Congruence Between Molecular Data and Morphology: Phylogenetic Position of Senodoniini (Coleoptera: Elateridae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Taxon Sampling and Laboratory Procedures
2.2. Dataset Assembling, Alignment, and Phylogenetic Analyses
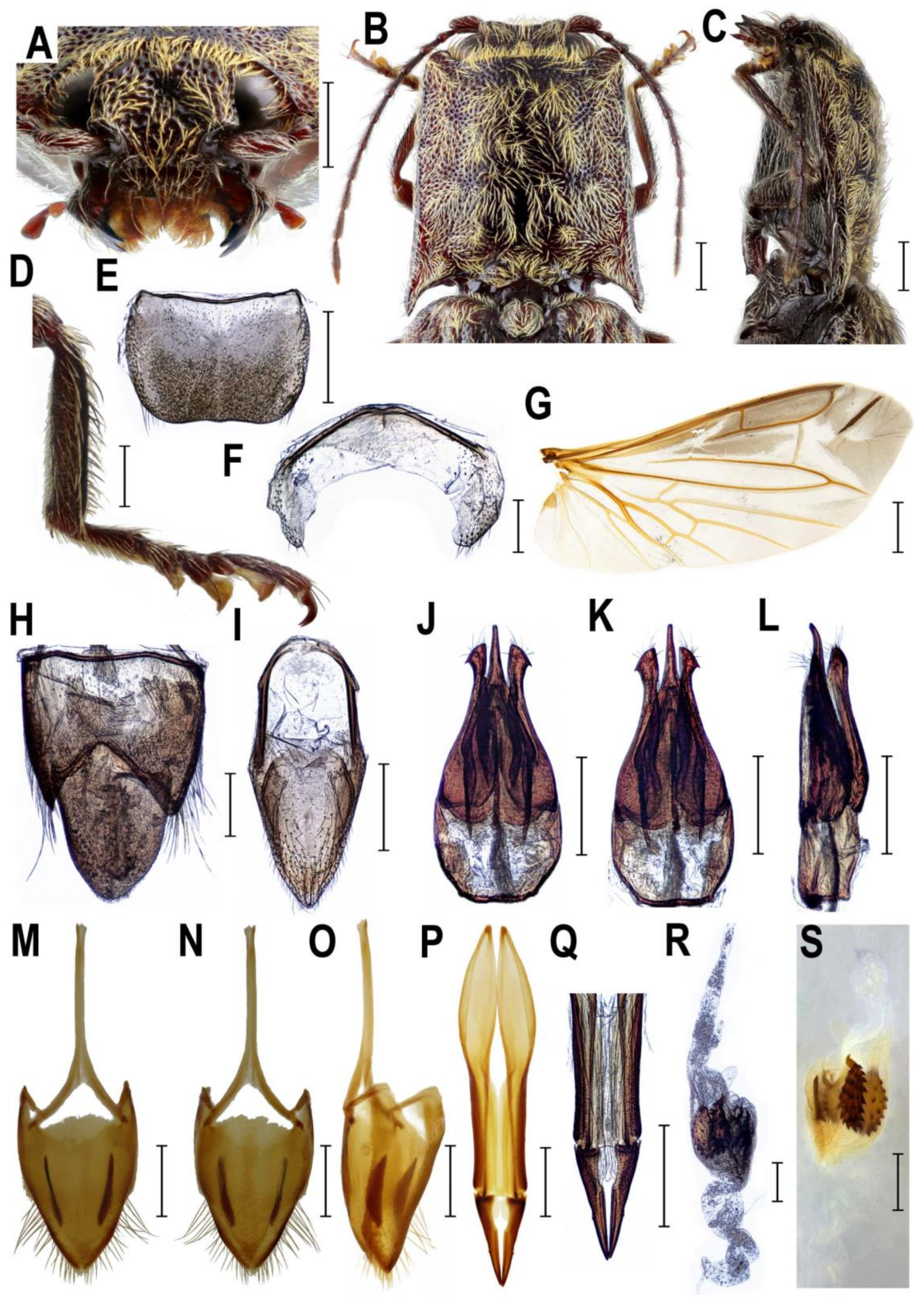
2.3. Morphology
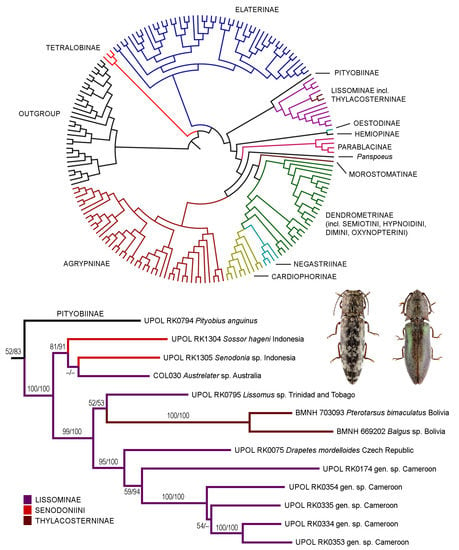
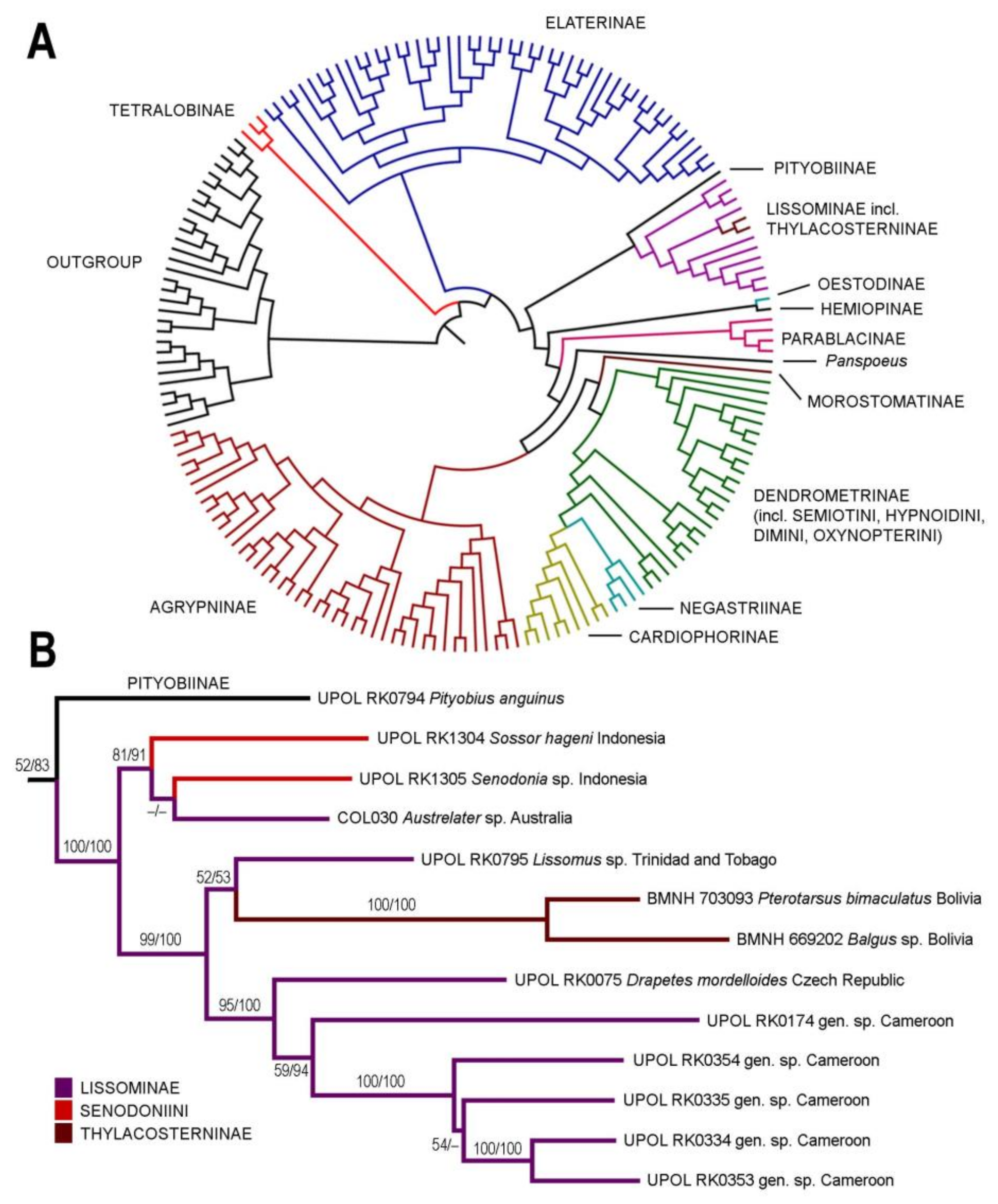
3. Results
3.1. Alignment Parameters and Phylogenetic Analyses
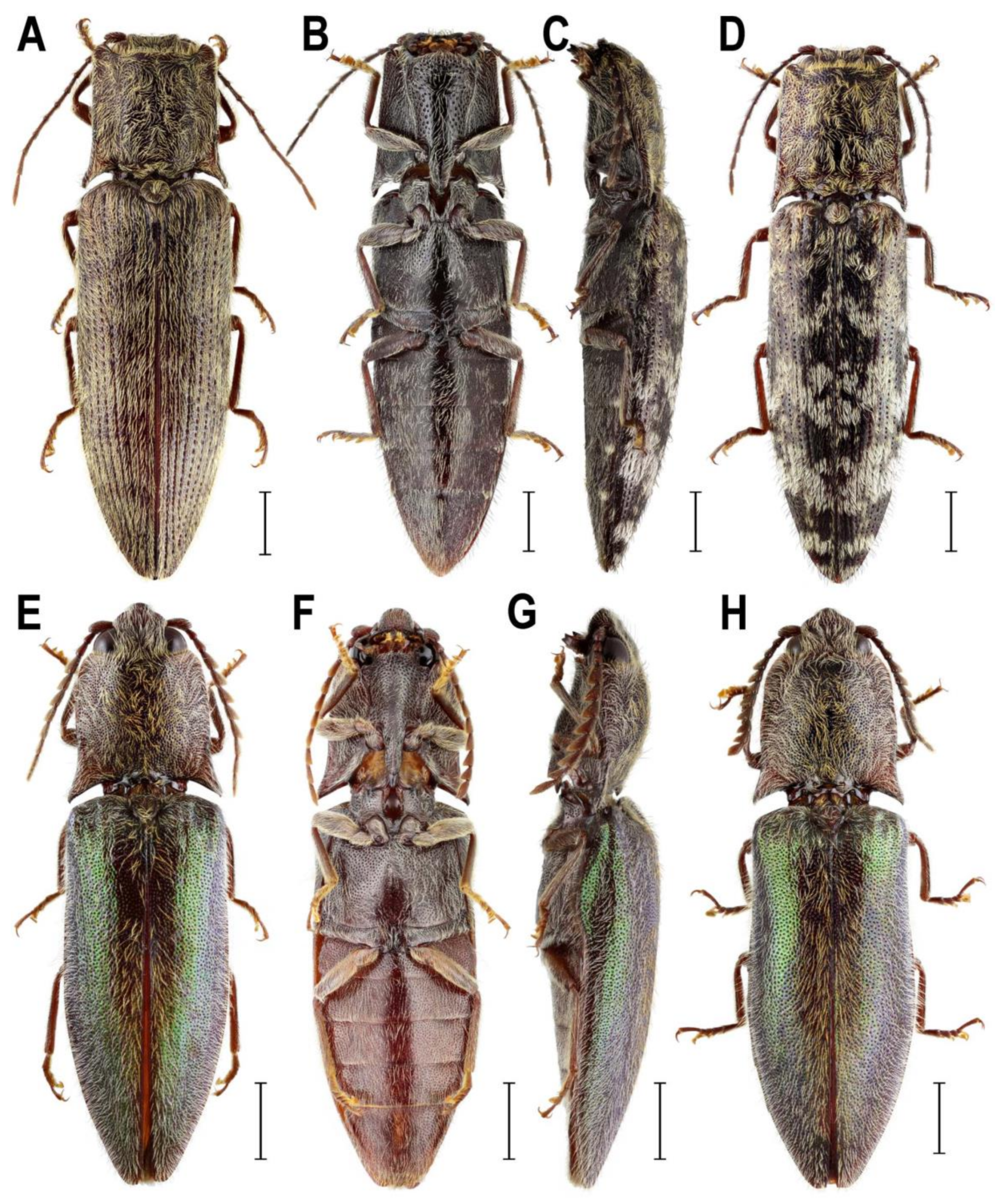
3.2. Taxonomy
- Family Elateridae Leach, 1815
- Subfamily Lissominae Laporte, 1835
- Tribe Protelaterini Schwarz, 1902
- = Senodoniini Schenkling, 1927, syn. nov.
- = Sphaenelaterini Stibick, 1979
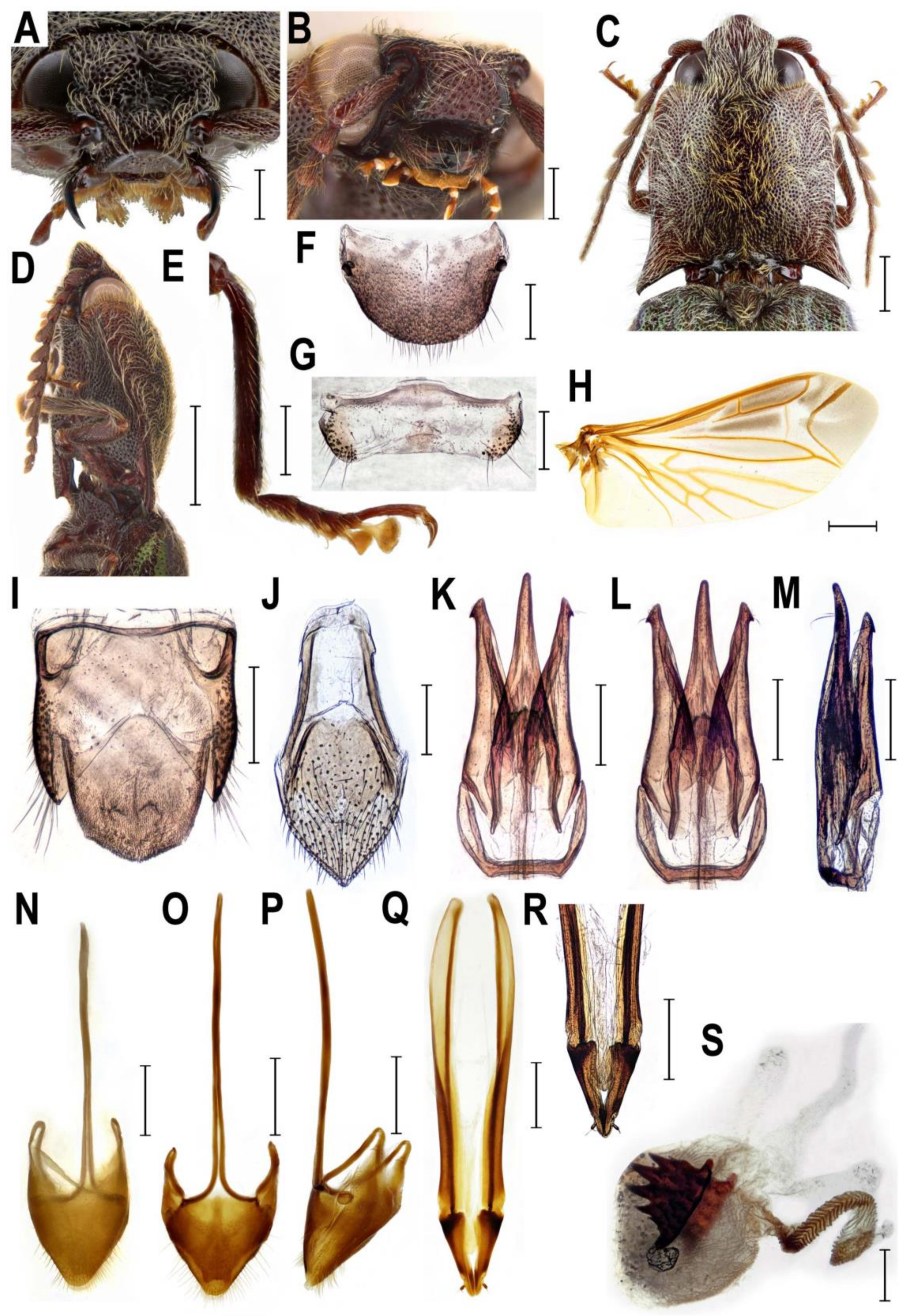
3.2.1. Genus Senodonia Laporte, 1838
- Senodonia Laporte, 1838: 12. Type species: Senodonia quadricollis Laporte, 1838, by monotypy.
