limitations, and future perspectives. Anal. Chim. Acta 2017, 984, 42–65. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacı, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in solid phase microextraction and perspective on future directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Sajid, M.; Khaled Nazal, M.; Rutkowska, M.; Szczepańska, N.; Namieśnik, J.; Płotka-Wasylka, J. Solid Phase Microextraction: apparatus, sorbent materials, and application. Crit. Rev. Anal. Chem. 2019, 49, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, G.; Huang, S.; Qiu, J.; Jiang, R.; Zhu, F.; Ouyang, G. Application of in vivo solid-phase microextraction in environmental analysis. TrAC Trends Anal. Chem. 2016, 85, 26–35. [Google Scholar] [CrossRef]
- Shirey, R.E. 4-SPME Commercial devices and fibre coatings. In Handbook of Solid Phase Microextraction; Pawliszyn, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 99–133. ISBN 978-0-12-416017-0. [Google Scholar]
- Zhu, F.; Xu, J.; Ke, Y.; Huang, S.; Zeng, F.; Luan, T.; Ouyang, G. Applications of in vivo and in vitro solid-phase microextraction techniques in plant analysis: A review. Anal. Chim. Acta 2013, 794, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, B.; Cong, H.; Yuan, H.; Peng, Q. Recent development and application of solid phase extraction materials. Rev. Adv. Mater. Sci. 2017, 49, 87–111. [Google Scholar]
- Kfoury, N.; Scott, E.; Orians, C.; Robbat, A. Direct contact sorptive extraction: A robust method for sampling plant volatiles in the field. J. Agric. Food Chem. 2017, 65, 8501–8509. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.; Li, D. Microextraction techniques for the determination of volatile and semivolatile organic compounds from plants: A review. Anal. Chim. Acta 2013, 799, 8–22. [Google Scholar] [CrossRef]
- Lim, D.K.; Mo, C.; Lee, D.-K.; Long, N.P.; Lim, J.; Kwon, S.W. Non-destructive profiling of volatile organic compounds using HS-SPME/GC–MS and its application for the geographical discrimination of white rice. J. Food Drug Anal. 2018, 26, 260–267. [Google Scholar] [CrossRef]
- Giunti, G.; Palmeri, V.; Algeri, G.M.; Campolo, O. VOC emissions influence intra- and interspecific interactions among stored-product Coleoptera in paddy rice. Sci. Rep. 2018, 8, 2052. [Google Scholar] [CrossRef] [PubMed]
- Būda, V.; Apšegaitė, V.; Blažytė-Čereškienė, L.; Butkienė, R.; Nedveckytė, I.; Pečiulytė, D. Response of moth Plodia interpunctella to volatiles of fungus-infected and uninfected wheat grain. J. Stored Prod. Res. 2016, 69, 152–158. [Google Scholar] [CrossRef]
- Usseglio, V.L.; Pizzolitto, R.P.; Rodriguez, C.; Zunino, M.P.; Zygadlo, J.A.; Areco, V.A.; Dambolena, J.S. Volatile organic compounds from the interaction between Fusarium verticillioides and maize kernels as a natural repellents of Sitophilus zeamais. J. Stored Prod. Res. 2017, 73, 109–114. [Google Scholar] [CrossRef]
- Soto, V.C.; Maldonado, I.B.; Jofré, V.P.; Galmarini, C.R.; Silva, M.F. Direct analysis of nectar and floral volatile organic compounds in hybrid onions by HS-SPME/GC–MS: Relationship with pollination and seed production. Microchem. J. 2015, 122, 110–118. [Google Scholar] [CrossRef]
- San Román, I.; Bartolomé, L.; Gee, W.S.; Alonso, R.M.; Beck, J.J. Comparison of ex situ volatile emissions from intact and mechanically damaged walnuts. Food Res. Int. 2015, 72, 198–207. [Google Scholar] [CrossRef]
- Smith, L.; Beck, J.J. Duration of emission of volatile organic compounds from mechanically damaged plant leaves. J. Plant Physiol. 2015, 188, 19–28. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, F.; Chen, S.; Guan, Z.; Jiang, J.; Fang, W.; Chen, F. Effects of aphid herbivory on volatile organic compounds of Artemisia annua and Chrysanthemum morifolium. Biochem. Syst. Ecol. 2015, 60, 225–233. [Google Scholar] [CrossRef]
- Santos, S.A.P.; Mota, L.; Malheiro, R.; Silva, F.; Campos, M.; Guedes de Pinho, P.; Pereira, J.A. Changes in volatile compounds of Dittrichia viscosa caused by the attack of the gall-forming dipteran Myopites stylatus. Ind. Crops Prod. 2016, 87, 71–77. [Google Scholar] [CrossRef]
- Cordero, C.; Zebelo, S.A.; Gnavi, G.; Griglione, A.; Bicchi, C.; Maffei, M.E.; Rubiolo, P. HS-SPME-GC×GC-qMS volatile metabolite profiling of Chrysolina herbacea frass and Mentha spp. leaves. Anal. Bioanal. Chem. 2012, 402, 1941–1952. [Google Scholar] [CrossRef]
- Özgenç, Ö.; Durmaz, S.; Çelik, G.; Korkmaz, B.; Yaylı, N. Comparative phytochemical analysis of volatile organic compounds by SPME-GC-FID/MS from six coniferous and nine deciduous tree bark species grown in Turkey. S. Afr. J. Bot. 2017, 113, 23–28. [Google Scholar] [CrossRef]
- Nunes, C.E.P.; Gerlach, G.; Bandeira, K.D.O.; Gobbo-Neto, L.; Pansarin, E.R.; Sazima, M. Two orchids, one scent? Floral volatiles of Catasetum cernuum and Gongora bufonia suggest convergent evolution to a unique pollination niche. Flora 2017, 232, 207–216. [Google Scholar] [CrossRef]
