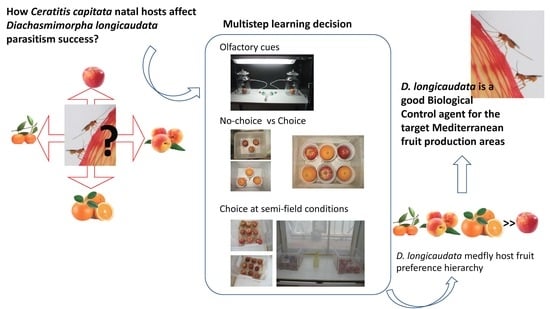
Diachasmimorpha longicaudata Parasitism Response to Medfly Host Fruit and Fruit Infestation Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects and Fruits
2.2. Olfactory Response Trials
2.3. Laboratory Trials
2.4. Semi-Field Trials
2.5. Data Analysis
3. Results
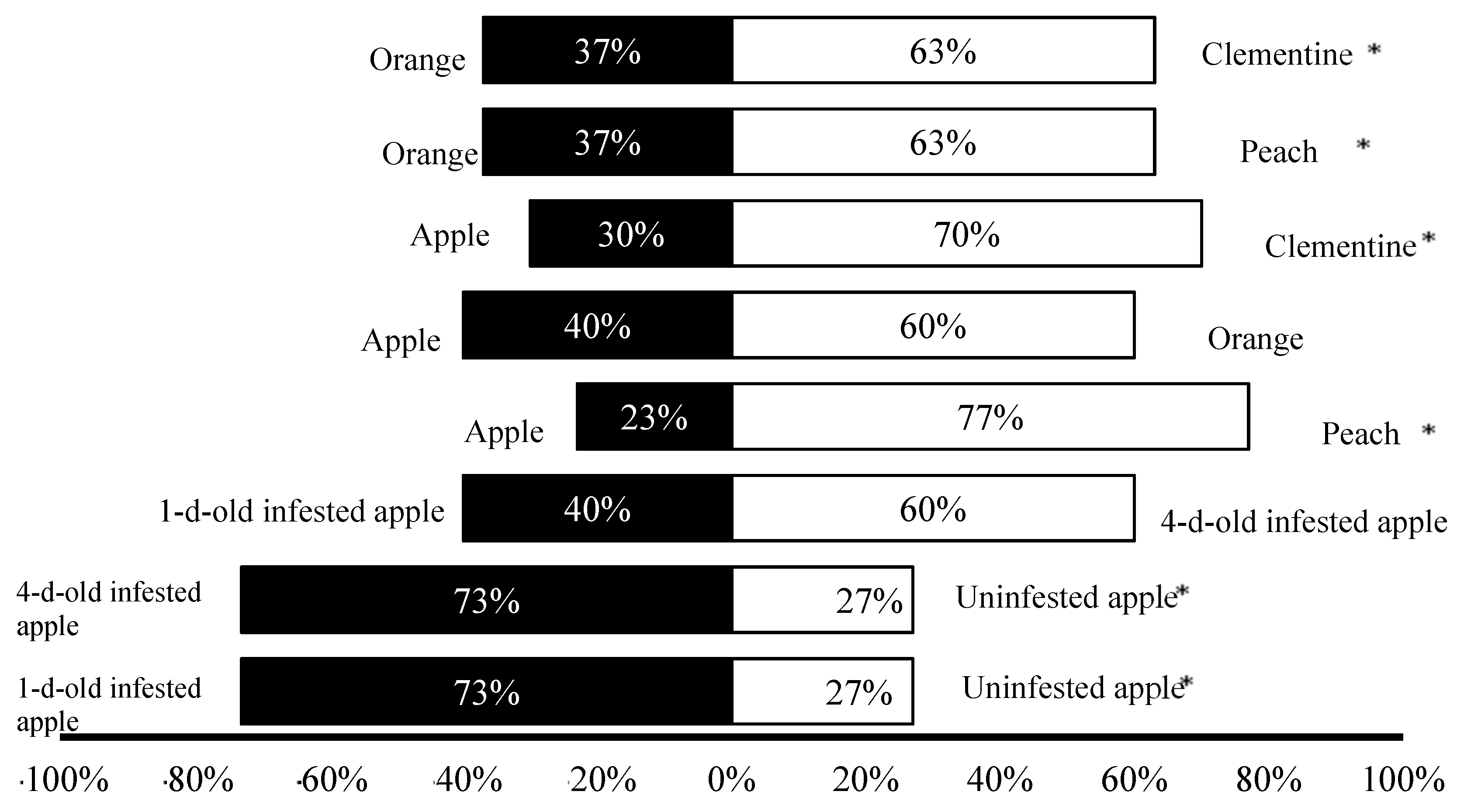
3.1. Olfactory Response Trials
3.2. Laboratory Trials
3.3. Semi-Field Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liquido, N.J.; Cunningham, R.T.; Nakagawa, S. Host plants of Mediterranean fruit fly (Diptera: Tephritidae) on the island of Hawaii (1949–1985 Survey). J. Econ. Entomol. 1990, 83, 1863–1878. [Google Scholar] [CrossRef]
- Malacrida, A.R.; Gomulski, L.M.; Bonizzoni, M.; Bertin, S.; Gasperi, G.; Guglielmino, C.R. Globalization and fruitfly invasion and expansion: The medfly paradigm. Genetica 2007, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Magaña, C.; Hernández-Crespo, P.; Ortego, F.; Castañera, P. Resistance to malathion in field populations of Ceratitis capitata. J. Econ. Entomol. 2007, 100, 1836–1843. [Google Scholar] [CrossRef]
- Michaud, J.P. Toxicity of fruit fly baits to beneficial insects in citrus. J. Insect Sci. 2003, 3, 1–9. [Google Scholar]
- Michaud, J.P.; Grant, A.K. IPM-compatibility of foliar insecticides for citrus: Indices derived from toxicity to beneficial insects from four orders. J. Insect Sci. 2003, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, A.; Abbes, K.; Harbi, A.; Duyck, P.-F.; Chermiti, B. Attract-and-kill systems efficiency against Ceratitis capitata (Diptera: Tephritidae) and effects on non-target insects in peach orchards. J. Appl. Entomol. 2016, 140, 28–36. [Google Scholar] [CrossRef]
- Gerson, U.; Cohen, E. Resurgences of spider mites (Acari: Tetranychidae) induced by synthetic pyrethroids. Exp. Appl. Acarol. 1989, 6, 29–46. [Google Scholar] [CrossRef]
- De Clerq, P.; Bale, J.S. Risks of invertebrte biological control agents—Harmonia axyridis as a case study. In Regulation of Biological Control Agents; Ehlers, R.U., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 243–257. [Google Scholar]
- Ehlers, R.U. Regulation of Biological Control Agents; Ehlers, R.-U., Ed.; Springer: Dordrecht, The Netherlands, 2011; ISBN 978-90-481-3663-6. [Google Scholar]
- Purcell, M.F. Contribution of biological control to integrated pest management of tephritid fruit flies in the tropics and subtropics. Integr. Pest Manag. Rev. 1998, 3, 63–83. [Google Scholar] [CrossRef]