- =Allotrius Laporte, 1840: 231. Type species: Allotrius quadricollis Laporte, 1838, by monotypy.
- =Hemiolimerus Candèze, 1863: 227. Type species: Hemiolimerus emodi Candèze, 1863, by monotypy.
- =Orientis Vats & Kashyap, 1992: 252. Type species: Orientis montanus Vats & Kashyap, 1992, by monotypy.
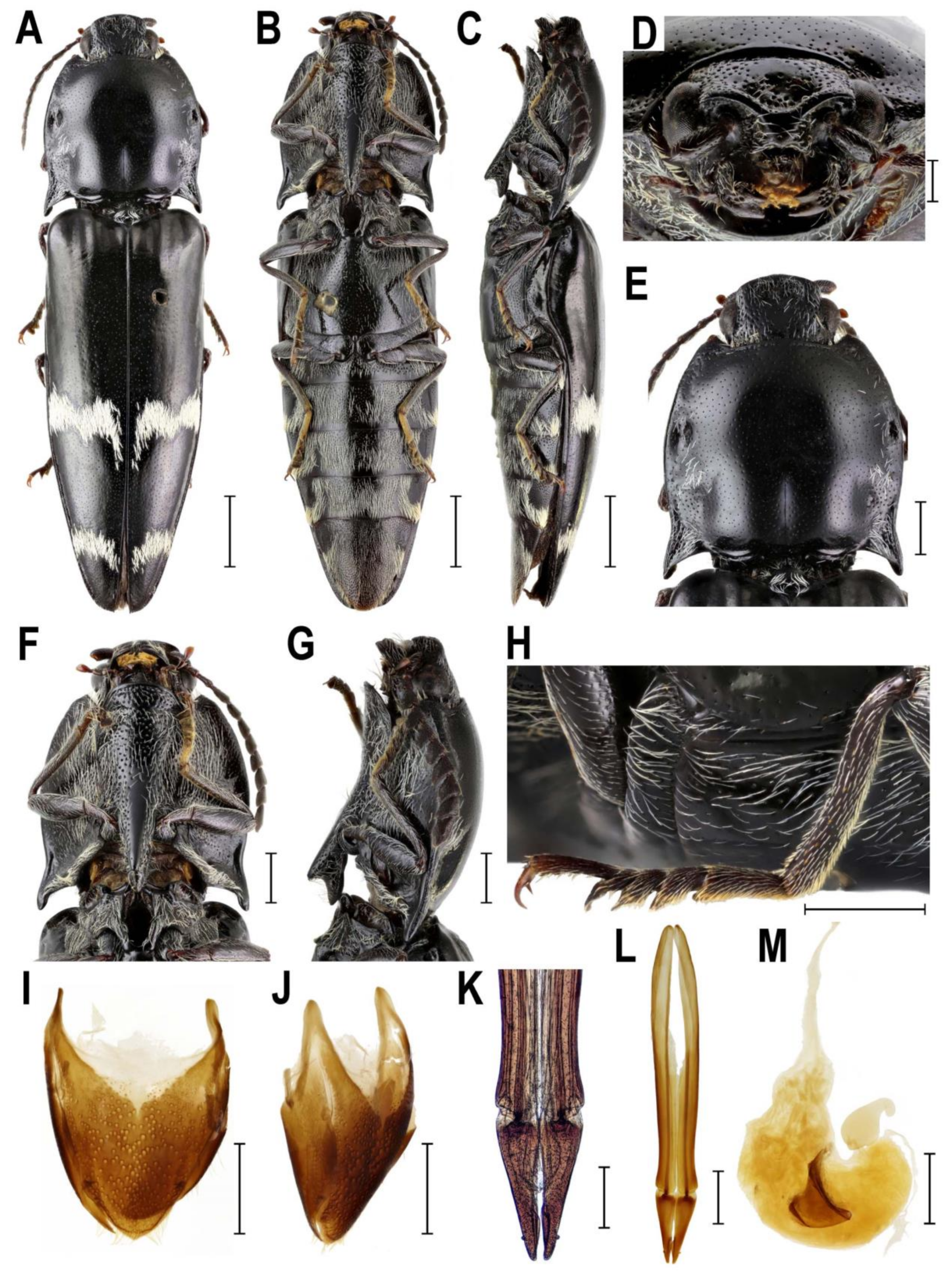
3.2.2. Genus Sossor Candèze, 1883
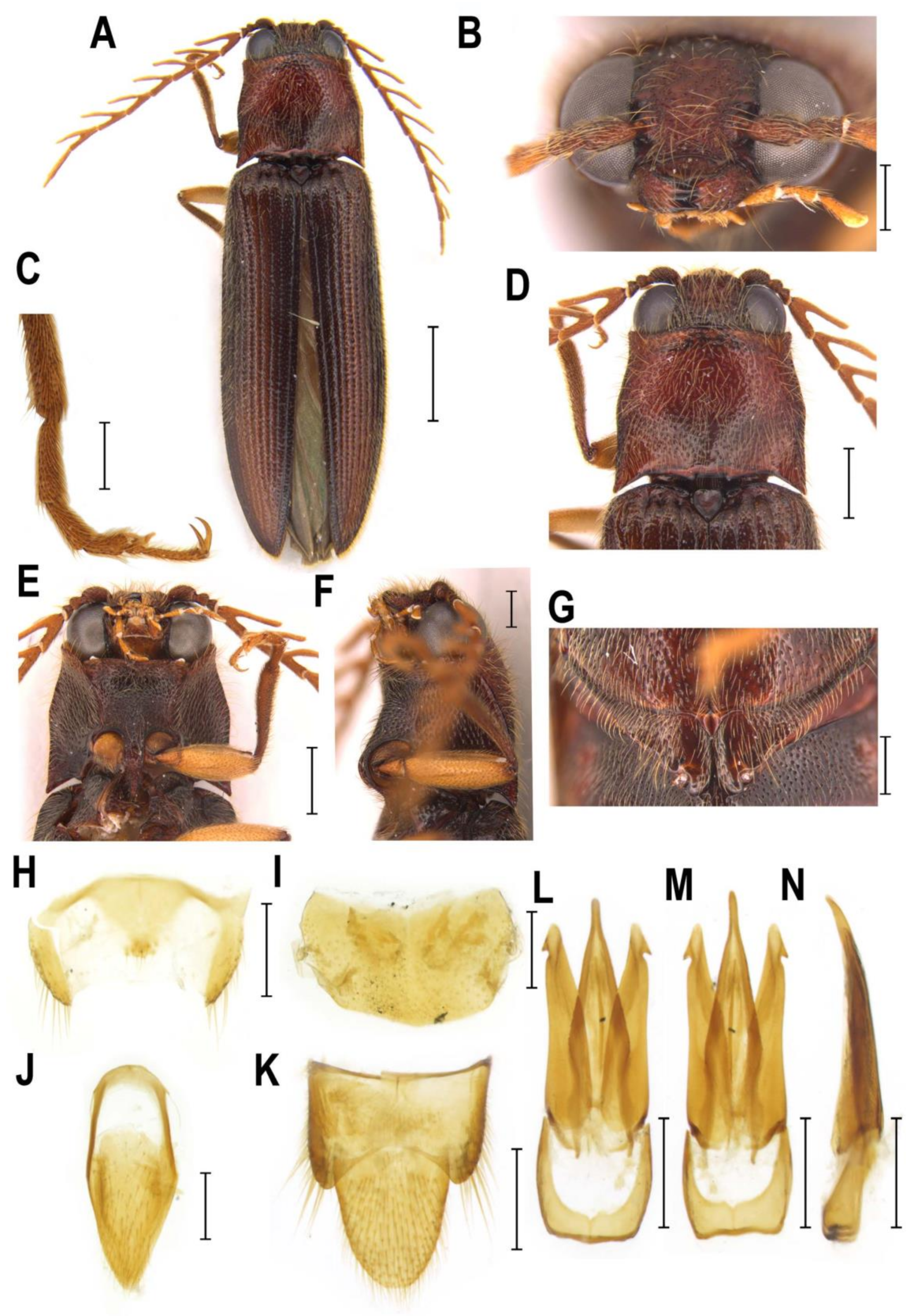
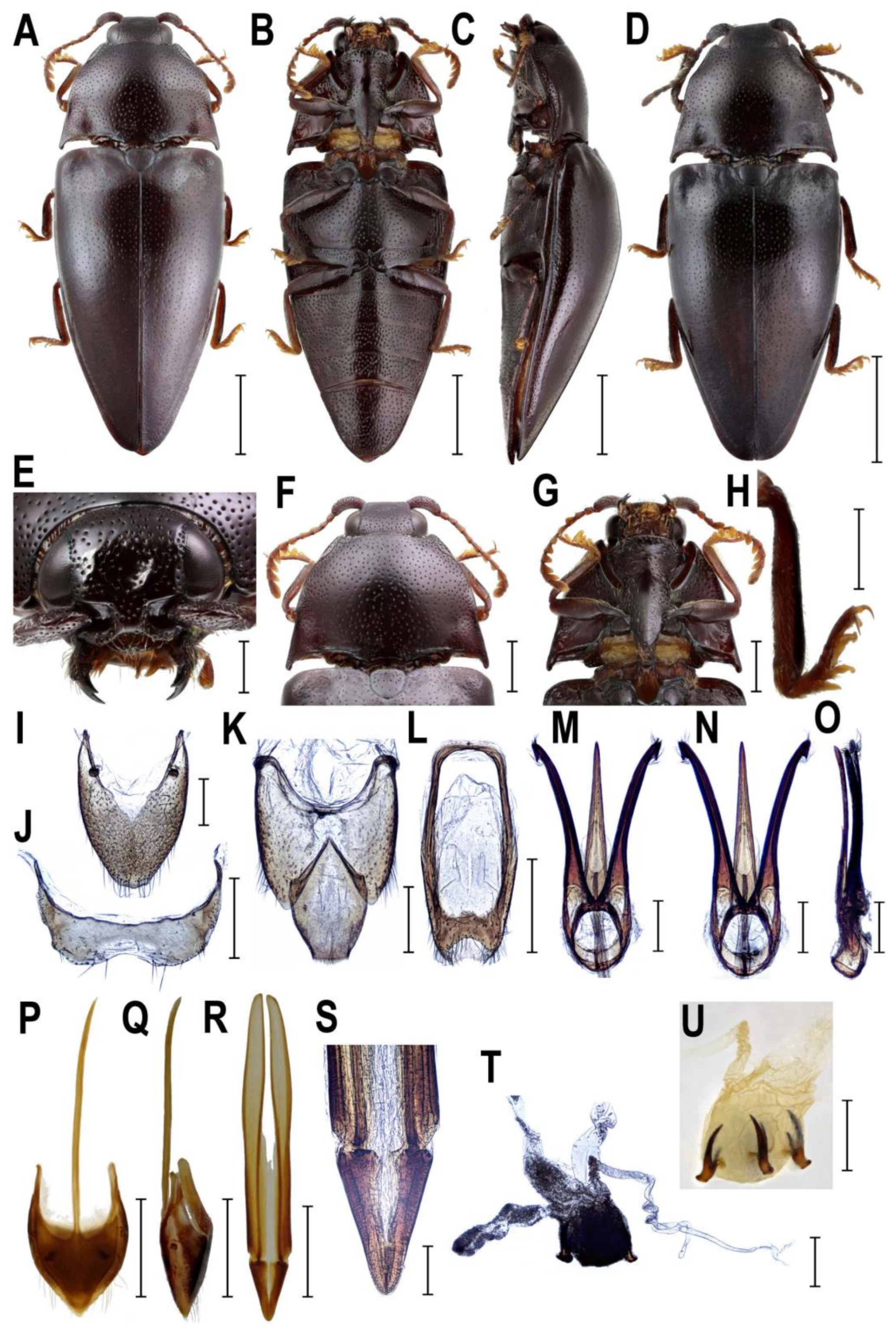
3.2.3. Genus Rostricephalus Fleutiaux, 1918
4. Discussion
4.1. Systematic Position and Morphology of Senodoniini
4.2. Monophyly, Phylogeny, and Classification of Lissominae
4.3. Systematic Position and Morphology of Rostricephalus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costa, C.; Lawrence, J.F.; Rosa, S.P. Elateridae Leach, 1815. In Handbook of Zoology, Arthropoda: Insecta; Coleoptera, Beetles; Volume 2: Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia partim); Leschen, R.A.B., Beutel, R.G., Lawrence, J.F., Eds.; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2010; pp. 75–103. [Google Scholar]
- Sagegami-Oba, R.; Oba, Y.; Ôhira, H. Phylogenetic relationships of click beetles (Coleoptera: Elateridae) inferred from 28S ribosomal DNA: Insights into the evolution of bioluminescence in Elateridae. Mol. Phylogenet. Evol. 2007, 42, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Douglas, H. Phylogenetic relationships of Elateridae inferred from adult morphology, with special reference to the position of Cardiophorinae. Zootaxa 2011, 2900, 1–45. [Google Scholar] [CrossRef]
- Kundrata, R.; Bocak, L. The phylogeny and limits of Elateridae (Insecta, Coleoptera): Is there a common tendency of click beetles to soft-bodiedness and neoteny? Zool. Scr. 2011, 40, 364–378. [Google Scholar] [CrossRef]
- Kundrata, R.; Gunter, N.L.; Douglas, H.; Bocak, L. Next step toward a molecular phylogeny of click-beetles (Coleoptera: Elateridae): Redefinition of Pityobiinae, with a description of a new subfamily, Parablacinae, from the Australasian Region. Austral Entomol. 2016, 55, 291–302. [Google Scholar] [CrossRef]
- Kundrata, R.; Gunter, N.L.; Janosikova, D.; Bocak, L. Molecular evidence for the subfamilial status of Tetralobinae (Coleoptera: Elateridae), with comments on parallel evolution of some phenotypic characters. Arthropod Syst. Phyl. 2018, 76, 137–145. [Google Scholar]
- Schenkling, S. Elateridae II. In Coleopterorum Catalogus. Vol. XI. Pars 88; Junk, W., Schenkling, S., Eds.; W. Junk: Berlin, Germany, 1927; pp. 265–636. [Google Scholar]
- Schimmel, R.; Platia, G. Die Arten des supraspezifischen Taxons Senodoniinae Schenkling, 1927 (Coleoptera: Elateridae). Entomol. Basiliensia 1992, 15, 229–254. [Google Scholar]
