Varied Effects of Tending Ant Species on the Development of Facultatively Myrmecophilous Lycaenid Butterfly Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Capturing and Rearing of Study Insects
2.2. Lycaenid Rearing Experiment
2.3. Statistical Analysis
3. Results
3.1. Mass of the Lycaenids in Each Developmental Stage
3.2. Mass Gain and Feces Mass during the Larval Stage
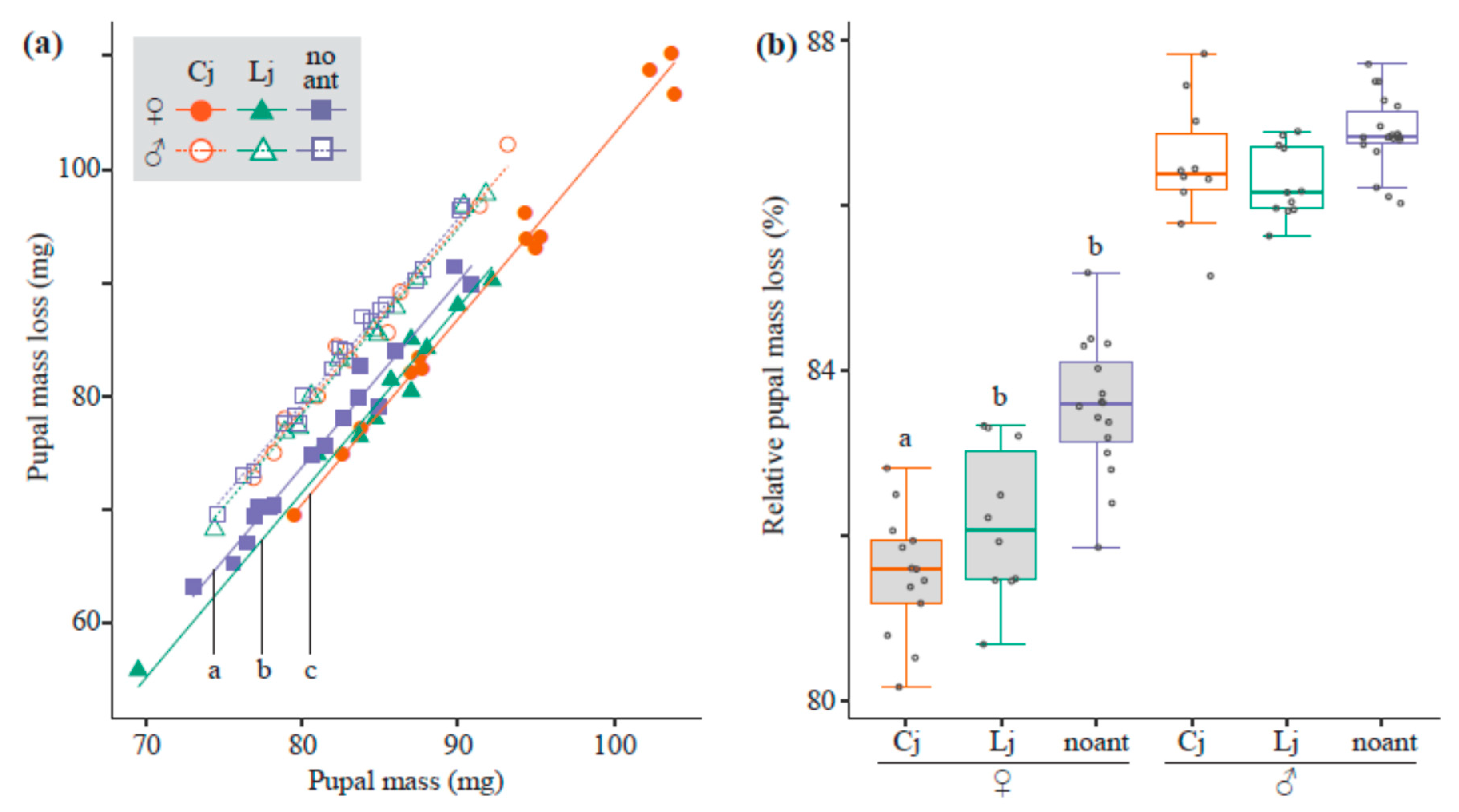
3.3. Mass Loss from Pupae to Adults
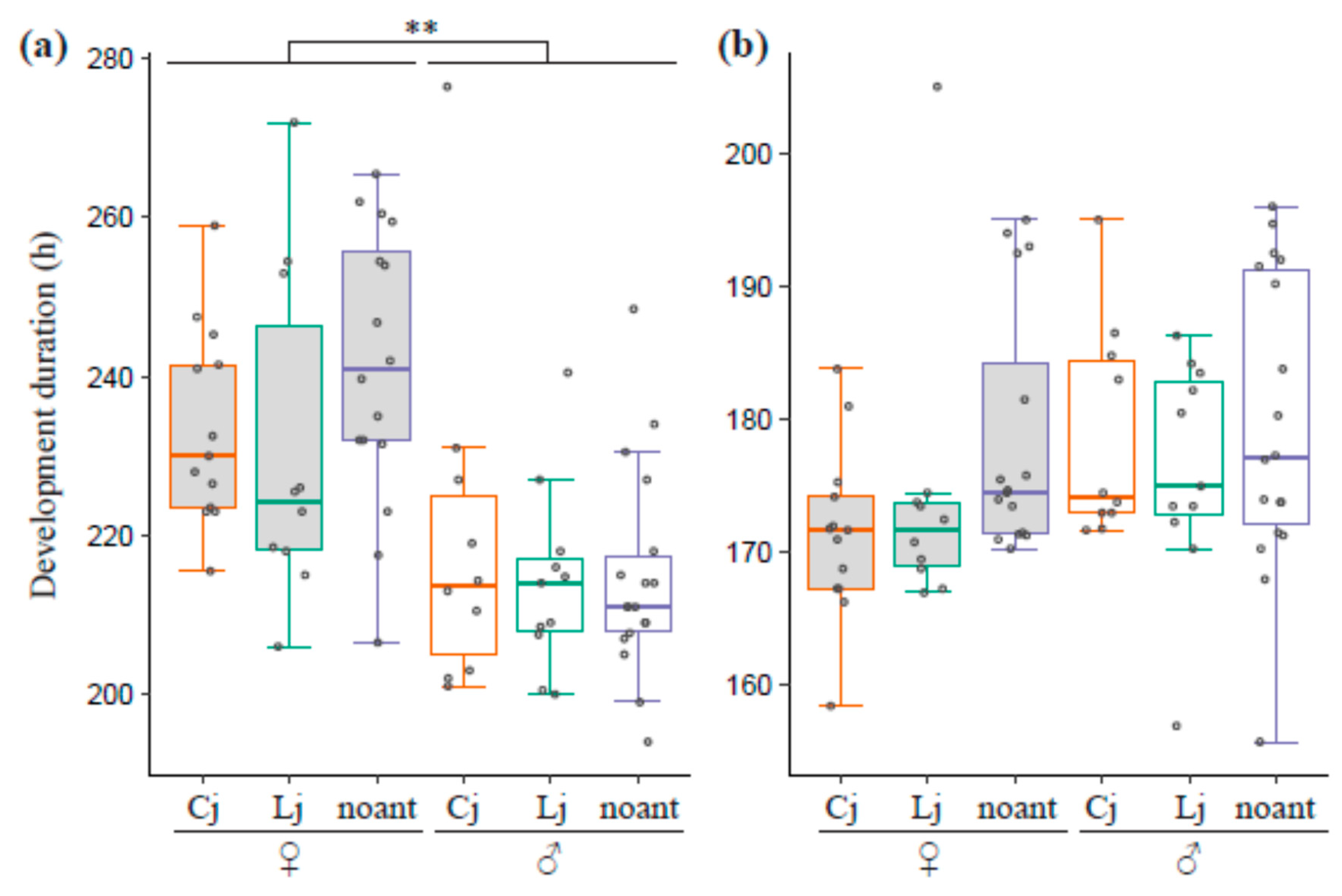
3.4. Developmental Time for Larvae and Pupae
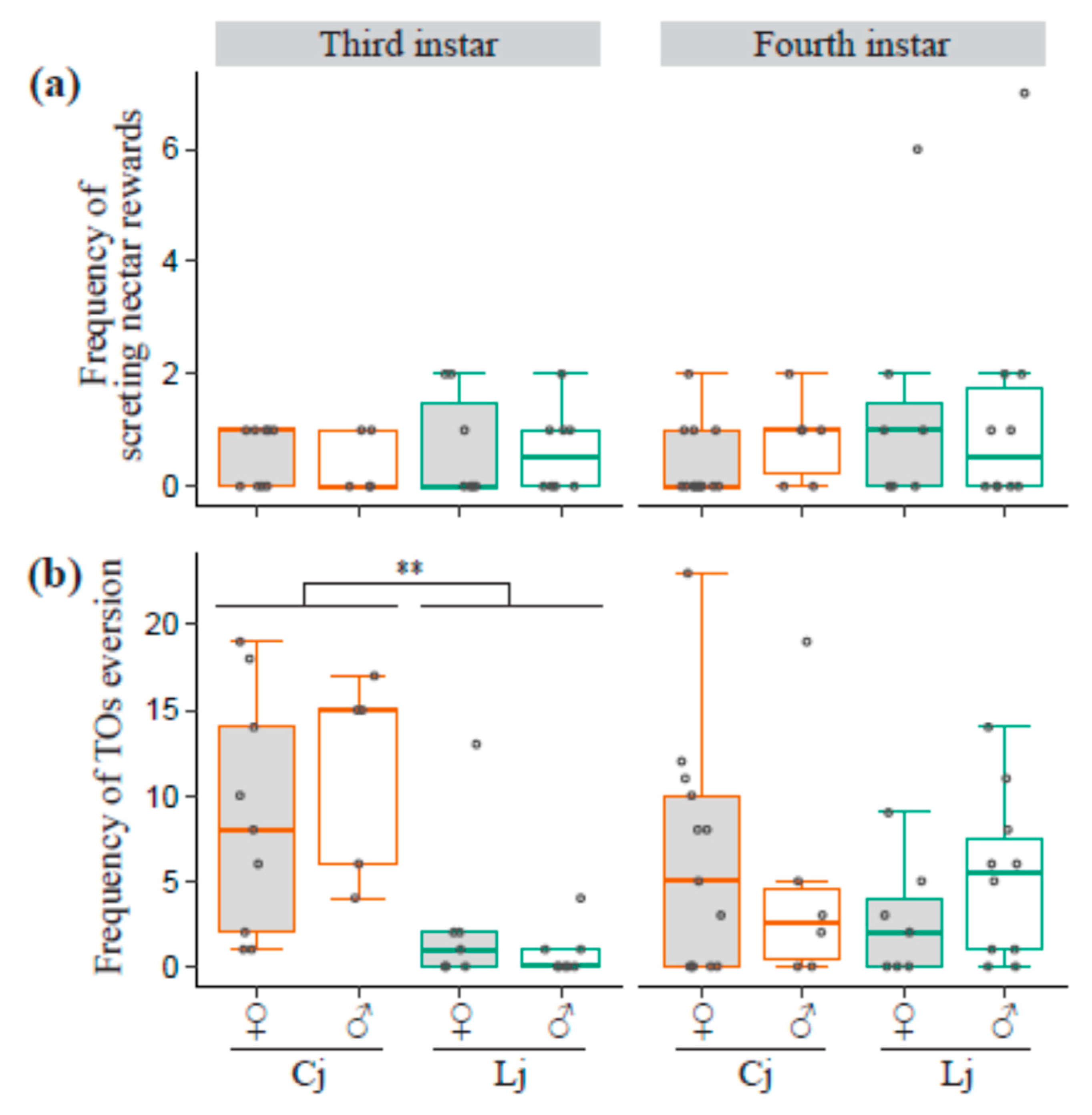
3.5. Frequency of Secreting Reward and TO Eversion
3.6. Number of Tending Ants
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Honěk, A. Intraspecific variation in body size and fecundity in insects: A general relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- García-Barros, E. Body size, egg size, and their interspecific relationships with ecological and life history traits in butterflies (Lepidoptera: Papilionoidea, Hesperioidea). Biol. J. Linn. Soc. 2000, 70, 251–284. [Google Scholar] [CrossRef]
- Jiménez-Pérez, A.; Wang, Q. Sexual Selection in Cnephasia jactatana (Lepidoptera: Tortricidae) in Relation to Age, Virginity, and Body Size. Ann. Entomol. Soc. Am. 2004, 97, 819–824. [Google Scholar] [CrossRef]
- Jiménez-Pérez, A.; Wang, Q. Effect of body weight on reproductive performance in Cnephasia jactatana (Lepidoptera: Tortricidae). J. Insect Behav. 2004, 17, 511–522. [Google Scholar] [CrossRef]
- Carroll, A.L. Interactions between body size and mating history influence the reproductive success of males of a tortricid moth, Zeiraphera canadensis. Can. J. Zool. 1994, 72, 2124–2132. [Google Scholar] [CrossRef]
- Van Dongen, S.; Matthysen, E.; Sprengers, E.; Dhondt, A.A. Mate selection by male winter moths Operophtera brumata (Lepidoptera, Geometridae): Adaptive male choice or female control? Behaviour 1998, 135, 29–42. [Google Scholar]