- Ovruski, S.M.; Aluja, M. Hymenopteran parasitoids on fruit-infesting Tephritidae (Diptera) in Latin America and the Southern United States. Integr. Pest Manag. Rev. 2000, 5, 81–107. [Google Scholar] [CrossRef]
- Kitthawee, S.; Dujardin, J.-P. The Diachasmimorpha longicaudata complex: Reproductive isolation and geometric patterns of the wing. Biol. Control 2009, 51, 191–197. [Google Scholar] [CrossRef]
- Bautista, R.C.; Harris, E.J. Effect of insectary rearing on host preference and oviposition behaviour of the fruit fly parasitoid Diachasmimorpha longicaudata. Entomol. Exp. Appl. 1997, 83, 213–218. [Google Scholar] [CrossRef]
- Montoya, P.; Liedo, P.; Benrey, B.; Barrera, J.F.; Cancino, J.; Aluja, M. Functional response and superparasitism by Diachasmimorpha longicaudata (Hymenoptera: Braconidae), a parasitoid of fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2006, 93, 47–54. [Google Scholar] [CrossRef]
- Van Nieuwenhove, G.A.; Ovruski, S.M. Influence of Anastrepha fraterculus (Diptera: Tephritidae) larval instars on the production of Diachasmimorpha longicaudata (Hymneoptera: Braconidae) progeny and their sex ratio. Fla Entomol. 2011, 94, 863–868. [Google Scholar] [CrossRef]
- Ovruski, S.M.; Bezdjian, L.P.; Van Nieuwenhove, G.A.; Albornoz-Medina, P.; Schliserman, P. Host preference by Diachasmimorpha longicaudata (Hymneoptera: Braconidae) reared on larvae of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae). Fla Entomol. 2011, 94, 195–200. [Google Scholar] [CrossRef]
- Segura, D.F.; Viscarret, M.M.; Ovruski, S.M.; Cladera, J.L. Response of the fruit fly parasitoid Diachasmimorpha longicaudata to host and host-habitat volatile cues. Entomol. Exp. Appl. 2012, 143, 164–176. [Google Scholar] [CrossRef]
- Hafsi, A.; Facon, B.; Ravigné, V.; Chiroleu, F.; Quilici, S.; Chermiti, B.; Duyck, P.-F. Host plant range of a fruit fly community (Diptera: Tephritidae): Does fruit composition influence larval performance? BMC Ecol. 2016, 16, 40. [Google Scholar] [CrossRef]
- Lewis, W.J.; Stapel, J.O.; Cortesero, A.M.; Takasu, K. Understanding how parasitoids balance food and host needs: Importance to biological control. Biol. Control 1998, 11, 175–183. [Google Scholar] [CrossRef]
- Benelli, G.; Carpita, A.; Simoncini, S.; Raspi, A.; Canale, A. For sex and more: Attraction of the tephritid parasitoid Psyttalia concolor (Hymenoptera: Braconidae) to male sex pheromone of the olive fruit fly, Bactrocera oleae. J. Pest Sci. 2014, 87, 449–457. [Google Scholar] [CrossRef]
- Giunti, G.; Benelli, G.; Palmeri, V.; Canale, A. Bactrocera oleae-induced olive VOCs routing mate searching in Psyttalia concolor males: Impact of associative learning. Bull. Entomol. Res. 2018, 108, 40–47. [Google Scholar] [CrossRef]