- Schimmel, R. Das Monophylum Diminae Candèze, 1863 (Insecta: Coleoptera: Elateridae). Pollichia-Buch 1996, 33, 1–370. [Google Scholar]
- Kundrata, R.; Musalkova, M.; Kubaczkova, M. Annotated catalogue of the click-beetle tribe Dimini (Coleoptera: Elateridae: Dendrometrinae). Zootaxa 2018, 4412, 1–75. [Google Scholar] [CrossRef]
- Schimmel, R. Neue Megapenthini-, Physorhinini-, Diminae- und Senodoniina-Arten aus Südostasien (Insecta: Coleoptera, Elateridae). Mitt. Pollichia 2006, 92, 107–130. [Google Scholar]
- Bouchard, P.; Bousquet, Y.; Davies, A.E.; Alonso-Zarazaga, M.A.; Lawrence, J.F.; Lyal, C.H.C.; Newton, A.F.; Reid, C.A.M.; Schmitt, M.; Ślipiński, S.A.; et al. Family-group names in Coleoptera (Insecta). ZooKeys 2011, 88, 1–972. [Google Scholar] [CrossRef]
- Kundrata, R.; Musalkova, M.; Prosvirov, A.S. Annotated catalogue of the click-beetle tribe Senodoniini (Coleoptera: Elateridae: Dendrometrinae). Zootaxa 2018, 4532, 273–287. [Google Scholar] [CrossRef]