- Tammaru, T.; Ruohomäki, K.; Saikkonen, K. Components of male fitness in relation to body size in Epirrita autumnata (Lepidoptera, Geometridae). Ecol. Entomol. 1996, 21, 185–192. [Google Scholar] [CrossRef]
- Lederhouse, R.C. The effect of female mating frequency on egg fertility in the black swallowtail, Papilio polyxenes asterius (Papilionidae). J. Lepid. Soc. 1981, 35, 266–277. [Google Scholar]
- Jones, R.E. Temperature, Size and Egg Production in the Cabbage Butterfly, Pieris Rapae L. Aust. J. Zool. 1982, 30, 223–232. [Google Scholar] [CrossRef]
- Elgar, M.A.; Pierce, N.E. Mating success and fecundity in an ant-tended lycaenid butterfly. In Reproductive Success Studies of Iondividual Variation in Contrasting Breeding Systems; Clutton-Brock, T., Ed.; Chicago University Press: Chicago, IL, USA, 1988; pp. 59–75. [Google Scholar]
- Trager, M.D.; Daniels, J.C. Size Effects on Mating and Egg Production in the Miami Blue Butterfly. J. Insect Behav. 2011, 24, 34–43. [Google Scholar] [CrossRef]
- Bernays, E.A. Feeding by lepidopteran larvae is dangerous. Ecol. Entomol. 1997, 22, 121–123. [Google Scholar] [CrossRef]
- Gotthard, K. Increased risk of predation as a cost of high growth rate: An experimental test in a butterfly. J. Anim. Ecol. 2000, 69, 896–902. [Google Scholar] [CrossRef]
- Pierce, N.E.; Kitching, R.L.; Buckley, R.C.; Taylor, M.F.J. The costs and benefits of cooperation between the Australian lycaenid butterfly, Jalmenus evagoras, and its attendant ants. Behav. Ecol. Sociobiol. 1987, 21, 237–248. [Google Scholar] [CrossRef]
- Robbins, R.K. Cost and evolution of a facultative mutualism between ants and lycaenid larvae (Lepidoptera). Oikos 1991, 3, 363–369. [Google Scholar] [CrossRef][Green Version]
- Fiedler, K.; Hölldobler, B. Ants and Polyommatus icarus immatures (Lycaenidae)—Sex-related developmental benefits and costs of ant attendance. Oecologia 1992, 91, 468–473. [Google Scholar] [CrossRef]
- Cushman, J.H.; Rashbrook, V.K.; Beattie, A.J. Assessing benefits to both participants in a lycaenid-ant association. Ecology 1994, 4, 1031–1041. [Google Scholar] [CrossRef]