- Rout, P.K.; Ramachandra Rao, Y.; Naik, S. Analysis of floral volatiles by using headspace-solid phase microextraction: A review. Asian J. Chem. 2012, 24, 945–956. [Google Scholar]
- Rodríguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Rosales Martinez, A.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef] [PubMed]
- Deasy, W.; Shepherd, T.; Alexander, C.J.; Birch, A.N.E.; Evans, K.A. Development and validation of a SPME-GC-MS method for in situ passive sampling of root volatiles from glasshouse-grown broccoli plants undergoing below-ground herbivory by larvae of cabbage root fly, Delia radicum L. Phytochem. Anal. 2016, 27, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, P.; Zhang, J.; Yang, D.; Li, Z.; Zhang, X.; Zhu, S.; Yu, Y.; Chen, N. Identification of odorant binding proteins in Carpomya vesuviana and their binding affinity to the male-borne semiochemicals and host plant volatiles. J. Insect Physiol. 2017, 100, 100–107. [Google Scholar] [CrossRef]
- Clancy, M.V.; Zytynska, S.E.; Senft, M.; Weisser, W.W.; Schnitzler, J.-P. Chemotypic variation in terpenes emitted from storage pools influences early aphid colonisation on tansy. Sci. Rep. 2016, 6, 38087. [Google Scholar] [CrossRef]
- Zagrobelny, M.; Simonsen, H.T.; Olsen, C.E.; Bak, S.; Møller, B.L. Volatiles from the burnet moth Zygaena filipendulae (Lepidoptera) and associated flowers, and their involvement in mating communication. Physiol. Entomol. 2015, 40, 284–295. [Google Scholar] [CrossRef]
- Kolb, B.; Liebhardt, B.; Ettre, L.S. Cryofocusing in the combination of gas chromatography with equilibrium headspace sampling. Chromatographia 1986, 21, 305–311. [Google Scholar] [CrossRef]
- Dieu Hien, V.T.; Lin, C.; Thanh, V.C.; Kim Oanh, N.T.; Thanh, B.X.; Weng, C.E.; Yuan, C.S.; Rene, E.R. An overview of the development of vertical sampling technologies for ambient volatile organic compounds (VOCs). J. Environ. Manag. 2019, 247, 401–412. [Google Scholar] [CrossRef]
- Boggia, L.; Sgorbini, B.; Bertea, C.M.; Cagliero, C.; Bicchi, C.; Maffei, M.E.; Rubiolo, P. Direct Contact—Sorptive Tape Extraction coupled with Gas Chromatography—Mass Spectrometry to reveal volatile topographical dynamics of lima bean (Phaseolus lunatus L.) upon herbivory by Spodoptera littoralis Boisd. BMC Plant Biol. 2015, 15, 102. [Google Scholar] [CrossRef]
- Ernst, M.; Silva, D.B.; Silva, R.R.; Vêncio, R.Z.N.; Lopes, N.P. Mass spectrometry in plant metabolomics strategies: From analytical platforms to data acquisition and processing. Nat. Prod. Rep. 2014, 31, 784. [Google Scholar] [CrossRef] [PubMed]
- Héberger, K. Quantitative structure—(chromatographic) retention relationships. J. Chromatogr. A 2007, 1158, 273–305. [Google Scholar] [CrossRef] [PubMed]
- Zellner, B.A.; Bicchi, C.; Dugo, P.; Rubiolo, P.; Dugo, G.; Mondello, L. Linear retention indices in gas chromatographic analysis: A review. Flavour Fragr. J. 2008, 23, 297–314. [Google Scholar] [CrossRef]
- Castello, G. Retention index systems: Alternatives to the n-alkanes as calibration standards. J. Chromatogr. A 1999, 842, 51–64. [Google Scholar] [CrossRef]
- Dallüge, J.; Beens, J.; Brinkman, U.A.T. Comprehensive two-dimensional gas chromatography: A powerful and versatile analytical tool. J. Chromatogr. A 2003, 1000, 69–108. [Google Scholar] [CrossRef]
- Adahchour, M.; Beens, J.; Vreuls, R.J.J.; Brinkman, U.A.T. Recent developments in comprehensive two-dimensional gas chromatography (GC × GC): I. Introduction and instrumental set-up. TrAC Trends Anal. Chem. 2006, 25, 438–454. [Google Scholar] [CrossRef]
- Kalinová, B.; Jiroš, P.; Žd’árek, J.; Wen, X.; Hoskovec, M. GC×GC/TOF MS technique—A new tool in identification of insect pheromones: Analysis of the persimmon bark borer sex pheromone gland. Talanta 2006, 69, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F. Ionization-based detectors for gas chromatography. J. Chromatogr. A 2015, 1421, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, P.; Sgorbini, B.; Liberto, E.; Cordero, C.; Bicchi, C. Essential oils and volatiles: Sample preparation and analysis. A review. Flavour Fragr. J. 2010, 25, 282–290. [Google Scholar] [CrossRef]
- Heuskin, S.; Rozet, E.; Lorge, S.; Farmakidis, J.; Hubert, P.; Verheggen, F.; Haubruge, E.; Wathelet, J.-P.; Lognay, G. Validation of a fast gas chromatographic method for the study of semiochemical slow release formulations. J. Pharm. Biomed. Anal. 2010, 53, 962–972. [Google Scholar] [CrossRef]
- Bartelt, R.J. Calibration of a commercial solid-phase microextraction device for measuring headspace concentrations of organic volatiles. Anal. Chem. 1997, 69, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Musteata, F.M.; Pawliszyn, J. In vivo sampling with solid phase microextraction. J. Biochem. Biophys. Methods 2007, 70, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.K.; Brown, S.; Clarke, S.; Röse, U.S.R.; Starks, P.T. The European wool-carder bee (Anthidium manicatum) eavesdrops on plant volatile organic compounds (VOCs) during trichome collection. Behav. Process. 2017, 144, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Martínez, J.R.; Ruíz, C.A.; Arias, G.; Durán, C.; Salgar, W.; Cala, M. Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal component analysis. J. Sep. Sci. 2010, 33, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Shen, L.; Yang, J.; Cheng, H.; Jiang, S.; Jiang, C.; Wang, H. Fumigant, contact, and repellent activities of essential oils against the darkling beetle, Alphitobius diaperinus. J. Insect Sci. 2014, 14, 1–11. [Google Scholar] [CrossRef]