- Stuhl, C.; Sivinski, J.; Teal, P.; Paranhos, B.; Aluja, M. A compound produced by fruigivorous Tephritidae (Diptera) larvae promotes oviposition behavior by the biological control agent Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Environ. Entomol. 2011, 40, 727–736. [Google Scholar] [CrossRef]
- Quilici, S.; Rousse, P. Location of host and host habitat by fruit fly parasitoids. Insects 2012, 3, 1220–1235. [Google Scholar] [CrossRef]
- Segura, D.F.; Nussenbaum, A.L.; Viscarret, M.M.; Devescovi, F.; Bachmann, G.E.; Corley, J.C.; Ovruski, S.M.; Cladera, J.L. Innate host habitat preference in the parasitoid Diachasmimorpha longicaudata: Functional significance and modifications through learning. PLoS ONE 2016, 11, e0152222. [Google Scholar] [CrossRef]
- Harbi, A.; Beitia, F.J.; Tur, C.; Chermiti, B.; Verdú, M.J.; Sabater-Muñoz, B. Field releases of the larval parasitoid Diachasmimorpha longicaudata in Spain: First results on dispersal pattern. Acta Hortic. 2015, 1065, 1057–1062. [Google Scholar] [CrossRef]
- Harbi, A. Diachasmimorpha longicaudata as Biological Control agent of the Mediterranean fruit fly, Ceratitis capitata: Biotic and Abiotic Factors Affecting Its Implementation in Citrus Crops of the Mediterranean Basin. PhD Thesis, Universitat Jaume I, Castelló de la Plana, Spain, 2017. [Google Scholar]
- Harbi, A.; Beitia, F.; Sabater-Muñoz, B.; Falcó, J.V.; Chermiti, B. First record of Pachycrepoideus vindemmiae (Rondani) (Hymenoptera: Pteromalidae) parasitizing pupae of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) in Tunisia. Afr. Entomol. 2015, 23, 514–518. [Google Scholar] [CrossRef]
- Harbi, A.; Abbes, K.; Sabater-Muñoz, B.; Beitia, F.; Chermiti, B. Residual toxicity of insecticides used in Tunisian citrus orchards on the imported parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae): Implications for IPM program of Ceratitis capitata (Diptera: Tephritidae). Span. J. Agric. Res. 2017, 15, e1008. [Google Scholar] [CrossRef]
- Juan-Blasco, M.; San Andrés, V.; Martínez-Utrillas, M.A.; Argilés, R.; Pla, I.; Urbaneja, A.; Sabater-Muñoz, B. Alternatives to ginger root oil aromatherapy for improved mating performance of sterile Ceratitis capitata (Diptera: Tephritidae) males. J. Appl. Entomol. 2013, 137, 244–251. [Google Scholar] [CrossRef]
