Occurrence and Distribution of Apolygus lucorum on Weed Hosts and Tea Plants in Tea Plantation Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experimental Design
2.2. Plot Surveys
2.3. Statistical Analysis
3. Results
3.1. Dominant Mirid Bug Species in Tea Agro-Ecosystems
3.2. Distribution of A. lucorum on Main Hosts in Tea Agro-Ecosystems
3.3. Population Change of A. lucorum on Tea Plants and Weed Hosts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, H.; Liu, B.; Lu, Y.; Wyckhuys, K.A.G. Seasonal alterations in host range and fidelity in the polyphagous mirid bug, Apolygus lucorum (Heteroptera: Miridae). PLoS ONE 2015, 10, e0117153. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, X.; Yuan, W.; Wu, K. Activities of digestive enzymes in the omnivorous pest Apolygus lucorum (Hemiptera: Miridae). J. Econ. Entomol. 2017, 110, 101–110. [Google Scholar] [PubMed]
- Jiang, Y.Y.; Lu, Y.H.; Zeng, J. Forecast and Management of Mirid Bugs in Multiple Agroecosystems of China; China Agriculture Press: Beijing, China, 2015; pp. 16–18. [Google Scholar]
- Zhang, Z.; Zhou, C.; Xu, Y.; Huang, X.; Zhang, L.; Mu, W. Effects of intercropping tea with aromatic plants on population dynamics of arthropods in Chinese tea plantations. J. Pest Sci. 2017, 90, 227–237. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Y.; Liang, G. A method for field assessment of plant injury elicited by the salivary proteins of Apolygus lucorum. Entomol. Exp. Appl. 2013, 149, 292–297. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, K.; Guo, Y. Flight potential of Lygus lucorum (Meyer-Dür) (Heteroptera: Miridae). Environ. Entomol. 2007, 36, 1007–1013. [Google Scholar] [PubMed]
- Lu, Y.H.; Wu, K.M.; Wyckhuys, K.A.G.; Guo, Y.Y. Overwintering hosts of Apolygus lucorum (Hemiptera: Miridae) in northern China. Crop Prot. 2010, 29, 1026–1033. [Google Scholar] [CrossRef]
- Lu, Y.H.; Jiao, Z.B.; Wu, K.M. Early season host plants of Apolygus lucorum (Heteroptera: Miridae) in northern China. J. Econ. Entomol. 2012, 105, 1603–1611. [Google Scholar] [CrossRef]
- Pan, H.; Lu, Y.; Wyckhuys, K.A.G.; Wu, K. Preference of a polyphagous mirid bug, Apolygus lucorum (Meyer-Dür) for flowering host plants. PLoS ONE 2013, 8, e68980. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Qiu, F.; Feng, H.Q.; Li, H.B.; Yang, Z.C.; Wyckhuys, K.A.G.; Wu, K.M. Species composition and seasonal abundance of pestiferous plant bugs (Hemiptera: Miridae) on Bt cotton in China. Crop Prot. 2008, 27, 465–472. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wu, K.M.; Jiang, Y.Y.; Xia, B.; Li, P.; Feng, H.Q.; Wyckhuys, K.A.G.; Guo, Y.Y. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 2010, 328, 1151–1154. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wu, K.M. Mirid bugs in China: Pest status and management strategies. Outlooks Pest Manag. 2011, 22, 248–252. [Google Scholar] [CrossRef]
- Wu, K.M.; Lu, Y.H.; Feng, H.Q.; Jiang, Y.Y.; Zhao, J.Z. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 2008, 321, 1676–1678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, Y.; van der Werf, W.; Huang, J.; Wu, F.; Zhou, K.; Deng, X.; Jiang, Y.; Wu, K.; Rosegrant, M.W. Multidecadal, county-level analysis of the effects of land use, Bt cotton, and weather on cotton pests in China. Proc. Natl. Acad. Sci. USA 2018, 115, 7700–7709. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, H.; Dong, S.; Song, D. Study on the annual population dynamics of Apolygus lucorum in the tea plantation in northern China. J. Shandong Agric. Univ. 2017, 48, 24–27. [Google Scholar]
- Pan, H.; Liu, B.; Lu, Y.; Desneux, N. Identification of the key weather factors affecting overwintering success of Apolygus lucorum eggs in dead host tree branches. PLoS ONE 2014, 9, e94190. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.W.; Pang, H.S.; Lu, Y.H.; Yang, Y.Z. Nymphal performance correlated with adult preference for flowering host plants in a polyphagous mirid bug, Apolygus lucorum (Heteroptera: Miridae). Arthropod-Plant Interact. 