- Szmigielski, R.; Cieslak, M.; Rudziński, K.J.; Maciejewska, B. Identification of volatiles from Pinus silvestris attractive for Monochamus galloprovincialis using a SPME-GC/MS platform. Environ. Sci. Pollut. Res. 2012, 19, 2860–2869. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, G.R.; Oldham, N.J. Pheromone analysis using capillary gas chromatographic techniques. J. Chromatogr. A 1999, 843, 199–236. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Stanly, C.; Fiume, I.; Vékey, K. Chromatography and its hyphenation to mass spectrometry for extracellular vesicle analysis. J. Chromatogr. A 2016, 1439, 26–41. [Google Scholar] [CrossRef]
- Tsizin, S.; Bokka, R.; Keshet, U.; Alon, T.; Fialkov, A.B.; Tal, N.; Amirav, A. Comparison of electrospray LC–MS, LC–MS with Cold EI and GC–MS with Cold EI for sample identification. Int. J. Mass Spectrom. 2017, 422, 119–125. [Google Scholar] [CrossRef]
- De Villiers, A.; Venter, P.; Pasch, H. Recent advances and trends in the liquid-chromatography—Mass spectrometry analysis of flavonoids. J. Chromatogr. A 2016, 1430, 16–78. [Google Scholar] [CrossRef]
- Mossi, A.J.; Astolfi, V.; Kubiak, G.; Lerin, L.; Zanella, C.; Toniazzo, G.; de Oliveira, D.; Treichel, H.; Devilla, I.A.; Cansian, R.; et al. Insecticidal and repellency activity of essential oil of Eucalyptus sp. against Sitophilus zeamais Motschulsky (Coleoptera, Curculionidae). J. Sci. Food Agric. 2011, 91, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wu, J.; Wang, J.; Wang, X. Discrimination of American ginseng and Asian ginseng using electronic nose and gas chromatography—Mass spectrometry coupled with chemometrics. J. Ginseng Res. 2017, 41, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.I.; Vaughan, M.J.; Bonello, P.; McSpadden Gardener, B.; Grotewold, E.; Alonso, A.P. Identification of biochemical features of defective Coffea arabica L. beans. Food Res. Int. 2017, 95, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Besada, C.; Sanchez, G.; Gil, R.; Granell, A.; Salvador, A. Volatile metabolite profiling reveals the changes in the volatile compounds of new spontaneously generated loquat cultivars. Food Res. Int. 2017, 100, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jang, M.; Shin, E.; Kim, J.; Lee, S.H.; Park, C.G. Fumigant and contact toxicity of 22 wooden essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2016, 133, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, M.; Wu, W.; Wang, A.; BAO, T.; Zheng, C.; Chou, L.; Tzeng, H.; Tu, S. The floral scent of Ficus pumila var. pumila and its effect on the choosing behavior of pollinating wasps of Wiebesia pumilae. Acta Ecol. Sin. 2016, 36, 321–326. [Google Scholar]
- Ramilo, P.; Guerrero, J.R.; Micó, E.; Galante, E. Volatile organic compounds emitted by Quercus pyrenaica Willd. and its relationship with saproxylic beetle assemblages. Arthropod. Plant. Interact. 2017, 11, 221–234. [Google Scholar] [CrossRef]
- Jofré, N.; Pildain, M.B.; Cirigliano, A.M.; Cabrera, G.M.; Corley, J.C.; Martínez, A.S. Host selection by Ibalia leucospoides based on temporal variations of volatiles from the hosts’ fungal symbiont. J. Appl. Entomol. 2016, 140, 736–743. [Google Scholar] [CrossRef]
- Altamar-Varón, P.; Pérez-Maldonado, D.; Rodríguez-Caicedo, D.; Guerrero-Perilla, C.; Coy-Barrera, E. Chemical composition of the low-polar fraction of the Copitarsia uncilata Burgos & Leiva (Lepidoptera: Noctuidae) eversible pheromone gland. Neotrop. Entomol. 2016, 45, 734–739. [Google Scholar] [PubMed]
- Simon, A.G.; Mills, D.K.; Furton, K.G. Chemotyping the temporal volatile organic compounds of an invasive fungus to the United States, Raffaelea lauricola. J. Chromatogr. A 2017, 1487, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Šanda, M.; Žáček, P.; Streinz, L.; Dračínský, M.; Koutek, B. Profiling and characterization of volatile secretions from the European stink bug Graphosoma lineatum (Heteroptera: Pentatomidae) by two-dimensional gas chromatography/time-of-flight mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 881, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Holmelid, B.; Øvstedal, D.O. Bumblebee death associated with Tilia x europaea L. Biochem. Syst. Ecol. 2019, 82, 16–23. [Google Scholar] [CrossRef]
- Ho, Y.-N.; Shu, L.-J.; Yang, Y.-L. Imaging mass spectrometry for metabolites: technical progress, multimodal imaging, and biological interactions. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1387. [Google Scholar] [CrossRef] [PubMed]