- Fiedler, K.; Saam, C. Does ant-attendance influence development in 5 European Lycaenidae butterfly species? (Lepidoptera). Nota Lepid. 1994, 17, 5–24. [Google Scholar]
- Wagner, D. Species-specific effects of tending ants on the development of lycaenid butterfly larvae. Oecologia 1993, 96, 276–281. [Google Scholar] [CrossRef]
- Kaminski, L.A.; Rodrigues, D. Species-specific levels of ant attendance mediate performance costs in a facultative myrmecophilous butterfly. Physiol. Entomol. 2011, 36, 208–214. [Google Scholar] [CrossRef]
- Trager, M.D.; Thom, M.D.; Daniels, J.C. Ant-related oviposition and larval performance in a myrmecophilous lycaenid. Int. J. Ecol. 2013, 2013, 152139. [Google Scholar] [CrossRef]
- Leimar, O.; Axen, A.H. Strategic behaviour in an interspecific mutualism: Interactions between lycaenid larvae and ants. Anim. Behav. 1993, 46, 1177–1182. [Google Scholar] [CrossRef]
- Axén, A.H.; Leimar, O.; Hoffman, V. Signalling in a mutualistic interaction. Anim. Behav. 1996, 52, 321–333. [Google Scholar] [CrossRef]
- Pierce, N.E.; Braby, M.F.; Heath, A.; Lohman, D.J.; Mathew, J.; Rand, D.B.; Travassos, M.A. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu. Rev. Entomol. 2002, 47, 733–771. [Google Scholar] [CrossRef]
- Fiedler, K.; Hölldobler, B.; Seufert, P. Butterflies and ants: The communicative domain. Experientia 1996, 52, 14–24. [Google Scholar] [CrossRef]
- Gnatzy, W.; Jatho, M.; Kleinteich, T.; Gorb, S.N.; Hustert, R. The eversible tentacle organs of Polyommatus caterpillars (Lepidoptera, Lycaenidae): Morphology, fine structure, sensory supply and functional aspects. Arthropod Struct. Dev. 2017, 46, 788–804. [Google Scholar] [CrossRef]
- Malicky, H. New aspects on the association between lycaenid larvae (lycaenidae) and ants (formicidae, hymenoptera). J. Lepdopterists Soc. 1970, 24, 190–202. [Google Scholar]
- Fiedler, K.; Seufert, P.; Pierce, N.E.; Pearson, J.G.; Baumgarten, H. Exploitation of lycaenid-ant mutualisms by braconid parasitoids. J. Res. Lepid. 1992, 31, 153–168. [Google Scholar]