2013, 7, 83–91. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, W.; Yang, F.; Yang, Y.; Lu, Y. A PCR-based analysis of plant DNA reveals the feeding preferences of Apolygus lucorum (Heteroptera: Miridae). Arthropod-Plant Interact. 2018, 12, 567–574. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Romeis, J.; van Rijn, P. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 2007, 52, 301–323. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, W.F.; Yang, F.; Xu, B.; Yang, Y.Z. The specific host plant DNA detection suggests a potential migration of Apolygus lucorum from cotton to mungbean fields. PLoS ONE 2017, 12, e0177789. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Ann. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Khan, Z.R.; Ampong-Nyarko, K.; Chiliswa, P.; Hassanali, A.; Kimani, S.; Lwande, W.; Overholt, W.A.; Picketta, J.A.; Smart, L.E.; Woodcock, C.M. Intercropping increases parasitism of pests. Nature 1997, 388, 631–632. [Google Scholar] [CrossRef]
- Khan, Z.R.; Pickett, J.A.; van den Berg, J.; Wadhams, L.J.; Woodcock, C.M. Exploiting chemical ecology and species diversity: Stem borer and striga control for maize and sorghum in Africa. Pest Manag. Sci. 2000, 56, 957–962. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wu, K.M.; Wyckhuys, K.A.G.; Guo, Y.Y. Potential of mungbean, Vigna radiatus as a trap crop for managing Apolygus lucorum (Hemiptera: Miridae) on Bt cotton. Crop Prot. 2009, 28, 77–81. [Google Scholar] [CrossRef]
- Pan, H.; Lu, Y.; Xiu, C.; Geng, H.; Cai, X.; Sun, X.; Zhang, Y.; Williams, L., III; Wyckhuys, K.A.G.; Wu, K. Volatile fragrances associated with flowers mediate host plant alternation of a polyphagous mirid bug. Sci. Rep. 2015, 5, 14805. [Google Scholar] [CrossRef] [PubMed]

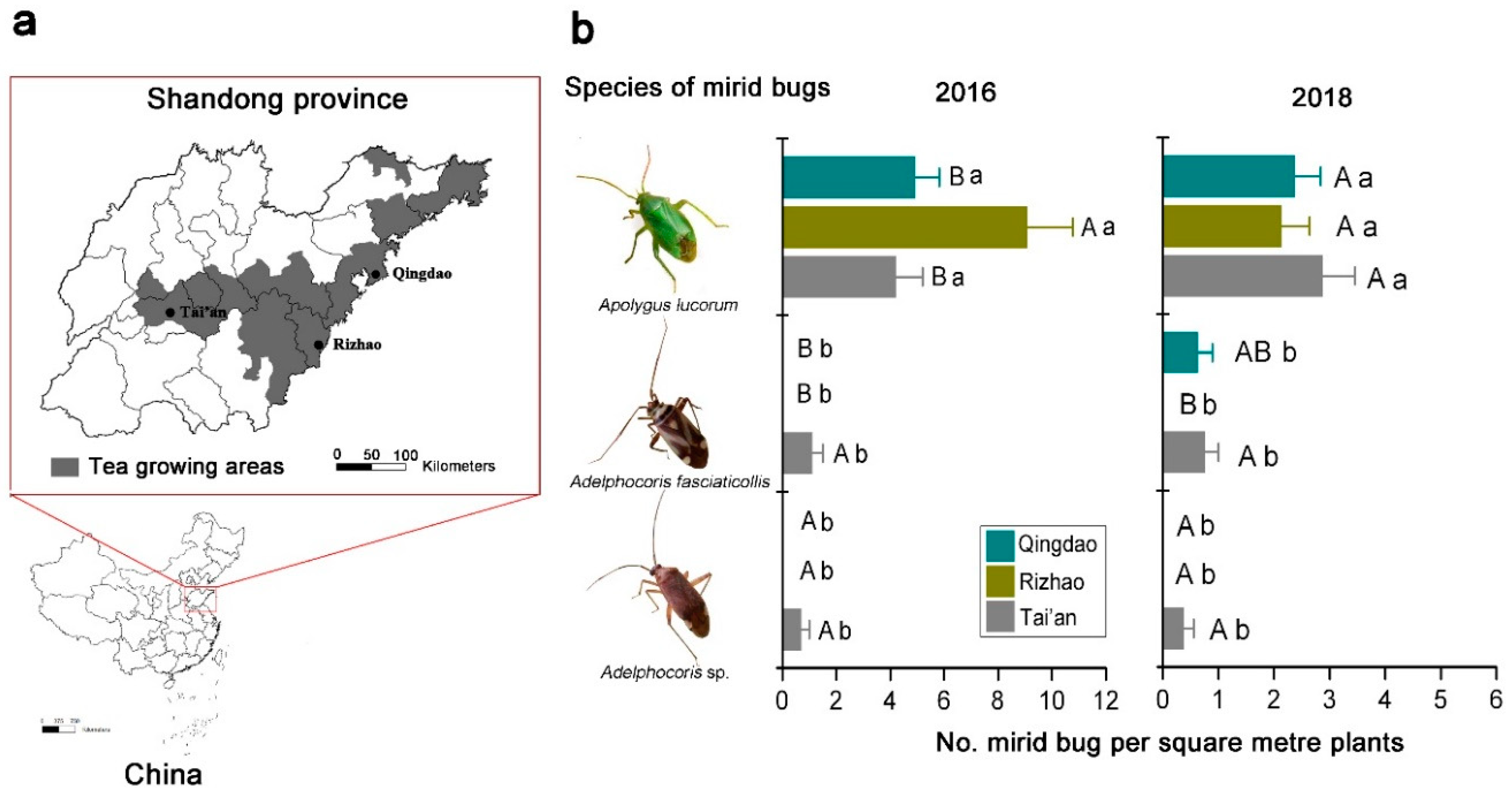
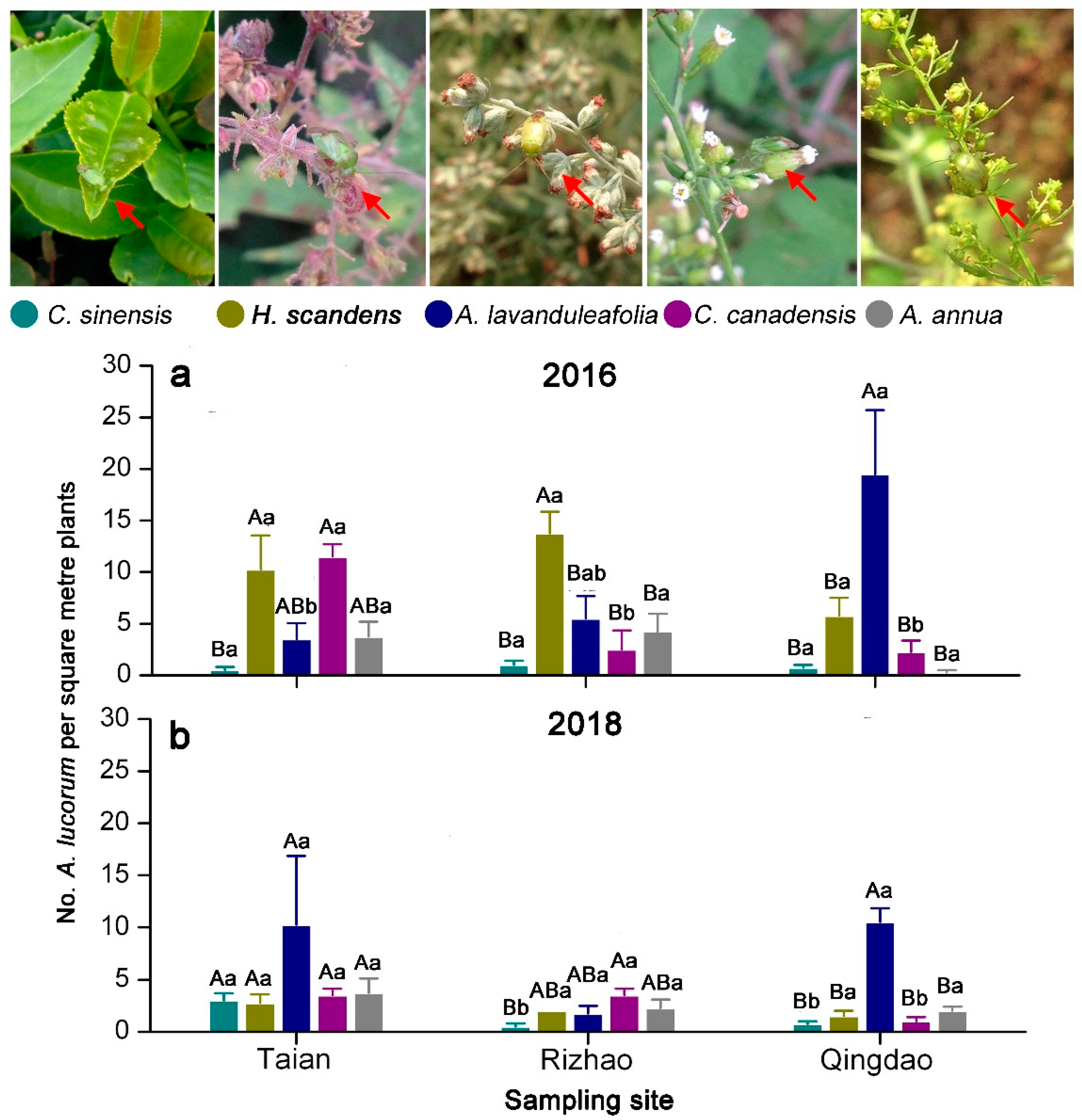
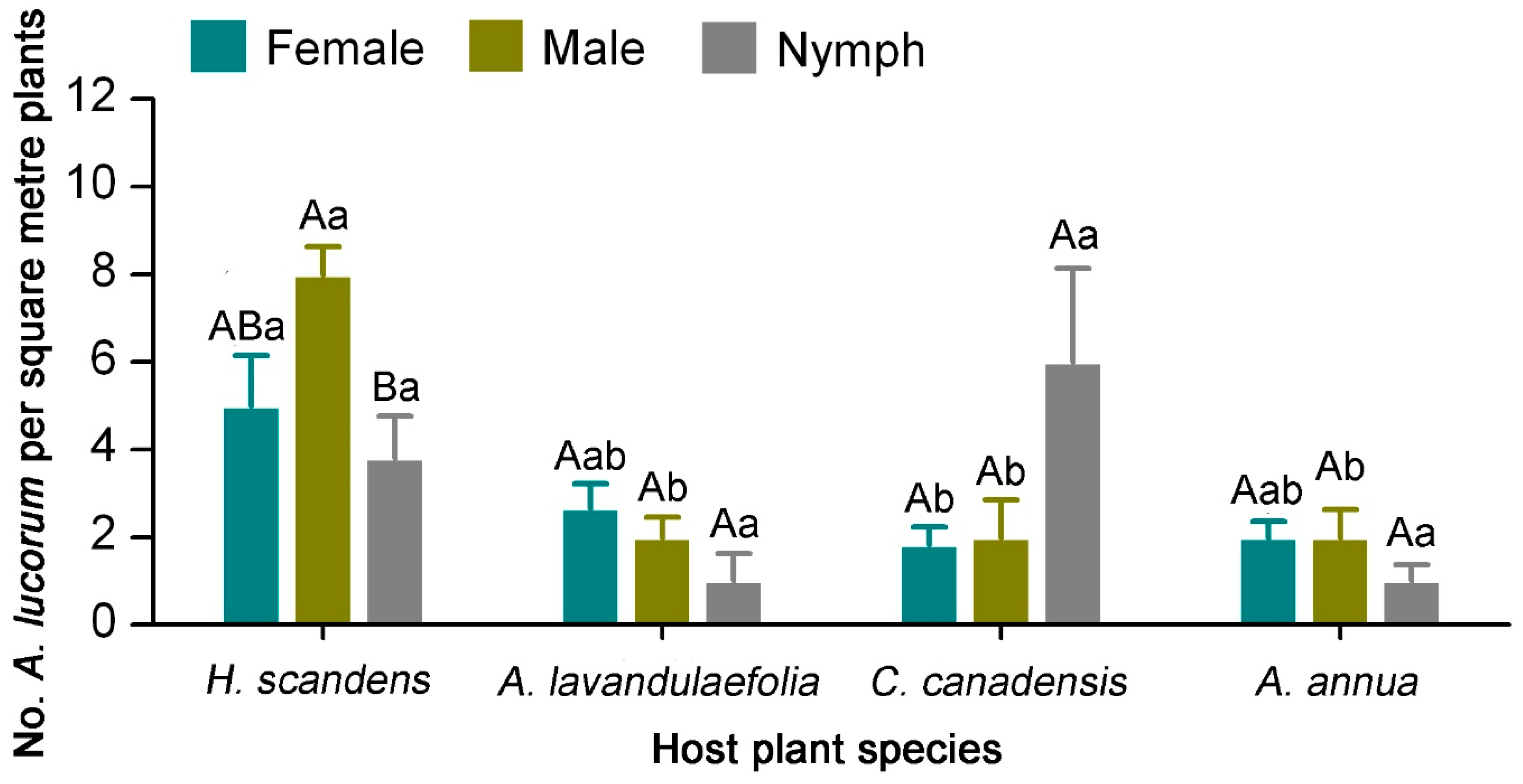
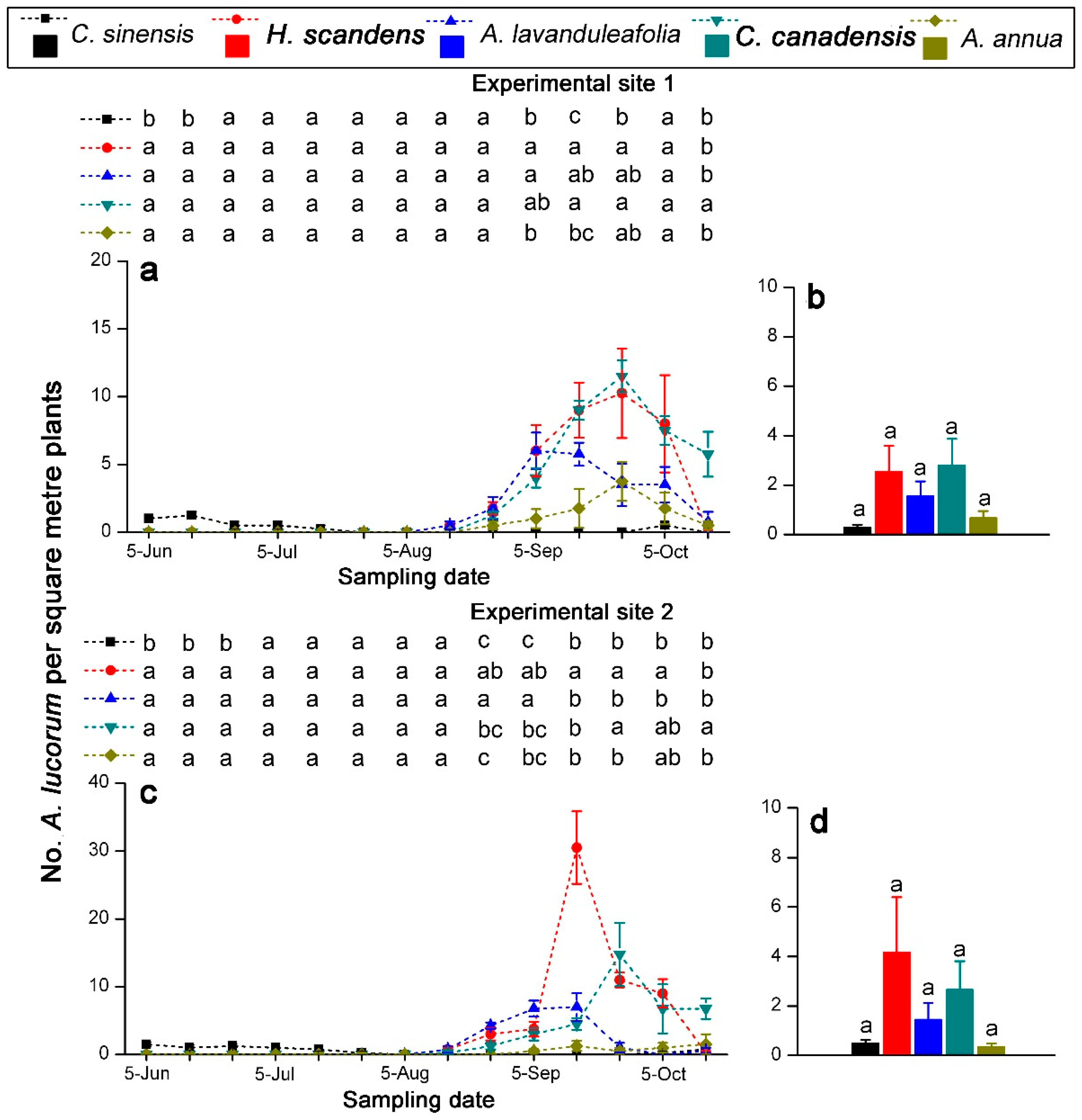
| Source | df | 2016 | 2018 | ||
|---|---|---|---|---|---|
| F | p-Values | F | p-Values | ||
| Mirid bug species | 2, 63 | 45.267 | <0.001 | 34.225 | <0.001 |
| Tea-producing area | 2, 63 | 4.506 | 0.015 | 26.430 | 0.051 |
| Mirid bug species × tea-producing area | 4, 63 | 11.735 | <0.001 | 41.279 | <0.001 |
| Source | df | 2016 | 2018 | ||
|---|---|---|---|---|---|
| F | p-Values | F | p-Values | ||
| Host plant species | 4, 45 | 5.485 | 0.001 | 4.749 | 0.002 |
| Tea-producing area | 2, 45 | 0.035 | 0.965 | 2.383 | 0.102 |
| Host plant species × tea-producing area | 8, 45 | 6.190 | <0.001 | 1.554 | 0.166 |