- Svatoš, A. Mass spectrometric imaging of small molecules. Trends Biotechnol. 2010, 28, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Rejšek, J.; Vrkoslav, V.; Hanus, R.; Vaikkinen, A.; Haapala, M.; Kauppila, T.J.; Kostiainen, R.; Cvačka, J. The detection and mapping of the spatial distribution of insect defense compounds by desorption atmospheric pressure photoionization Orbitrap mass spectrometry. Anal. Chim. Acta 2015, 886, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.M.; Pretzel, J.; Becker, K.; Spengler, B. High-resolution AP-SMALDI mass spectrometry imaging of Drosophila melanogaster. Int. J. Mass Spectrom. 2017, 416, 1–19. [Google Scholar] [CrossRef]
- Giannoukos, S.; Marshall, A.; Taylor, S.; Smith, J. Molecular communication over gas stream channels using portable mass spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 2371–2383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chingin, K.; Zhong, D.; Luo, L.; Frankevich, V.; Chen, H. Deciphering the chemical origin of the semen-like floral scents in three angiosperm plants. Phytochemistry 2018, 145, 137–145. [Google Scholar] [CrossRef]
- Nozière, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgić, I.; Hamilton, J.F.; et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev. 2015, 115, 3919–3983. [Google Scholar] [CrossRef] [PubMed]
- Smelcerovic, A.; Djordjevic, A.; Lazarevic, J.; Stojanovic, G. Recent advances in analysis of essential oils. Curr. Anal. Chem. 2013, 9, 61–70. [Google Scholar] [CrossRef]
- Nojima, S.; Kiemle, D.J.; Webster, F.X.; Apperson, C.S.; Schal, C. Nanogram-scale preparation and NMR analysis for mass-limited small volatile compounds. PLoS ONE 2011, 6, e18178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reitz, M.; Gerhardt, H.; Schmitt, C.; Betz, O.; Albert, K.; Lämmerhofer, M. Analysis of chemical profiles of insect adhesion secretions by gas chromatography—Mass spectrometry. Anal. Chim. Acta 2015, 854, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cantrell, C.L.; Oi, D.; Grodowitz, M.J. Update on the defensive chemicals of the little black ant, Monomorium minimum (Hymenoptera: Formicidae). Toxicon 2016, 122, 127–132. [Google Scholar] [CrossRef]
- Sun, Z.-F.; Zhou, L.-N.; Meng, Y.; Zhang, T.; Du, Z.-T.; Zheng, H. Concise asymmetric synthesis of the sex pheromone of the tea tussock moth. Tetrahedron Asymmetry 2017, 28, 1562–1567. [Google Scholar] [CrossRef]
- Pilli, R.A.; Riatto, V.B. The asymmetric synthesis of (+)-sitophilure, the natural form of the aggregation pheromone of Sitophilus oryzae L. and Sitophilus zeamais M. J. Braz. Chem. Soc. 1999, 10, 363–368. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Yang, Y.Q.; Gao, H.H.; Hou, R.Y.; Wan, X.C. Synthesis of the enantiomers of (3Z,9Z)-cis-6,7-epoxy-3,9-octadecadiene, one of the major components of the sex pheromone of Ectropis oblique Prout. Tetrahedron Asymmetry 2017, 28, 758–761. [Google Scholar] [CrossRef]
- Chiluwal, K.; Kim, J.; Bae, S.D.; Maharjan, R.; Park, C.G. Attractiveness of male azuki bean beetle to the synthetic blends of 2E- and 2Z-homofarnesals. J. Asia Pac. Entomol. 2017, 20, 1183–1189. [Google Scholar] [CrossRef]
- You, C.; Zhang, W.; Guo, S.; Wang, C.; Yang, K.; Liang, J.; Wang, Y.; Geng, Z.; Du, S.; Deng, Z. Chemical composition of essential oils extracted from six Murraya species and their repellent activity against Tribolium castaneum. Ind. Crops Prod. 2015, 76, 681–687. [Google Scholar] [CrossRef]
- Padoan Gonçalves, G.L.; de Cássia Domingues, V.; do Prado Ribeiro, L.; Batista Fernandes, J.; das Graças Fernandes, M. de F.; Rossi Forim, M.; Vendramim, J.D. Compounds from Duguetia lanceolata St.-Hil. (Annonaceae) bioactive against Zabrotes subfasciatus (Boheman) (Coleoptera: Chrysomelidae: Bruchinae). Ind. Crops Prod. 2017, 97, 360–367. [Google Scholar] [CrossRef]
- Park, C.G.; Shin, E.; Kim, J. Insecticidal activities of essential oils, Gaultheria fragrantissima and Illicium verum, their components and analogs against Callosobruchus chinensis adults. J. Asia Pac. Entomol. 2016, 19, 269–273. [Google Scholar] [CrossRef]
- Delort, E.; Jaquier, A.; Decorzant, E.; Chapuis, C.; Casilli, A.; Frérot, E. Comparative analysis of three Australian finger lime (Citrus australasica) cultivars: Identification of unique citrus chemotypes and new volatile molecules. Phytochemistry 2015, 109, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, J.; Chatterjee, S.; Gamre, S.; Chattopadhyay, S.; Variyar, P.S.; Sharma, A. Analysis of free and bound aroma compounds of pomegranate (Punica granatum L.). LWT Food Sci. Technol. 2014, 59, 461–466. [Google Scholar] [CrossRef]
- Uenishi, J.; Kawahama, R.; Izaki, Y.; Yonemitsu, O. A facile preparation of geometrically pure alkenyl, alkynyl, and aryl conjugated Z-alkenes: Stereospecific synthesis of bombykol. Tetrahedron 2000, 56, 3493–3500. [Google Scholar] [CrossRef]
- Mori, K.; Yang, C.Y. Pheromone synthesis. Part 259: Synthesis of seven methyl-branched hydrocarbons as the pheromone candidates for female Korean apricot wasp, Eurytoma maslovskii. Tetrahedron 2016, 72, 4593–4607. [Google Scholar] [CrossRef]