- Martins, D.S.; Skouri, W.; Chermiti, B.; Aboussaid, H.; Messoussi, S.E.; Oufdou, K.; Carboneu, E.; Sabater-Muñoz, B.; Beitia, F. Analysis of Two Larval-Pupal Parasitoids (Hymenoptera, Braconidae) in the Biological Control of Ceratitis capitata (Wiedemann) in Spanish Mediterranean Areas. In Proceeding of the 8th International Symposium on Fruit Flies of Economic Importance (ISFFEI), Valencia, Spain, 26 September–1 October 2010; Sabater-Muñoz, B., Navarro-Llopis, V., Urbaneja-García, A., Eds.; Editorial Universitat Politecnica de Valencia: Valencia, Spain, 2010; pp. 252–258. [Google Scholar]
- Sabater-Muñoz, B.; Martins, D.S.; Skouri, W.; Laurin, C.; Tur, C.; Beitia, F.J. Primeros ensayos sobre la utilización de Diachasmimorpha tryony (Hymenoptera: Braconidae) para el control biológico de Ceratitis capitata (Diptera: Tephritidae) en la Comunidad Valenciana. Levante Agric. Rev. Int. Citricos 2009, 398, 372–376. [Google Scholar]
- Harbi, A.; Beitia, F.; Ferrara, F.; Chermiti, B.; Sabater-Muñoz, B. Functional response of Diachasmimorpha longicaudata (Ashmead) over Ceratitis capitata (Wiedemann): Influence of temperature, fruit location and host density. Crop Prot. 2018, 109, 115–122. [Google Scholar] [CrossRef]
- Pérez-Hedo, M.; Urbaneja, A. Prospects for predatory mirid bugs as biocontrol agents of aphids in sweet peppers. J. Pest Sci. (2004) 2015, 88, 65–73. [Google Scholar] [CrossRef]
- McGregor, R.R.; Gillespie, D.R. Olfactory responses of the omnivorous generalist predator Dicyphus hesperus to plant and prey odours. Entomol. Exp. Appl. 2004, 112, 201–205. [Google Scholar] [CrossRef]
- Montoya, P.; Ruiz, L.; Pérez-Lachaud, G.; Cancino, J.; Liedo, P. Field superparasitism by Diachasmimorpha longicaudata attacking Anastrepha spp. larvae on mango fruits. Biol. Control 2013, 64, 160–165. [Google Scholar] [CrossRef]
- Ovruski, S.; Van Nieuwenhove, G.; Bezdjian, L.; Albornoz-Medina, P.; Schliserman, P. Evaluation of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) as a mortality factor of Ceratitis capitata (Diptera: Tephritidae) infesting Citrus species under laboratory and field-cage conditions. Biocontrol Sci. Technol. 2012, 22, 187–202. [Google Scholar] [CrossRef]
- Purcell, M.F.; Herr, J.C.; Messing, R.H.; Wong, T.T.Y. Interactions between augmentatively released Diachasmimorpha longicaudata (Hymenoptera: Braconidae) and a complex of Opiine parasitoids in a commercial guava orchard. Biocontrol Sci. Technol. 1998, 8, 139–151. [Google Scholar] [CrossRef]
- Silva, J.W.P.; Bento, J.M.S.; Zucchi, R.A. Olfactory response of three parasitoid species (Hymenoptera: Braconidae) to volatiles of guavas infested or not with fruit fly larvae (Diptera: Tephritidae). Biol. Control 2007, 41, 304–311. [Google Scholar] [CrossRef]
- Greany, P.D.; Tumlinson, J.H.; Chambers, D.L.; Boush, G.M. Chemically mediated host finding by Biosteres (Opius) longicaudatus, a parasitoid of tephritid fruit fly larvae. J. Chem. Ecol. 1977, 3, 189–195. [Google Scholar] [CrossRef]
