Host Associations of Culex (Melanoconion) atratus (Diptera: Culicidae) and Culex (Melanoconion) pilosus from Florida, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling Protocol
2.2. Blood Meal Analysis
2.3. Statistical Analysis
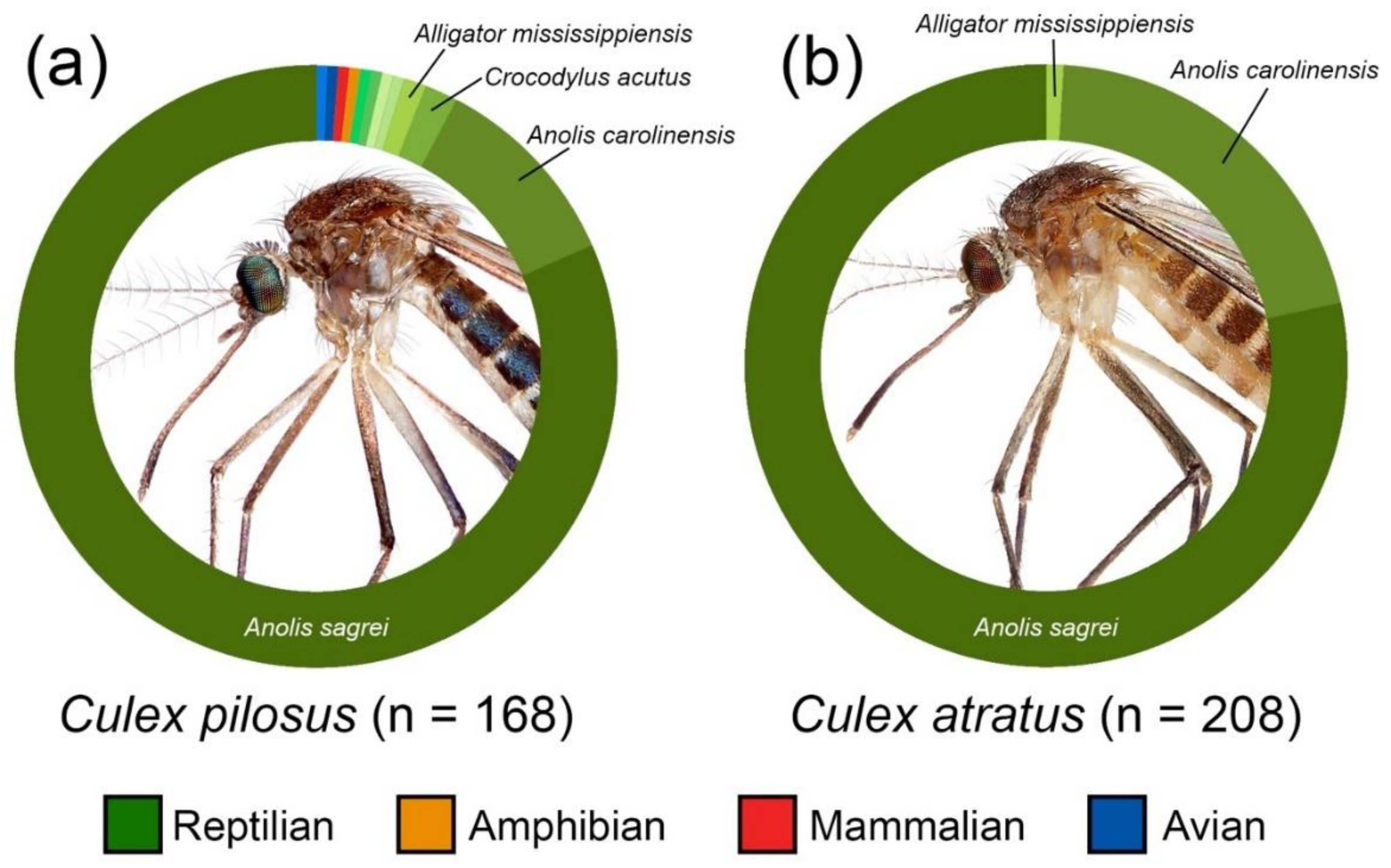
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torres-Gutierrez, C.; Sallum, M.A.B. Catalog of the subgenus Melanoconion of Culex (Diptera: Culicidae) for South America. Zootaxa 2014, 4028, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Darsie, R.F.; Ward, R.A. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico; University Press of Florida: Gainesville, FL, USA, 2005; p. 384. [Google Scholar]
- Blosser, E.M.; Burkett-Cadena, N.D. Culex (Melanoconion) panocossa from peninsular Florida, USA. Acta Trop. 2017, 167, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Bingham, A.M.; Burkett-Cadena, N.D.; Hassan, H.K.; Unnasch, T.R. Vector competence and capacity of Culex erraticus (Diptera: Culicidae) for eastern equine encephalitis virus in the southeastern United States. J. Med. Entomol. 2015, 53, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Vittor, A.Y.; Armien, B.; Gonzalez, P.; Carrera, J.-P.; Dominguez, C.; Valderrama, A.; Glass, G.E.; Beltran, D.; Cisneros, J.; Wang, E.; et al. Epidemiology of emergent Madariaga encephalitis in a region with endemic Venezuelan equine encephalitis: Initial host studies and human cross-sectional study in Darien, Panama. PLoS Negl. Trop. Dis. 2016, 10, e0004554. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Ferro, C.; Barrera, R.; Boshell, J.; Navarro, J.C. Venezuelan equine encephalitis. Annu. Rev. Entomol. 2004, 49, 141–174. [Google Scholar] [CrossRef]
- Coffey, L.L.; Weaver, S.C. Susceptibility of Ochlerotatus taeniorhynchus and Culex nigripalpus for Everglades virus. Am. J. Trop. Med. Hyg. 2005, 73, 11–16. [Google Scholar] [CrossRef]
- Harbach, R.E. Classification within the cosmopolitan genus Culex (Diptera: Culicidae): The foundation for molecular systematics and phylogenetic research. Acta Trop. 2011, 120, 1–14. [Google Scholar] [CrossRef]
- Lane, J. Neotropical Culicidae, Vols. I and II; University of São Paulo: São Paulo, Brazil, 1953; p. 112. [Google Scholar]
- Belkin, J.N.; Heinemann, S.J.; Page, W.A. The Culicidae of Jamaica (Mosquito studies XXI). Contrib. Am. Entomol. Inst. 1970, 6, 1–319. [Google Scholar]
- Pecor, J.E.; Mallampalli, V.L.; Harbach, R.E.; Peyton, E.L. Catalog and illustrated review of the subgenus Melanoconion of Culex (Diptera: Culicidae). Contrib. Am. Entomol. Inst. 1992, 27, 1–234. [Google Scholar]
- Darsie Jr., R.F.; Morris, C.D. Keys to the Adult Females and Fourth Instar Larvae of the Mosquitoes of Florida (Diptera, Culicidae); Florida Mosquito Control Association: Lehigh Acres, FL, USA, 2003; p. 156. [Google Scholar]