- Shklyaruck, D.; Matiushenkov, E. Stereoselective synthesis of (3S,5S,6S)-tetrahydro-6-isopropyl-3,5-dimethylpyran-2-one; A C5-epimer of a component of a natural sex pheromone of the wasp Macrocentrus grandii, the larval parasitoid of the European corn borer Ostrinia nubilalis. Tetrahedron Asymmetry 2011, 22, 1448–1454. [Google Scholar] [CrossRef]
- Yang, X.; Luo, S.; Hua, C.; Zhai, H. Efficient synthesis of beetle aggregation pheromone frontalin and its analogues. Tetrahedron 2003, 59, 8551–8553. [Google Scholar] [CrossRef]
- Akasaka, K.; Tamogami, S.; Beeman, R.W.; Mori, K. Pheromone synthesis. Part 245: Synthesis and chromatographic analysis of the four stereoisomers of 4,8-dimethyldecanal, the male aggregation pheromone of the red flour beetle, Tribolium castaneum. Tetrahedron 2011, 67, 201–209. [Google Scholar] [CrossRef]
- Pan, H.; Lu, Y.; Xiu, C.; Geng, H.; Cai, X.; Sun, X.; Zhang, Y.; Williams, L., III; Wyckhuys, K.A.G.; Wu, K. Volatile fragrances associated with flowers mediate host plant alternation of a polyphagous mirid bug. Sci. Rep. 2015, 5, 14805. [Google Scholar] [CrossRef]
- Olsson, S.B.; Hansson, B.S. Electroantennogram and Single Sensillum Recording in insect antennae. In Pheromone Signaling: Methods and Protocols; Touhara, K., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 157–177. ISBN 978-1-62703-619-1. [Google Scholar]
- Pellegrino, M.; Nakagawa, T.; Vosshall, L.B. Single Sensillum recordings in the insects Drosophila melanogaster and Anopheles gambiae. J. Vis. Exp. 2010, 36, e1725. [Google Scholar]
- Li, H.; You, Y.; Zhang, L. Single sensillum recordings for locust palp Sensilla Basiconica. J. Vis. Exp. 2018, 136, e57863. [Google Scholar] [CrossRef]
- Liu, F.; Liu, N. Using single sensillum recording to detect olfactory neuron responses of bed bugs to semiochemicals. J. Vis. Exp. 2016, 107, e53337. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.L.; Oh, H.W.; Park, K.C. Antennal sensillum morphology and electrophysiological responses of olfactory receptor neurons in trichoid sensilla of the diamondback moth (Lepidoptera: Plutellidae). Fla. Entomol. 2016, 99, 146–158. [Google Scholar] [CrossRef]
- Yao, C.A. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 2005, 25, 8359–8367. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Guo, M.; Wang, B.; Liu, Y.; Dong, S.; Wang, G. Sensillar expression and responses of olfactory receptors reveal different peripheral coding in two Helicoverpa species using the same pheromone components. Sci. Rep. 2016, 6, 18742. [Google Scholar] [CrossRef] [PubMed]
- Getz, W.M.; Akers, R.P. Olfactory response characteristics and tuning structure of placodes in the honey bee Apis mellifera L. Apidologie 1993, 24, 195–217. [Google Scholar] [CrossRef]
- Clyne, P.; Grant, A.; O’Connell, R.; Carlson, J.R. Odorant response of individual sensilla on the Drosophila antenna. Invertebr. Neurosci. 1997, 3, 127–135. [Google Scholar] [CrossRef]
- Willis, M.A.; Avondet, J.L.; Zheng, E. The role of vision in odor-plume tracking by walking and flying insects. J. Exp. Biol. 2011, 214, 4121–4132. [Google Scholar] [CrossRef]
- Groot, A.T.; Dekker, T.; Heckel, D.G. The genetic basis of pheromone evolution in moths. Annu. Rev. Entomol. 2016, 61, 99–117. [Google Scholar] [CrossRef]
- Zhang, J.; Walker, W.B.; Wang, G. Pheromone reception in moths: From molecules to behaviors. In Progress in Molecular Biology and Translational Science; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 130, pp. 109–128. [Google Scholar]
- Butenandt, A.; Beckmann, R.; Stamm, D.; Hecker, E. Über den sexuallockstoff den seidenspinners Bombyx mori. Reindarstellung und konstitution. Z. Naturforsch. C 1959, 14, 283–284. [Google Scholar]
- Sakurai, T.; Namiki, S.; Kanzaki, R. Molecular and neural mechanisms of sex pheromone reception and processing in the silkmoth Bombyx mori. Front. Physiol. 2014, 5, 125. [Google Scholar] [CrossRef]
- Namiki, S.; Kanzaki, R. The neurobiological basis of orientation in insects: insights from the silkmoth mating dance. Curr. Opin. Insect Sci. 2016, 15, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Grünbaum, D.; Willis, M.A. Spatial memory-based behaviors for locating sources of odor plumes. Mov. Ecol. 2015, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Cardé, R.T.; Willis, M.A. Navigational strategies used by insects to find distant, wind-borne sources of odor. J. Chem. Ecol. 2008, 34, 854–866. [Google Scholar] [CrossRef]
- Calatayud, P.-A.; Ahuya, P.; Le Ru, B. Importance of the experimental setup in research on attractiveness of odours in moths: An example with Busseola fusca. Entomol. Exp. Appl. 2014, 152, 72–76. [Google Scholar] [CrossRef]
- Carnohan, L.P.; Kaufman, P.E.; Allan, S.A.; Gezan, S.A.; Weeks, E.N.I. Laboratory and field evaluation of brown dog tick behavioral responses to potential semiochemicals. Ticks Tick. Borne Dis. 2017, 8, 226–234. [Google Scholar] [CrossRef]
- Castro, A.M.; Tapias, J.; Ortiz, A.; Benavides, P.; Góngora, C.E. Identification of attractant and repellent plants to coffee berry borer, Hypothenemus hampei. Entomol. Exp. Appl. 2017, 164, 120–130. [Google Scholar] [CrossRef]