- Duan, J.J.; Messing, R.H. Response of two opiine fruit fly parasitoids (Hymenoptera: Braconidae) to the Lantana gall fly (Diptera: Tephritidae). Env. Entomol. 1996, 25, 1428–1437. [Google Scholar] [CrossRef]
- Duan, J.J.; Ahmad, M.; Joshi, K.; Messing, R.H. Evaluation of the impact of the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) on a nontarget tephritid, Eutreta xanthochaeta (Diptera: Tephritidae). Biol. Control 1997, 8, 58–64. [Google Scholar] [CrossRef]
- Duan, J.J.; Messing, R.H. Effects of host substrate and vibration cues on ovipositor-probing behavior in two larval parasitoids of tephritid fruit flies. J. Insect Behav. 2000, 13, 175–186. [Google Scholar] [CrossRef]
- Montoya, P.; Pérez-Lachaud, G.; Liedo, P. Superparasitism in the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) and the implications for mass rearing and augmentative release. Insects 2012, 3, 900–911. [Google Scholar] [CrossRef]
- Vargas, R.I.; Ramadan, M.; Hussain, T.; Mochizuki, N.; Bautista, R.C.; Stark, J.D. Comparative demography of six fruit fly (Diptera: Tephritidae) parasitoids (Hymenoptera: Braconidae). Biol. Control 2002, 25, 30–40. [Google Scholar] [CrossRef]
- Vargas, R.I.; Leblanc, L.; Putoa, R.; Piñero, J.C. Population dynamics of three Bactrocera spp. fruit flies (Diptera: Tephritidae) and two introduced natural enemies, Fopius arisanus (Sonan) and Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae), after an invasion by Bactrocera dorsalis (Hen). Biol. Control 2012, 60, 199–206. [Google Scholar] [CrossRef]
- Vargas, R.I.; Stark, J.D.; Banks, J.; Leblanc, L.; Manoukis, N.C.; Peck, S. Spatial dynamics of two oriental fruit fly (Diptera: Tephritidae) parasitoids, Fopius arisanus and Diachasmimorpha longicaudata (Hymenoptera: Braconidae), in a guava orchard in Hawaii. Environ. Entomol. 2013, 42, 888–901. [Google Scholar] [CrossRef]
- Giunti, G.; Canale, A.; Messing, R.H.; Donati, E.; Stefanini, C.; Michaud, J.P.; Benelli, G. Parasitoid learning: Current knowledge and implications for biological control. Biol. Control 2015, 90, 208–219. [Google Scholar] [CrossRef]
- Leyva, J.L.; Browning, H.W.; Gilstrap, F.E. Effect of host fruit species, size, and color on parasitization of Anastrepha ludens (Diptera: Tephritidae) by Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Environ. Entomol. 1991, 20, 1469–1474. [Google Scholar] [CrossRef]