- Urcola, J.I.; Fischer, S. First record and larval habitat description of Culex (Melanoconion) pilosus from Buenos Aires Province, Argentina. J. Am. Mosq. Control Assoc. 2015, 31, 271–274. [Google Scholar] [CrossRef]
- Grabham, M. Notes on some new mosquitoes from Jamaica, West Indies. Can. Entomol. 1907, 39, 25–26. [Google Scholar] [CrossRef]
- Hill, R.B.; Hill, C.M. The mosquitoes of Jamaica. Bull. Inst. Jam. 1948, 4, 1–60. [Google Scholar]
- Page, W.A. Observations on man-biting mosquitoes in Jamaica. In Proceedings of the Royal Entomological Society of London; Series A, General Entomology. Blackwell Publishing Ltd.: Oxford, UK, 1967; Volume 42, pp. 180–186. [Google Scholar]
- Edman, J.D. Host-feeding patterns of Florida mosquitoes (Diptera: Culicidae) VI. Culex (Melanoconion). J. Med. Entomol. 1979, 15, 521–525. [Google Scholar] [CrossRef]
- Chamberlain, R.W.; Sudia, W.D.; Coleman, P.H.; Work, T.H. Venezuelan equine encephalitis virus from south Florida. Science 1964, 17, 272–274. [Google Scholar] [CrossRef]
- Chamberlain, R.W.; Sudia, W.D.; Work, T.H.; Coleman, P.H.; Newhouse, V.F.; Johnston, J.G., Jr. Arbovirus studies in south Florida, with emphasis on Venezuelan equine encephalomyelitis virus. Am. J. Epidemiol. 1969, 89, 197–210. [Google Scholar] [CrossRef]
- Williams, M.R.; Savage, H.M. Identification of Culex (Melanoconion) species of the United States using female cibarial armature (Diptera: Culicidae). J. Med. Entomol. 2009, 46, 745–752. [Google Scholar] [CrossRef]
- Williams, M.R.; Savage, H.M. Development of multiplexed species specific polymerase chain reaction assays for identification of the Culex (Melanoconion) species (Diptera: Culicidae) of the southeastern United States based on rDNA. J. Med. Entomol. 2011, 48, 961–966. [Google Scholar] [CrossRef]
- Cupp, E.W.; Stokes, G.M. Identification of blood meals from mosquitoes collected in light traps and dog baited traps. Mosq. News 1973, 33, 39–41. [Google Scholar]
- Reeves, L.E.; Krysko, K.L.; Avery, M.L.; Gillett-Kaufman, J.L.; Kawahara, A.Y.; Connelly, C.R.; Kaufman, P.E. Interactions between the invasive Burmese python, Python bivittatus Kuhl, and the local mosquito community in Florida, USA. PLoS ONE 2018, 13, e0190633. [Google Scholar] [CrossRef]
- Burkett-Cadena, N.D. A wire-frame shelter for collecting resting mosquitoes. J. Am. Mosq. Control Assoc. 2011, 27, 153–155. [Google Scholar] [CrossRef]
- Burkett-Cadena, N.D.; Hoyer, I.; Blosser, E.; Reeves, L.E. Human-powered pop-up resting shelter for sampling cavity-resting mosquitoes. Acta Trop. 2019, 190, 288–292. [Google Scholar] [CrossRef]
- Bingham, A.M.; Burkett-Cadena, N.D.; Hassan, H.K.; McClure, C.J.W.; Unnasch, T.R. Field investigations of winter transmission of Eastern equine encephalitis virus in Florida. Am. J. Trop. Med. Hyg. 2014, 91, 685–693. [Google Scholar] [CrossRef]