- Najar-Rodriguez, A.J.; Galizia, C.G.; Stierle, J.; Dorn, S. Behavioral and neurophysiological responses of an insect to changing ratios of constituents in host plant-derived volatile mixtures. J. Exp. Biol. 2010, 213, 3388–3397. [Google Scholar] [CrossRef]
- Torres, M.F.; Sanchez, A. Neotropical ant-plant Triplaris americana attracts Pseudomyrmex mordax ant queens during seedling stages. Insectes Soc. 2017, 64, 255–261. [Google Scholar] [CrossRef]
- Kepler, R.M.; Bruck, D.J. Examination of the interaction between the black vine weevil (Coleoptera: Curculionidae) and an entomopathogenic fungus reveals a new tritrophic interaction. Environ. Entomol. 2006, 35, 1021–1029. [Google Scholar] [CrossRef]
- Quiroz, A.; Ortega, F.; Ramirez, C.C.; Wadhams, L.J.; Pinilla, K. Response of the beetle Hylastinus obscurus Marsham (Coleoptera: Scolytidae) to red clover (Trifolium pratense L.) volatiles in a laboratory olfactometer. Environ. Entomol. 2005, 34, 690–695. [Google Scholar] [CrossRef]
- Fan, J.; Xue, W.; Duan, H.; Jiang, X.; Zhang, Y.; Yu, W.; Jiang, S.; Sun, J.; Chen, J. Identification of an intraspecific alarm pheromone and two conserved odorant-binding proteins associated with (E)-β-farnesene perception in aphid Rhopalosiphum padi. J. Insect Physiol. 2017, 101, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, F.; Vidoni, F.; Frilli, F. Semiochemicals affecting the host-related behaviour of the dry bean beetle Acanthoscelides obtectus (Say). J. Stored Prod. Res. 2008, 44, 108–114. [Google Scholar] [CrossRef]
- Ponzio, C.; Weldegergis, B.T.; Dicke, M.; Gols, R. Compatible and incompatible pathogen-plant interactions differentially affect plant volatile emissions and the attraction of parasitoid wasps. Funct. Ecol. 2016, 30, 1779–1789. [Google Scholar] [CrossRef]
- Vargas, R.R.; Troncoso, A.J.; Tapia, D.H.; Olivares-Donoso, R.; Niemeyer, H.M. Behavioural differences during host selection between alate virginoparae of generalist and tobacco-specialist Myzus Persicae. Entomol. Exp. Appl. 2005, 116, 43–53. [Google Scholar] [CrossRef]
- Badeke, E.; Haverkamp, A.; Hansson, B.S.; Sachse, S. A challenge for a male noctuid moth? Discerning the female sex pheromone against the background of plant volatiles. Front. Physiol. 2016, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.D.; Cardé, R.T. Male pheromone blend preference function measured in choice and no-choice wind tunnel trials with almond moths, Cadra cautella. Anim. Behav. 2008, 75, 259–266. [Google Scholar] [CrossRef]
- Curkovic, T.; Brunner, J.F. Evaluation of permethrin for attracticide development against Choristoneura rosaceana and Pandemis pyrusana (Lepidoptera: Tortricidae) males. Crop Prot. 2006, 25, 973–976. [Google Scholar] [CrossRef]
- Dalen, M.; Knudsen, G.K.; Norli, H.R.; Thöming, G. Sources of volatiles mediating host location behaviour of Glypta haesitator, a larval parasitoid of Cydia nigricana. Biol. Control 2015, 90, 128–140. [Google Scholar] [CrossRef]
- Withers, T.M.; Mansfield, S. Choice or no-choice tests? Effects of experimental design on the expression of host range. In Proceedings of the Second International Symposium on Biological Control of Arthropods, Davos, Switzerland, 12–16 September 2005; Volume 2, pp. 620–633.
- Murray, T.J.; Withers, T.M.; Mansfield, S. Choice versus no-choice test interpretation and the role of biology and behavior in parasitoid host specificity tests. Biol. Control 2010, 52, 153–159. [Google Scholar] [CrossRef]
- Kłyś, M.; Malejky, N.; Nowak-Chmura, M. The repellent effect of plants and their active substances against the beetle storage pests. J. Stored Prod. Res. 2017, 74, 66–77. [Google Scholar] [CrossRef]
- Nenaah, G.E. Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst). Ind. Crops Prod. 2014, 53, 252–260. [Google Scholar] [CrossRef]
- Bossou, A.D.; Ahoussi, E.; Ruysbergh, E.; Adams, A.; Smagghe, G.; De Kimpe, N.; Avlessi, F.; Sohounhloue, D.C.K.; Mangelinckx, S. Characterization of volatile compounds from three Cymbopogon species and Eucalyptus citriodora from Benin and their insecticidal activities against Tribolium castaneum. Ind. Crops Prod. 2015, 76, 306–317. [Google Scholar] [CrossRef]
- Benelli, G.; Giunti, G.; Messing, R.H.; Wright, M.G. Visual and olfactory female-borne cues evoke male courtship in the aphid parasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). J. Insect Behav. 2013, 26, 695–707. [Google Scholar] [CrossRef]
- Benelli, G.; Revadi, S.; Carpita, A.; Giunti, G.; Raspi, A.; Anfora, G.; Canale, A. Behavioral and electrophysiological responses of the parasitic wasp Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae) to Ceratitis capitata-induced fruit volatiles. Biol. Control 2013, 64, 116–124. [Google Scholar] [CrossRef]
- Green, P.W.C. Insect-derived compounds affect the behaviour of Liposcelis bostrychophila: Effects of combination and structure. J. Stored Prod. Res. 2011, 47, 262–266. [Google Scholar] [CrossRef]