- DeVries, P.J.; Baker, I. Butterfly exploitation of an ant-plant mutualism: Adding insult to herbivory. J. New York Entomol. Soc. 1989, 97, 332–340. [Google Scholar]
- Pierce, N.E.; Nash, D.R.; Baylis, M.; Carper, E.R. Variation in the attractiveness of Iycaenid butterfly larvae to ants. In Ant-Plant Interactions; Huxley, C.R., Cutler, D.F., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 131–142. ISBN 0198546394. [Google Scholar]
- Daniels, H.; Gottsberger, G.; Fiedler, K. Nutrient composition of larval nectar secretions from three species of myrmecophilous butterflies. J. Chem. Ecol. 2005, 31, 2805–2821. [Google Scholar] [CrossRef]
- Baylis, M.; Pierce, N.E. Lack of compensation by final instar larvae of the myrmecophilous Iycaenid butterfly, Jalmenus evagoras, for the loss of nutrients to ants. Physiol. Entomol. 1992, 17, 107–114. [Google Scholar] [CrossRef]
- Fiedler, K.; Hummel, V. Myrmecophily in the brown argus butterfly, Aricia agestis (Lepidoptera: Lycaenidae): Effects of larval age, ant number and persistence of contact with ants. Zoology 1995, 99, 128–137. [Google Scholar]
- Trager, M.D.; Daniels, J.C. Ant tending of miami blue butterfly larvae (Lepidoptera: Lycaenidae): Partner diversity and effects on larval performance. Fla. Entomol. 2009, 92, 474–482. [Google Scholar]
- Mizuno, T.; Hagiwara, Y.; Akino, T. Chemical tactic of facultative myrmecophilous lycaenid pupa to suppress ant aggression. Chemoecology 2018, 28, 173–182. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Baylis, M.; Pierce, N.E. The Effects of Ant Mutualism on the Foraging and Diet of Lycaenid Caterpillars. In Caterpillars: Ecological and Evolutionary Constraints on Foraging; Stamp, N.E., Casey, T.M., Eds.; Chapman & Hall: New York, NY, USA, 1993; pp. 404–421. [Google Scholar]
- Thomas, J.A. The Ecology and Conservation of Lysandra bellargus (Lepidoptera: Lycaenidae) in Britain. J. Anim. Ecol. 1983, 20, 59–83. [Google Scholar] [CrossRef]