- Carrasco, M.; Montoya, P.; Cruz-lopez, L.; Rojas, J.C. Response of the Fruit Fly Parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) to Mango Fruit Volatiles. Env. Entomol. 2005, 34, 576–583. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Papadopoulos, N.T. Are citrus species favorable hosts for the Mediterranean fruit fly? A demographic perspective. Entomol. Exp. Appl. 2009, 132, 1–12. [Google Scholar] [CrossRef]
- Papadopoulos, N.T.; Papachristos, D.P.; Ioannou, C. Citrus fruits and the Mediterranean fruit fly. Acta Hortic. 2015, 1009–1018. [Google Scholar] [CrossRef]
- Lawrence, P.O. Host vibration? A cue to host location by the parasite, Biosteres longicaudatus. Oecologia 1981, 48, 249–251. [Google Scholar] [CrossRef]
- Pérez, J.; Rojas, J.C.; Montoya, P.; Liedo, P.; González, F.J.; Castillo, A. Size, shape and hue modulate attraction and landing responses of the braconid parasitoid Fopius arisanus to fruit odour-baited visual targets. BioControl 2012, 57, 405–414. [Google Scholar] [CrossRef]
- Suárez, L.; Biancheri, M.J.B.; Sánchez, G.; Murúa, F.; Funes, C.F.; Kirschbaum, D.S.; Molina, D.; Laría, O.; Ovruski, S.M. Effects of releasing two Diachasmimorpha longicaudata population lines for the control of Ceratitis capitata infesting three key host fruit species. Biol. Control 2019, 133, 58–65. [Google Scholar] [CrossRef]
- Montoya, P.; Cancino, J.; Zenil, M.; Santiago, G.; Gutierrez, J.M. The Augmentative Biological Control Component in the Mexican National Campaign Against Anastrepha spp. Fruit Flies. In Area-Wide Control of Insect Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 661–670. ISBN 9781402060595. [Google Scholar]
- Sivinski, J.; Aluja, M. The roles of parasitoid foraging for hosts, food and mates in the augmentative control of Tephritidae. Insects 2012, 3, 668–691. [Google Scholar] [CrossRef]
- Bautista, R.C.; Harris, E.J. Effect of fruit substrates on parasitization of tephritid fruit flies (Diptera) by the parasitoid Biosteres arisanus (Hymenoptera: Braconidae). Env. Entomol. 1996, 25, 470–475. [Google Scholar] [CrossRef]
| No-choice test | Dual choice test | ||||||
|---|---|---|---|---|---|---|---|
| Parasitism (%) | Fertility | Sex-ratio | Parasitism (%) | Fertility | Sex-ratio | ||
| Apple/Orange | Apple | 12.92 ± 2.61 | 8.87 ± 1.63 | 33.42 ± 6.95 | 11.47 ± 2.19 | 8.60 ± 1.70 | 21.14 ± 4.80 |
| Orange | 12.10 ± 2.46 | 7.93 ± 1.66 | 33.02 ± 8.83 | 11.72 ± 1.93 | 7.20 ± 1.17 | 35.92 ± 6.44 | |
| t | 0.23 | 0.4 | 0.08 | 0.09 | 0.68 | 1.82 | |
| df | 1, 28 | 1, 28 | 1, 28 | 1, 28 | 1, 28 | 1, 28 | |
| P | 0.821 | 0.691 | 0.937 | 0.932 | 0.503 | 0.081 | |
| Apple/Clementine | Apple | 12.47 ± 2.83 | 9.53± 2.20 | 23.50 ± 7.97 | 4.86 ± 1.22 | 3.73 ± 0.95 | 5.77 ± 5.41 |