- Adams, T.F.; Wongchai, C.; Chaidee, A.; Pfeiffer, W. “Singing in the Tube”—audiovisual assay of plant oil repellent activity against mosquitoes (Culex pipiens). Parasitol. Res. 2016, 115, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Brezolin, A.N.; Martinazzo, J.; Muenchen, D.K.; de Cezaro, A.M.; Rigo, A.A.; Steffens, C.; Steffens, J.; Blassioli-Moraes, M.C.; Borges, M. Tools for detecting insect semiochemicals: a review. Anal. Bioanal. Chem. 2018, 410, 4091–4108. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yao, Y.; Zhang, Q.; Zhang, D.; Zhuang, S.; Li, H.; Liu, Q. Olfactory biosensor for insect semiochemicals analysis by impedance sensing of odorant-binding proteins on interdigitated electrodes. Biosens. Bioelectron. 2015, 67, 662–669. [Google Scholar] [CrossRef]
- Brito, N.F.; Moreira, M.F.; Melo, A.C.A. A look inside odorant-binding proteins in insect chemoreception. J. Insect Physiol. 2016, 95, 51–65. [Google Scholar] [CrossRef]
- Leal, W.S. Reverse chemical ecology at the service of conservation biology. Proc. Natl. Acad. Sci. USA 2017, 114, 12094–12096. [Google Scholar] [CrossRef]
- Leal, W.S.; Barbosa, R.M.R.; Xu, W.; Ishida, Y.; Syed, Z.; Latte, N.; Chen, A.M.; Morgan, T.I.; Cornel, A.J.; Furtado, A. Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. PLoS ONE 2008, 3, e3045. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.-J.; Zhou, W.-W.; Yu, H.-Z.; Mao, C.-G.; Zhang, C.-X.; Cheng, J.A.; Zhu, Z.-R. Cloning, expression and functional analysis of a general odorant-binding protein 2 gene of the rice striped stem borer, Chilo suppressalis (Walker) (Lepidoptera: Pyralidae). Insect Mol. Biol. 2009, 18, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-L.; He, P.; Zhang, L.; Fang, S.-Q.; Dong, S.-L.; Zhang, Y.-J.; Li, F. Large-scale identification of odorant-binding proteins and chemosensory proteins from expressed sequence tags in insects. BMC Genom. 2009, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, K.P.D.; Kempraj, V.; Aurade, R.M.; Roy, T.K.; Shivashankara, K.S.; Verghese, A. Computational reverse chemical ecology: virtual screening and predicting behaviorally active semiochemicals for Bactrocera dorsalis. BMC Genom. 2014, 15, 209. [Google Scholar]
- Sanes, J.T.; Plettner, E. Gypsy moth pheromone-binding protein-ligand interactions: pH profiles and simulations as tools for detecting polar interactions. Arch. Biochem. Biophys. 2016, 606, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Nardella, J.; Terrado, M.; Honson, N.S.; Plettner, E. Endogenous fatty acids in olfactory hairs influence pheromone binding protein structure and function in Lymantria dispar. Arch. Biochem. Biophys. 2015, 579, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L. Proteomic identification of a potential sex biomarker for 2 fruit fly species at pupal stage. J. Asia Pac. Entomol. 2017, 20, 125–131. [Google Scholar] [CrossRef]
- Zhu, J.; Pelosi, P.; Liu, Y.; Lin, K.; Yuan, H.; Wang, G. Ligand-binding properties of three odorant-binding proteins of the diamondback moth Plutella xylostella. J. Integr. Agric. 2016, 15, 580–590. [Google Scholar] [CrossRef]
- Li, H.-L.; Ni, C.-X.; Tan, J.; Zhang, L.-Y.; Hu, F.-L. Chemosensory proteins of the eastern honeybee, Apis cerana: Identification, tissue distribution and olfactory related functional characterization. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 194, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, J.; Song, X.; Wu, F.; Tang, M.; Hua, Q.; Zheng, H.; Hu, F. Sublethal doses of neonicotinoid imidacloprid can interact with honey bee chemosensory protein 1 (CSP1) and inhibit its function. Biochem. Biophys. Res. Commun. 2017, 486, 391–397. [Google Scholar] [CrossRef]
- Li, H.; Wu, F.; Zhao, L.; Tan, J.; Jiang, H.; Hu, F. Neonicotinoid insecticide interact with honeybee odorant-binding protein: Implication for olfactory dysfunction. Int. J. Biol. Macromol. 2015, 81, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, H.; Zhuang, S.; Zhang, D.; Zhang, Q.; Zhou, J.; Dong, S.; Liu, Q.; Wang, P. Olfactory biosensor using odorant-binding proteins from honeybee: Ligands of floral odors and pheromones detection by electrochemical impedance. Sens. Actuators B Chem. 2014, 193, 420–427. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, H.; Li, H.; Zhang, J.; Zhuang, S.; Zhang, F.; Jimmy Hsia, K.; Wang, P. Impedance sensing and molecular modeling of an olfactory biosensor based on chemosensory proteins of honeybee. Biosens. Bioelectron. 2013, 40, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Dudzik, D.; Barbas-Bernardos, C.; García, A.; Barbas, C. Quality assurance procedures for mass spectrometry untargeted metabolomics. A review. J. Pharm. Biomed. Anal. 2018, 147, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, T.S. From design to analysis: Effective statistical approaches for host range testing. In Proceedings of the Second International Symposium of Biological Control of Arthropod, Davos, Switzerland, 12–16 September, 2005; pp. 672–682.