| Source | df | Experimental Site 1 | Experimental Site 2 | ||
|---|---|---|---|---|---|
| F | p-Values | F | p-Values | ||
| Host plant | 4, 210 | 22.661 | <0.001 | 29.259 | <0.001 |
| Sampling date | 13, 210 | 29.679 | <0.001 | 26.430 | <0.001 |
| Host plant × sampling date | 52, 210 | 5.123 | <0.001 | 12.836 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Wang, H.; Hou, J.; Zhang, L.; Zhang, Z.; Cai, X. Occurrence and Distribution of Apolygus lucorum on Weed Hosts and Tea Plants in Tea Plantation Ecosystems. Insects 2019, 10, 167. https://doi.org/10.3390/insects10060167
Tian Y, Wang H, Hou J, Zhang L, Zhang Z, Cai X. Occurrence and Distribution of Apolygus lucorum on Weed Hosts and Tea Plants in Tea Plantation Ecosystems. Insects. 2019; 10(6):167. https://doi.org/10.3390/insects10060167
Chicago/Turabian StyleTian, Yueyue, Hanyue Wang, Jian Hou, Lixia Zhang, Zhengqun Zhang, and Xiaoming Cai. 2019. "Occurrence and Distribution of Apolygus lucorum on Weed Hosts and Tea Plants in Tea Plantation Ecosystems" Insects 10, no. 6: 167. https://doi.org/10.3390/insects10060167
APA StyleTian, Y., Wang, H., Hou, J., Zhang, L., Zhang, Z., & Cai, X. (2019). Occurrence and Distribution of Apolygus lucorum on Weed Hosts and Tea Plants in Tea Plantation Ecosystems. Insects, 10(6), 167. https://doi.org/10.3390/insects10060167