- Reeves, L.E.; Holderman, C.J.; Gillett-Kaufman, J.L.; Kawahara, A.Y.; Kaufman, P.E. Maintenance of host DNA integrity in field-preserved mosquito (Diptera: Culicidae) blood meals for identification by DNA barcoding. Parasit. Vectors 2016, 9, 503. [Google Scholar] [CrossRef]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Truett, A.A.; Walker, J.A.; Warman, M.L. Preparation of PCR quality mouse genomic DNA with sodium hydroxide and Tris (HotSHOT). Biotechniques 2000, 29, 52–54. [Google Scholar] [CrossRef]
- Blosser, E.M.; Stenn, T.; Acevedo, C.; Burkett-Cadena, N.D. Host use and seasonality of Culex (Melanoconion) iolambdis (Diptera: Culicidae) from eastern Florida, USA. Acta Trop. 2016, 164, 352–359. [Google Scholar] [CrossRef]
- Hoyer, I.J.; Blosser, E.M.; Acevedo, C.; Thompson, A.C.; Reeves, L.E.; Burkett-Cadena, N.D. Mammal decline, linked to invasive Burmese python, shifts host use of vector mosquito towards reservoir hosts of a zoonotic disease. Biol. Lett. 2017, 13, 20170353. [Google Scholar] [CrossRef]
- Hoyer, I.J.; Acevedo, C.; Wiggins, K.; Alto, B.W.; Burkett-Cadena, N.D. Patterns of abundance, host use, and Everglades virus infection in Culex (Melanoconion) cedecei mosquitoes, Florida, USA. Emerg. Infect. Dis. 2019, 25, 1093–1100. [Google Scholar] [CrossRef]
- Reeves, L.E.; Gillett-Kaufman, J.L.; Kawahara, A.Y.; Kaufman, P.E. Barcoding blood meals: New vertebrate-specific primer sets for assigning taxonomic identities to host DNA from mosquito blood meals. PLoS Negl. Trop. Dis. 2018, 12, e0006767. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical Methods for Research Workers; Oliver and Boyd: Edinburgh, UK, 1932. [Google Scholar]
- Ogo, S.H.; Bernardes, C.F.; Glass, M.L.; Torsoni, M.A.; Vercesi, A.E. Functional mitochondria in snake Bothrops alternus erythrocytes and modulation of HbO2 affinity by mitochondrial ATP. J. Comp. Physiol. B 1993, 163, 614–619. [Google Scholar] [CrossRef]
- Krysko, K.L.; Enge, K.M.; Moler, P.E. Atlas of Amphibians and Reptiles in Florida; Florida Fish and Wildlife Conservation Commission: Tallahassee, FL, USA, 2011; p. 524. [Google Scholar]
- Stroud, J.T.; Richardson, A.J.; Sim, J.; Airnes, A. Onward to the Mid-Atlantic: First records of Cuban brown anoles (Anolis sagrei) on Ascension Island. Reptiles Amphib. 2018, 25, 220–222. [Google Scholar]
- Branch, N.; Logan, L.; Beck, E.C.; Mulrennan, J.A. New distributional records for Florida mosquitoes. Fla. Entomol. 1958, 41, 155–163. [Google Scholar] [CrossRef]
- Campbell-Staton, S.C.; Goodman, R.M.; Backstrom, N.; Edwards, S.V.; Losos, J.B.; Kolbe, J.J. Out of Florida: mtDNA reveals patterns of migration and Pleistocene range expansion of the green anole lizard (Anolis carolinensis). Ecol. Evol. 2012, 2, 2274–2284. [Google Scholar] [CrossRef]
- Kamanth, A.; Stuart, Y.E.; Campbell, T.S. Behavioral partitioning by the native lizard Anolis carolinensis in the presence and absence of the invasive Anolis sagrei in Florida. Breviora 2013, 535, 1–10. [Google Scholar] [CrossRef]