- Wagner, D.; Martínez del Rio, C. Experimental tests of the mechanism for ant-enhanced growth in an ant-tended lycaenid butterfly. Oecologia 1997, 112, 424–429. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Fordyce, J.A. Induced indirect defence in a lycaenid-ant association: The regulation of a resource in a mutualism. Proc. R. Soc. B Biol. Sci. 2000, 267, 1857–1861. [Google Scholar] [CrossRef]
- Horvitz, C.C.; Schemske, D.W. Effects of ants and an ant-tended herbivore on seed production of a neotropical herb. Ecology 1984, 65, 1369–1378. [Google Scholar] [CrossRef]
- Thaler, J.S.; Mcart, S.H.; Kaplan, I. Compensatory mechanisms for ameliorating the fundamental trade-off between predator avoidance and foraging. Proc. Natl. Acad. Sci. USA 2012, 109, 12075–12080. [Google Scholar] [CrossRef]
- Berger, D.; Walters, R.; Gotthard, K. What keeps insects small?—Size dependent predation on two species of butterfly larvae. Evol. Ecol. 2006, 20, 575–589. [Google Scholar] [CrossRef]
- Mänd, T.; Tammaru, T.; Mappes, J. Size dependent predation risk in cryptic and conspicuous insects. Evol. Ecol. 2007, 21, 485–498. [Google Scholar] [CrossRef]
- Tammaru, T.; Ruohoma, K.; Gotthard, K. Compensatory responses in lepidopteran larvae: A test of growth rate maximisation. Oikos 2004, 107, 352–362. [Google Scholar] [CrossRef]
- Dmitriew, C.M. The evolution of growth trajectories: What limits growth rate? Biol. Rev. 2011, 86, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Pierce, N.E.; Nash, D.R. The imperial blue, Jalmenus evagoras (Lycaenidae). In Biology of Australian Butterflies; Kitching, R.L., Scheermeyer, E., Jones, R.E., Pierce, N.E., Eds.; CSIRO Publishing: Collingwood, Australia, 1999; pp. 279–317. [Google Scholar]
- Lundgren, L. The role of intra- and interspecific male:male interactions in Polyommatus icarus Rott. and some other species of blues (Lycaenidae). J. Res. Lepid. 1977, 16, 249–264. [Google Scholar]
- Fraser, A.M.; Axén, A.H.; Pierce, N.E. Assessing the quality of different ant species as partners of a myrmecophilous butterfly. Oecologia 2001, 129, 452–460. [Google Scholar] [CrossRef]