- Fleutiaux, E. Les Elateridae de l’Indochine française (6ͤ partie). Ann. Soc. Entomol. Fr. 1936, 105, 279–300. [Google Scholar]
- Fleutiaux, E. Révision des Élatérides (Coléoptères) de l’Indo-Chine Française. Notes Entomol. Chinoise 1947, 11, 233–420. [Google Scholar]
- Gurjeva, Y.L. Thoracic structure of click beetles (Coleoptera, Elateridae) and the significance of the structural characters for the system of the family. Entomol. Obozr. 1974, 53, 96–113. [Google Scholar]
- Stibick, J.N.L. Classification of the Elateridae (Coleoptera). Relationships and classification of the subfamilies and tribes. Pacific Insects 1979, 20, 145–186. [Google Scholar]
- Jiang, S.H.; Chen, X.Q.; Wu, S.J.; Meng, Z.Y.; Li, G.J. Molecular phylogenetic analysis of Elateridae (Insecta: Coleoptera) based on 28S rDNA gene fragments. Acta Entomol. Sinica 2009, 52, 74–83. [Google Scholar]
- Meng, Z.Y.; Lei, C.L.; Chen, X.Q.; Shi, T.X.; Chen, Q.J.; Jiang, S.H. Phylogenetic analysis of click beetles (Coleoptera: Elateridae) based upon 28S rDNA: Phylogeny and classification. Entomotaxonomia 2018, 40, 231–252. [Google Scholar]
- Meng, Z.Y.; Lei, C.L.; Chen, X.Q.; Jiang, S.H. Phylogenetic analysis of click beetles (Coleoptera: Elateridae) based on internal transcribed spacer-2 sequences. Chin. J. Appl. Entomol. 2017, 54, 767–779. [Google Scholar]
- Calder, A.A.; Lawrence, J.F.; Trueman, J.W.H. Austrelater, gen. nov. (Coleoptera: Elateridae), with a description of the larva and comments on elaterid relationships. Invertebr. Taxon. 1993, 7, 1349–1394. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Drummond, A.J. Tracer 1.5.—Institute of Evolutionary Biology University of Edinburgh, Edinburgh, 2007. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 15 March 2018).