| Clementine | 21.87 ± 3.33 | 16.20± 2.63 | 29.57 ± 5.56 | 18.14 ± 4.06 | 12.60 ±2.70 | 32.46 ± 5.63 | |
| t | 2.15 | 1.94 | 0.63 | 3.13 | 3.10 | 3.33 | |
| df | 1, 28 | 1, 28 | 1, 27 | 1, 28 | 1, 28 | 1, 28 | |
| P | 0.040* | 0.061 | 0.533 | 0.006** | 0.006** | 0.004** | |
| Apple/Peach | Apple | 8.85 ± 1.79 | 6.20 ± 1.38 | 52.85± 8.13 | 5.98 ± 1.23 | 3.73 ± 0.81 | 36.06 ± 9.85 |
| Peach | 19.27 ± 1.94 | 11.13 ± 1.02 | 48.55 ± 6.79 | 14.19 ± 2.30 | 7.13 ± 1.12 | 46.00 ± 6.53 | |
| t | −3.95 | −2.87 | 0.41 | −3.15 | −2.46 | -0.85 | |
| df | 1, 28 | 1, 28 | 1, 28 | 1, 28 | 1, 28 | 1, 28 | |
| P | 0.001** | 0.008** | 0.688 | 0.005** | 0.023* | 0.402 | |
| Dual-Choice Test | ||||
|---|---|---|---|---|
| Parasitism (%) | Fertility | Sex-ratio (%) | ||
| Apple/Orange | Apple | 2.62 ± 0.72 | 5.92 ± 1.63 | 80.22 ± 6.77 |
| Orange | 15.34 ± 3.37 | 35.00 ± 8.03 | 51.79 ± 7.24 | |
| t | −3.69 | −3.55 | 2.85 | |
| df | 1, 22 | 1, 22 | 1, 17 | |
| P | 0.003** | 0.004** | 0.011* | |
| Apple/Clementine | Apple | 0.81 ± 0.55 | 1.83 ± 1.24 | 6.33 ± 4.84 |
| Clementine | 6.55 ± 1.42 | 13.25 ± 2.50 | 32.75 ± 5.62 | |
| t | 3.78 | 4.09 | 3.02 | |
| df | 1, 22 | 1, 22 | 1, 13 | |
| P | 0.002** | 0.001** | 0.010* | |
| Apple/Peach | Apple | 2.79 ± 0.92 | 6.75 ± 2.33 | 29.64 ± 10.99 |
| Peach | 14.58 ± 2.83 | 32.08 ± 5.86 | 36.43 ± 5.05 | |
| t | −3.97 | −4.02 | −0.56 | |
| df | 1, 22 | 1, 22 | 1, 21 | |
| P | 0.002** | 0.001** | 0.584 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harbi, A.; de Pedro, L.; Ferrara, F.A.A.; Tormos, J.; Chermiti, B.; Beitia, F.; Sabater-Munoz, B. Diachasmimorpha longicaudata Parasitism Response to Medfly Host Fruit and Fruit Infestation Age. Insects 2019, 10, 211. https://doi.org/10.3390/insects10070211
Harbi A, de Pedro L, Ferrara FAA, Tormos J, Chermiti B, Beitia F, Sabater-Munoz B. Diachasmimorpha longicaudata Parasitism Response to Medfly Host Fruit and Fruit Infestation Age. Insects. 2019; 10(7):211. https://doi.org/10.3390/insects10070211
Chicago/Turabian StyleHarbi, Ahlem, Luis de Pedro, Fernando A. A. Ferrara, José Tormos, Brahim Chermiti, Francisco Beitia, and Beatriz Sabater-Munoz. 2019. "Diachasmimorpha longicaudata Parasitism Response to Medfly Host Fruit and Fruit Infestation Age" Insects 10, no. 7: 211. https://doi.org/10.3390/insects10070211
APA StyleHarbi, A., de Pedro, L., Ferrara, F. A. A., Tormos, J., Chermiti, B., Beitia, F., & Sabater-Munoz, B. (2019). Diachasmimorpha longicaudata Parasitism Response to Medfly Host Fruit and Fruit Infestation Age. Insects, 10(7), 211. https://doi.org/10.3390/insects10070211