- Molleman, F.; Javois, J.; Esperk, T.; Teder, T.; Davis, R.B.; Tammaru, T. Sexual differences in weight loss upon eclosion are related to life history strategy in Lepidoptera. J. Insect Physiol. 2011, 57, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Yamashita, M.; Sawada, H. Invasive status of Eragrostis curvula in the Lycaeides argyrognomon praeterinsularis habitat along the Abe River, and the effect of its removal. Jpn. J. Conserv. Ecol. 2009, 14, 25–35. [Google Scholar]


| Ant Tending Condition | Sex | Average Mass ± Standard Error (mg) | n | |||
|---|---|---|---|---|---|---|
| 3rd | 4th | Pupa | Adult | |||
| Cj | f | 2.3 ± 0.1 | 15.1 ± 0.6 | 92.1 ± 2.3 a | 17.0 ± 0.5 a | 13 |
| Lj | f | 2.3 ± 0.2 | 13.6 ± 0.7 | 84.9 ± 2.0 b | 15.2 ± 0.5 b | 10 |
| no-ant | f | 2.1 ± 0.1 | 15.3 ± 0.4 | 81.2 ± 1.3 b | 13.3 ± 0.3 c | 16 |
| Cj | m | 2.0 ± 0.1 | 13.6 ± 1.0 | 83.7 ± 1.7 A | 11.3 ± 0.3 A | 10 |
| Lj | m | 2.1 ± 0.1 | 14.7 ± 0.9 | 83.7 ± 1.6 A | 11.5 ± 0.3 A | 11 |
| no-ant | m | 2.3 ± 0.1 | 14.4 ± 0.7 | 82.6 ± 1.1 A | 10.9 ± 0.2 A | 17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizuno, T.; Hagiwara, Y.; Akino, T. Varied Effects of Tending Ant Species on the Development of Facultatively Myrmecophilous Lycaenid Butterfly Larvae. Insects 2019, 10, 234. https://doi.org/10.3390/insects10080234
Mizuno T, Hagiwara Y, Akino T. Varied Effects of Tending Ant Species on the Development of Facultatively Myrmecophilous Lycaenid Butterfly Larvae. Insects. 2019; 10(8):234. https://doi.org/10.3390/insects10080234
Chicago/Turabian StyleMizuno, Takafumi, Yasuo Hagiwara, and Toshiharu Akino. 2019. "Varied Effects of Tending Ant Species on the Development of Facultatively Myrmecophilous Lycaenid Butterfly Larvae" Insects 10, no. 8: 234. https://doi.org/10.3390/insects10080234
APA StyleMizuno, T., Hagiwara, Y., & Akino, T. (2019). Varied Effects of Tending Ant Species on the Development of Facultatively Myrmecophilous Lycaenid Butterfly Larvae. Insects, 10(8), 234. https://doi.org/10.3390/insects10080234