- Kundrata, R.; Kubaczkova, M.; Prosvirov, A.S.; Douglas, H.B.; Fojtikova, A.; Costa, C.; Bousquet, Y.; Alonso-Zarazaga, M.A.; Bouchard, P. World catalogue of the genus-group names in Elateridae (Insecta, Coleoptera). Part I: Agrypninae, Campyloxeninae, Hemiopinae, Lissominae, Oestodinae, Parablacinae, Physodactylinae, Pityobiinae, Subprotelaterinae, Tetralobinae. ZooKeys 2019, 839, 83–154. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Arias, E.T. Valdivelater, a new genus of Protelaterini (Elateridae: Lissominae) from the forests of Central and Southern Chile. Ann. Zool. 2009, 59, 319–328. [Google Scholar] [CrossRef]
- Arias-Bohart, E.T. Tunon, a new genus of Protelaterini (Elateridae: Lissominae) from southern Chile. Pan-Pac. Entomol. 2013, 89, 159–167. [Google Scholar] [CrossRef]
- Prosvirov, A.S.; Savitsky, V.Y. On the significance of genital characters in supraspecific systematics of the elaterid subfamily Agrypninae (Coleoptera, Elateridae). Entomol. Rev. 2011, 91, 755–772. [Google Scholar] [CrossRef]
- Dolin, V.G. Znachenie lichinochnykh priznakov i zhilkovaniya krylev v sistematike Elateroidea (Coleoptera). Doklad na pyat’desyat vtorom ezhegodnom chtenii pamyati N A Kholodkskogo 1 aprelya 1999 g; Zoologicheskii Institut: St. Petersburg, Russia, 2000; pp. 1–49. [Google Scholar]
- Suzuki, W. Notes on Rostricephalus vitalisi Fleutiaux (Coleoptera: Elateridae) from Taiwan. Coleopt. News 2003, 143, 13–15. [Google Scholar]
- Suzuki, W. Catalogue of the family Elateridae (Coleoptera) of Taiwan. Misc. Rep. Hiwa Mus. Nat. Hist. 1999, 38, 1–348. [Google Scholar]
- Suzuki, W. Record of Rostricephalus vitalisi (Coleoptera, Elateridae) from Laos. Elytra 2004, 32, 292. [Google Scholar]
- Douglas, H.; Kundrata, R.; Janosikova, D.; Bocak, L. Molecular and morphological evidence for new genera in the click-beetle subfamily Cardiophorinae (Coleoptera: Elateridae). Entomol. Sci. 2018, 21, 292–305. [Google Scholar] [CrossRef] [Green Version]
- Kundrata, R.; Bocak, L. Molecular phylogeny reveals the gradual evolutionary transition to soft-bodiedness in click-beetles and identifies Sub-Saharan Africa as a cradle of diversity for Drilini (Coleoptera: Elateridae). Zool. J. Linn. Soc. 2019. [Google Scholar] [CrossRef]
- Cate, P. Elateridae. In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2007; Volume 4, pp. 89–209. [Google Scholar]
- Dolin, V.G. Wing venation in click beetles and its significance for the taxonomy of the family. Zool. Zhurnal 1975, 54, 1618–1633. [Google Scholar]
- Lawrence, J.F.; Muona, J.; Teräväinen, M.; Ståhls, G.; Vahtera, V. Anischia, Perothops and the phylogeny of Elateroidea (Coleoptera: Elateriformia). Insect Syst. Evol. 2007, 38, 205–239. [Google Scholar] [CrossRef]
- Fleutiaux, E. Nouvelles contributions a la faune de l’Indo-Chine française (Coleoptera Serricornia). Ann. Soc. Entomol. Fr. 1918, 87, 175–278. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundrata, R.; Prosvirov, A.S.; Vondracek, D.; Sormova, E. Congruence Between Molecular Data and Morphology: Phylogenetic Position of Senodoniini (Coleoptera: Elateridae). Insects 2019, 10, 231. https://doi.org/10.3390/insects10080231
Kundrata R, Prosvirov AS, Vondracek D, Sormova E. Congruence Between Molecular Data and Morphology: Phylogenetic Position of Senodoniini (Coleoptera: Elateridae). Insects. 2019; 10(8):231. https://doi.org/10.3390/insects10080231
Chicago/Turabian StyleKundrata, Robin, Alexander S. Prosvirov, Dominik Vondracek, and Eliska Sormova. 2019. "Congruence Between Molecular Data and Morphology: Phylogenetic Position of Senodoniini (Coleoptera: Elateridae)" Insects 10, no. 8: 231. https://doi.org/10.3390/insects10080231
APA StyleKundrata, R., Prosvirov, A. S., Vondracek, D., & Sormova, E. (2019). Congruence Between Molecular Data and Morphology: Phylogenetic Position of Senodoniini (Coleoptera: Elateridae). Insects, 10(8), 231. https://doi.org/10.3390/insects10080231