- Pilipavičius, V.; Lazauskas, P. Optimal number of observation, treatment and replication in field experiments. Afr. J. Agric. Res. 2012, 7, 4368–4377. [Google Scholar] [CrossRef]
- Ramírez, C.C.; Fuentes-Contreras, E.; Rodríguez, L.C.; Niemeyer, H.M. Pseudoreplication and its frequency in olfactometric laboratory studies. J. Chem. Ecol. 2000, 26, 1423–1431. [Google Scholar] [CrossRef]
- Wajnberg, E.; Haccou, P. Statistical tools for analyzing data on behavioral ecology of insect parasitoids. In Behavioral Ecology of Insect Parasitoids; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 402–429. [Google Scholar]
- Thiery, D.; Visser, J.H. Misleading the Colorado potato beetle with an odor blend. J. Chem. Ecol. 1987, 13, 1139–1146. [Google Scholar] [CrossRef]
- Rivest, L.; Duchesne, T. A general angular regression model for the analysis. Appl. Stat. 2016, 65, 445–463. [Google Scholar]
- McCullagh, P.; Nelder, J.A. An outline of generalized linear models. In Generalized Linear Models; McCullagh, P., Ed.; Chapmann & Hall/CRC: New York, NY, USA; Routledge: Abingdon, UK, 1989; pp. 21–48. [Google Scholar]
- Poline, J.-B.; Brett, M. The general linear model and fMRI: Does love last forever? Neuroimage 2012, 62, 871–880. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Parastar, H. Recent trends in application of multivariate curve resolution approaches for improving gas chromatography–mass spectrometry analysis of essential oils. Talanta 2011, 85, 835–849. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. The analytical process to search for metabolomics biomarkers. J. Pharm. Biomed. Anal. 2018, 147, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Busto, O. Data fusion methodologies for food and beverage authentication and quality assessment—A review. Anal. Chim. Acta 2015, 891, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Nayak, P.; Mukherjee, A.K.; Pandit, E.; Pradhan, S.K. Application of statistical tools for data analysis and interpretation in rice plant pathology. Rice Sci. 2018, 25, 1–18. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Chapter 13-Spatial analysis. In Numerical Ecology; Legendre, P., Legendre, L.B.T.-D., In, E.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 24, pp. 785–858. ISBN 0167-8892. [Google Scholar]
- Bricchi, I.; Leitner, M.; Foti, M.; Mithöfer, A.; Boland, W.; Maffei, M.E. Robotic mechanical wounding (MecWorm) versus herbivore-induced responses: Early signaling and volatile emission in Lima bean (Phaseolus lunatus L.). Planta 2010, 232, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Ingrao, A.; Ali, J.; Szendrei, Z. Identification of plant semiochemicals and evaluation of their interactions with early spring insect pests of asparagus. J. Plant Interact. 2016, 11, 11–19. [Google Scholar] [CrossRef]
- Byers, K.J.R.P.; Bradshaw, H.D., Jr.; Riffell, J.A. Three floral volatiles contribute to differential pollinator attraction in monkeyflowers (Mimulus). J. Exp. Biol. 2014, 217, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, A.; Johnson, S.D. Floral scents of chafer-pollinated asclepiads and a potential hybrid. S. Afr. J. Bot. 2010, 76, 770–778. [Google Scholar] [CrossRef][Green Version]
- Conchou, L.; Anderson, P.; Birgersson, G. Host plant species differentiation in a polyphagous moth: Olfaction is enough. J. Chem. Ecol. 2017, 43, 794–805. [Google Scholar] [CrossRef]
| Semiochemical | Source | Type | Key Conversions/Reactions | Ref. |
|---|---|---|---|---|
| bombykol | Bombyx mori | Sex pheromone | Ni- and Pd-catalyzed cross coupling reactions | [138] |
| (Z)-15-methyl-7-nonacosene and (Z)-17-methyl-7-hentriacontene | Eurytoma maslovskii | Sex pheromone | Grignard reaction | [139] |
| (3S, 5S, 6S)-tetrahydro-6-isopropyl-3,5-dimethylpyran-2-one | Macrocentrus grandii | Sex pheromone | cationic cyclopropyl-allyl rearrangement, diastereoselective alkylation and diastereoselective hydrogenation | [140] |
| (3Z, 9Z)-cis-6,7-epoxy-3,9-octadecadiene | Ectropis obliqua | Sex pheromone | regioselective dienol epoxidation (sequential ring-opening) | [131] |
| 2E- and 2Z-homofarnesals (6:4 blend) | Callosobruchus chinensis | Sex pheromone | Ando’s Z-selective alkene synthesis | [132] |
| (R)-Lavandulyl propionate | Dysmicoccus grassii | Sex pheromone | Two-cycle enzymatic transesterification of racemic lavandulol using Porcine pancreas lipase | [46] |
| 10, 14-dimethyl-1-pentadecyl isobutyrate | Euproctis pseudoconspersa | Sex pheromone | Evans’ methylation and C–C bond formation by Julia-Kocienski coupling and Wittig olefination | [129] |
| (+)-sitophilure | Sitophilus oryzae L. and Sitophilus zeamais M. | Aggregation pheromone | Enzymatic reduction using S. cerevisiae | [130] |
| (±)-frontalin | Dendroctonus genus | Aggregation pheromone | Double dihydroxylation, mono-cleavage, and acid-catalyzed intramolecular acetalation | [141] |
| 4, 8-dimethyldecanal (four stereoisomers) | Tribolium castaneum | Aggregation pheromone | Organolithium-mediated reaction | [142] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa-Cornelio, R.; Cantor, F.; Coy-Barrera, E.; Rodríguez, D. Tools in the Investigation of Volatile Semiochemicals on Insects: From Sampling to Statistical Analysis. Insects 2019, 10, 241. https://doi.org/10.3390/insects10080241
Barbosa-Cornelio R, Cantor F, Coy-Barrera E, Rodríguez D. Tools in the Investigation of Volatile Semiochemicals on Insects: From Sampling to Statistical Analysis. Insects. 2019; 10(8):241. https://doi.org/10.3390/insects10080241
Chicago/Turabian StyleBarbosa-Cornelio, Ricardo, Fernando Cantor, Ericsson Coy-Barrera, and Daniel Rodríguez. 2019. "Tools in the Investigation of Volatile Semiochemicals on Insects: From Sampling to Statistical Analysis" Insects 10, no. 8: 241. https://doi.org/10.3390/insects10080241
APA StyleBarbosa-Cornelio, R., Cantor, F., Coy-Barrera, E., & Rodríguez, D. (2019). Tools in the Investigation of Volatile Semiochemicals on Insects: From Sampling to Statistical Analysis. Insects, 10(8), 241. https://doi.org/10.3390/insects10080241