- Edwards, J.R.; Lailvaux, S.P. Display behavior and habitat use in single and mixed populations of Anolis carolinensis and Anolis sagrei lizards. Ethology 2012, 118, 494–502. [Google Scholar] [CrossRef]
- Stuart, Y.E.; Campbell, T.S.; Hohenlohe, P.A.; Reynolds, R.G.; Revell, L.J.; Losos, J.B. Rapid evolution of a native species following invasion by a congener. Science 2014, 346, 463–466. [Google Scholar] [CrossRef]
- Christensen, H.A.; De Vasquez, A.M.; Boreham, M.M. Host-feeding patterns of mosquitoes (Diptera: Culicidae) from central Panama. Am. J. Trop. Med. Hyg. 1996, 55, 202–208. [Google Scholar] [CrossRef]
- Savage, H.M.; Aggarwal, D.; Apperson, C.S.; Katholi, C.R.; Gordon, E.; Hassan, H.K.; Anderson, M.; Charnetzky, D.; McMillen, L.; Unnasch, E.A.; et al. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens Complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector Borne Zoonotic Dis. 2007, 7, 365–386. [Google Scholar] [CrossRef]
- Burkett-Cadena, N.D.; Graham, S.P.; Hassan, H.K.; Guyer, C.; Eubanks, M.D.; Katholi, C.R.; Unnasch, T.R. Blood feeding patterns of potential arbovirus vectors of the genus Culex targeting ectothermic hosts. Am. J. Trop. Med. Hyg. 2008, 79, 809–815. [Google Scholar] [CrossRef]
- Tempelis, C.H.; Galindo, P. Host-feeding patterns of Culex (Melanoconion) and Culex (Aedinus) mosquitoes collected in Panama. J. Med. Entomol. 1975, 12, 205–209. [Google Scholar] [CrossRef]
- Cupp, E.W.; Zhang, D.; Yue, X.; Cupp, M.S.; Guyer, C.; Sprenger, T.R.; Unnasch, T.R. Identification of reptilian and amphibian blood meals from mosquitoes in an eastern equine encephalomyelitis virus focus in central Alabama. Am. J. Trop. Med. Hyg. 2004, 71, 272–276. [Google Scholar] [CrossRef]
- Perkins, S.L.; Rothschild, A.; Waltari, E. Infections of the malaria parasite, Plasmodium floridense, in the invasive lizard, Anolis Sagrei, in Florida. J. Herpetol. 2007, 41, 750–754. [Google Scholar] [CrossRef]
- Doan, T.M.; Devlin, B.G.; Greene, K.C. Malaria infection is lower in invasive anoles than native anoles in Central Florida, USA. J. Herpetol. 2019, 53, 22–26. [Google Scholar] [CrossRef]
- Klein, T.A.; Akin, D.C.; Young, D.G.; Telford, S.R., Jr. Sporogeny, development and ultrastructure of Plasmodium floridense in Culex erraticus. Int. J. Parasitol. 1988, 18, 711–719. [Google Scholar] [CrossRef]
- Cohen, S.B.; Lewoczko, K.; Huddleston, D.B.; Moody, E.; Mukherjee, S.; Dunn, J.R.; Jones, T.F.; Wilson, R.; Moncayo, A.C. Host feeding patterns of potential vectors of eastern equine encephalitis virus at an epizootic focus in Tennessee. Am. J. Trop. Med. Hyg. 2009, 81, 452–456. [Google Scholar] [CrossRef]


| Nearest Landmark | Habitat | Coordinates | Culex atratus | Culex pilosus |
|---|---|---|---|---|
| C-111E Canal | Hardwood hammock | 25.407291, −80.523353 | 0 | 2 |
| Anhinga Trail | Hardwood hammock | 25.402178, −80.615617 | 31 | 85 |
| Long Pine Key | Pine rocklands | 25.399878, −80.659990 | 27 | 65 |
| Pa-Hay-Okee | Sawgrass prairie | 25.412619, −80.782649 | 11 | 15 |
| Mahogany Hammock | Hardwood hammock | 25.338870, −80.818099 | 82 | 34 |
| Nine-Mile Pond | Mangrove | 25.253915, −80.798166 | 24 | 21 |
| Snake Bight | Mangrove | 25.200773, −80.874253 | 43 | 12 |
| Buttonwood Canal | Coastal prairie | 25.148854, −80.923344 | 41 | 11 |
| Flamingo | Coastal prairie | 25.144328, −80.921075 | 1 | 3 |
| TOTAL | 260 | 248 |
| Primer Name | Gene | Primer Sequence | Amplicon Size (bp) | Reference |
|---|---|---|---|---|
| VertCOI_7194_F | COI | 5′-CGM ATR AAY AAY ATR AGC TTC TGA Y-3′ | 395 | [32] |
| Mod_RepCOI_R | 5′-TTC DGG RTG NCC RAA RAA TCA-3′ | |||
| Mod_RepCOI_F | COI | 5′-TNT TYT CMA CYA ACC ACA AAG A-3′ | 244 | [32] |
| VertCOI_7216_R | 5′-CAR AAG CTY ATG TTR TTY ATD CG-3′ | |||
| Mod_RepCOI_F | COI | 5′-TNT TYT CMA CYA ACC ACA AAG A-3′ | 664 | [32] |
| Mod_RepCOI_R | 5′-TTC DGG RTG NCC RAA RAA TCA-3′ | |||
| 16L1 | 16s | 5′-CTGACCGTGCAAAGGTAGCGTAATCACT-3′ | 450 | [29,30,31] |
| H3056 | 5′-CTCCGGTCTGAACTCAGATCACGTAGG-3′ |
| Host Class | Host Species | Culex atratus | Culex pilosus | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Reptilia | Anolis carolinensis | 43 | 20.6 | 18 | 10.7 |
| Reptilia | Anolis sagrei | 163 | 78 | 137 | 81.5 |
| Reptilia | Anolis equestris | 0 | 0 | 1 | 0.6 |
| Reptilia | Coluber constrictor | 0 | 0 | 1 | 0.6 |
| Reptilia | Nerodia fasciata | 0 | 0 | 1 | 0.6 |
| Reptilia | Alligator mississippiensis | 2 | 1 | 2 | 1 |
| Reptilia | Crocodylus acutus | 0 | 0 | 3 | 1.7 |
| Reptilia | Terrapene carolina | 0 | 0 | 1 | 0.6 |
| Amphibia | Hyla cinerea | 0 | 0 | 1 | 0.6 |
| Aves | Cardinalis cardinalis | 0 | 0 | 1 | 0.6 |
| Aves | Nyctanassa violacea | 0 | 0 | 1 | 0.6 |
| Mammalia | Homo sapiens | 0 | 0 | 1 | 0.6 |
| Mixed blood meal | 0 | 0 | 1 | 0.6 | |
| No amplification | 52 | 80 | |||
| Total identified | 208 | 168 | |||
| TOTAL | 260 | 248 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reeves, L.E.; Hoyer, I.; Acevedo, C.; Burkett-Cadena, N.D. Host Associations of Culex (Melanoconion) atratus (Diptera: Culicidae) and Culex (Melanoconion) pilosus from Florida, USA. Insects 2019, 10, 239. https://doi.org/10.3390/insects10080239
Reeves LE, Hoyer I, Acevedo C, Burkett-Cadena ND. Host Associations of Culex (Melanoconion) atratus (Diptera: Culicidae) and Culex (Melanoconion) pilosus from Florida, USA. Insects. 2019; 10(8):239. https://doi.org/10.3390/insects10080239
Chicago/Turabian StyleReeves, Lawrence E., Isaiah Hoyer, Carolina Acevedo, and Nathan D. Burkett-Cadena. 2019. "Host Associations of Culex (Melanoconion) atratus (Diptera: Culicidae) and Culex (Melanoconion) pilosus from Florida, USA" Insects 10, no. 8: 239. https://doi.org/10.3390/insects10080239
APA StyleReeves, L. E., Hoyer, I., Acevedo, C., & Burkett-Cadena, N. D. (2019). Host Associations of Culex (Melanoconion) atratus (Diptera: Culicidae) and Culex (Melanoconion) pilosus from Florida, USA. Insects, 10(8), 239. https://doi.org/10.3390/insects10080239







