Early Olfactory Environment Influences Antennal Sensitivity and Choice of the Host-Plant Complex in a Parasitoid Wasp
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants
Parasitoid Conditioning
- -
- Simple environment, without host switching: Parasitoids from each population had been raised on their respective HPC for over 50 generations and were reared in two separate climatic chambers for at least two generations. Thus, females used for experiments were exposed only to the odor cues of their natal HPC during their larval and pupal development.
- -
- Simple environment, with host switching: Females originating from each population reared in a simple environment for many generations were then allowed to oviposit on the alternative HPC, in the corresponding chamber. Females hatching from the resulting mummies were used for experiments. Thus, experimental insects originated from one HPC strain, but in the last generation developed on the alternative HPC (and were exposed only to the odor cues of this HPC).
- -
- Complex environment: Parasitoids from each population were raised on their respective HPC in a mixed environment, i.e., they were raised on one HPC in the presence of the other HPC. Cages containing the two HPCs were placed next to each other in the same climatic chamber, resulting in a distance of only a few centimeters between mummies on their HPC and the alternative HPC and thus odor exposure to both HPCs. Females were used for experiments after at least 2 generations in their complex environment.
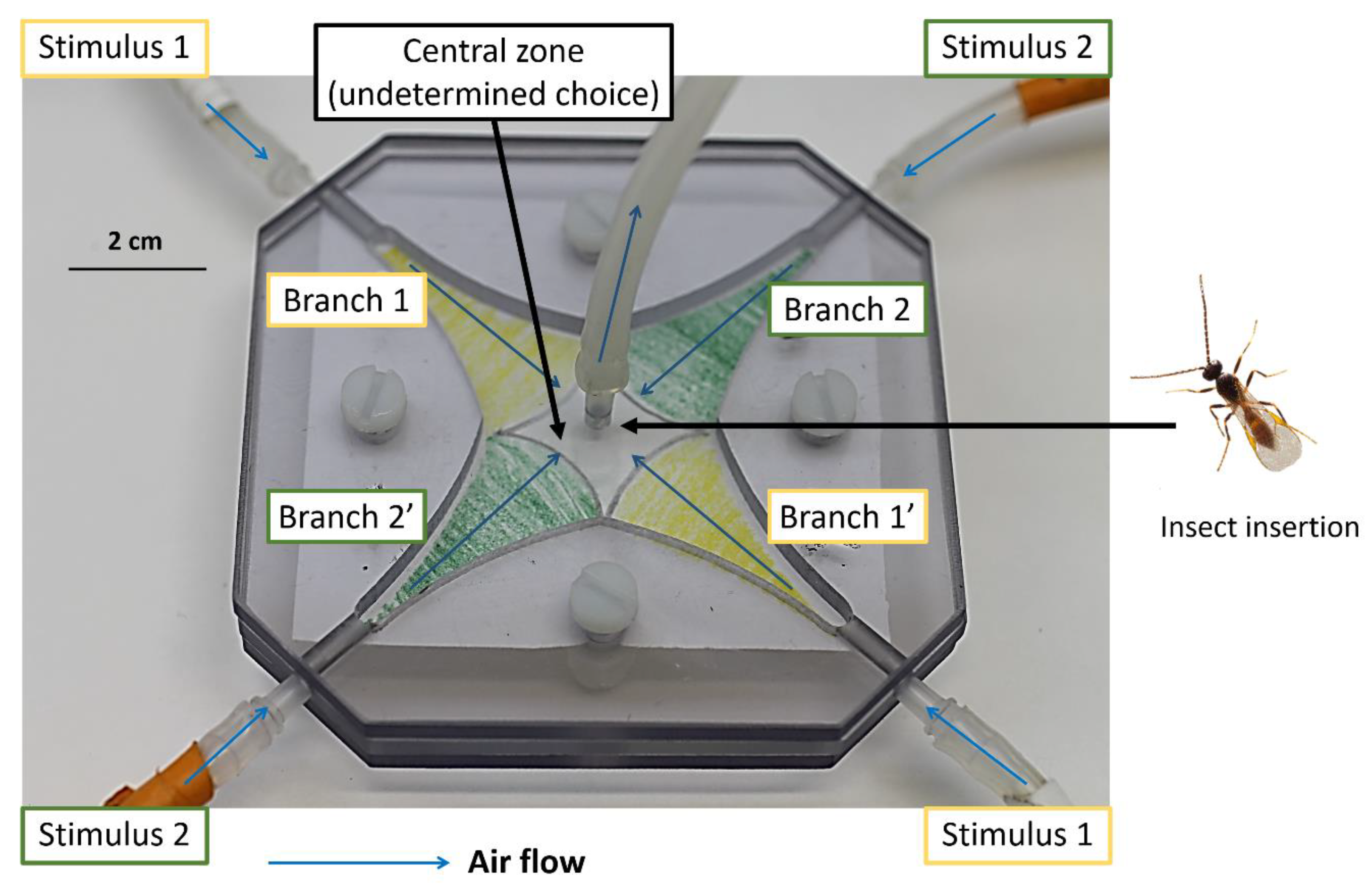
2.2. Behavioral Experiments
2.3. Electroantennography
2.4. Statistical Analysis
3. Results
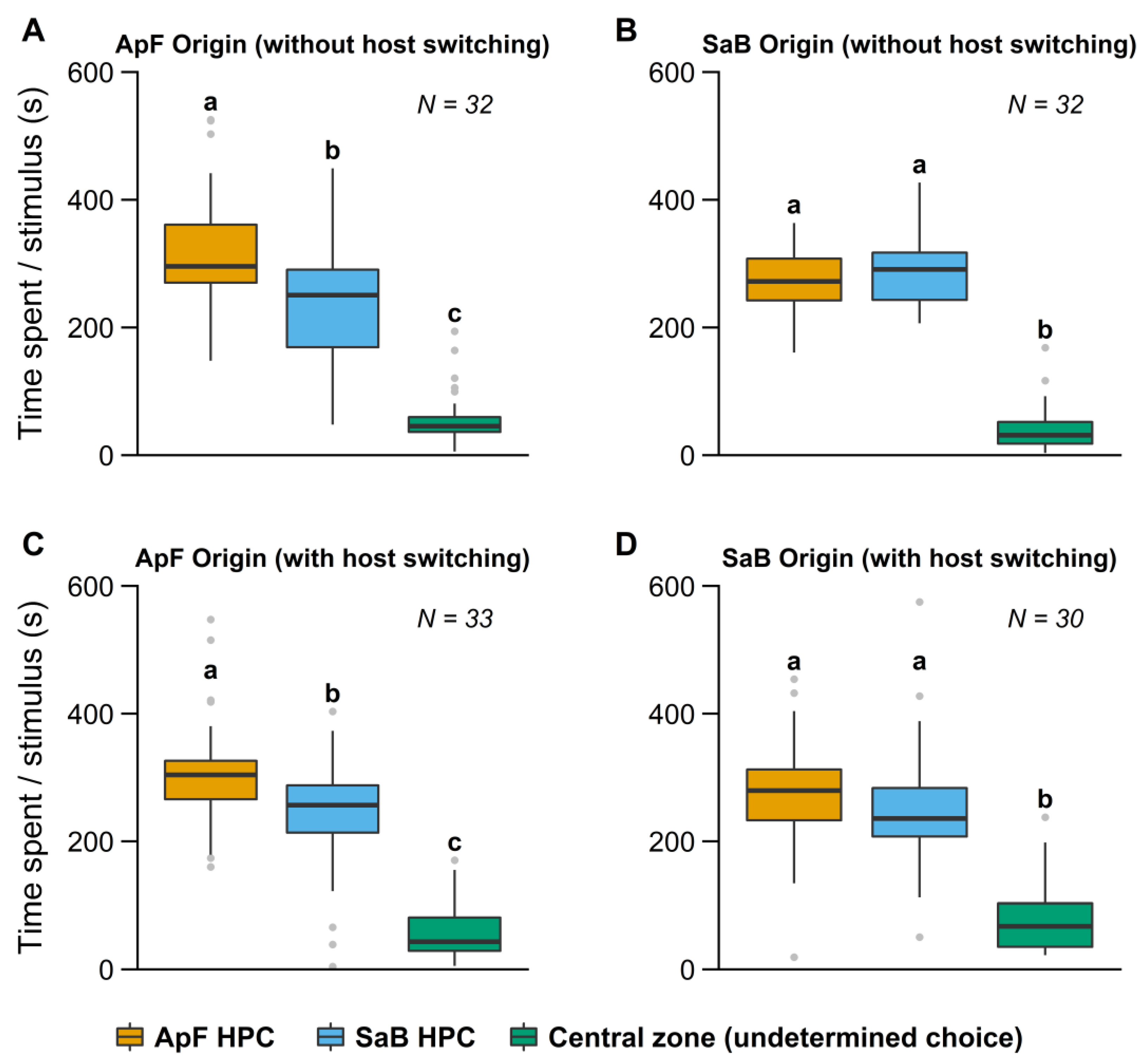
3.1. Influence of Early Experience in a Simple Environment
3.1.1. Influence of Early Experience on HPC Choice
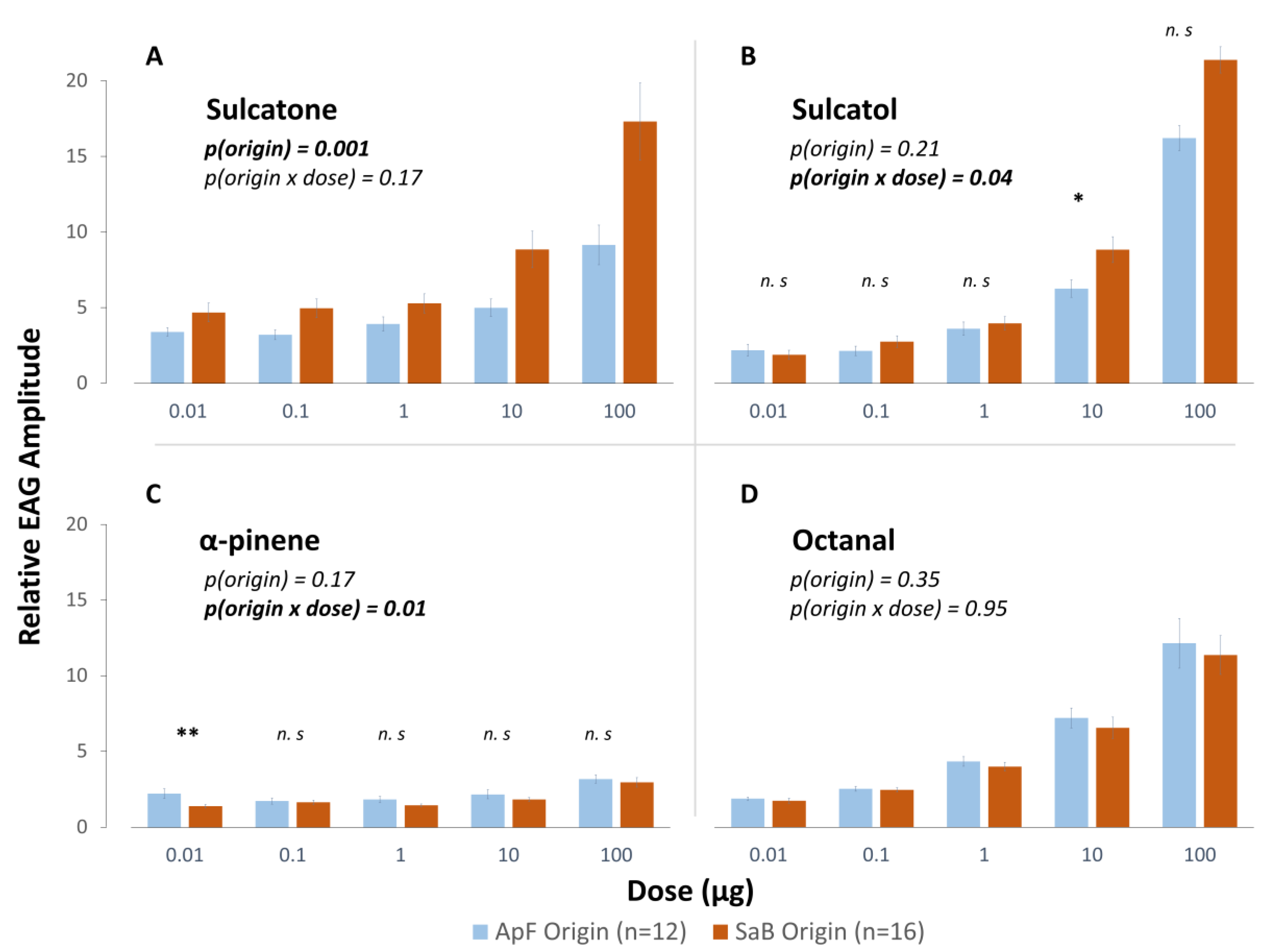
3.1.2. Influence of Early Experience on Antennal Sensitivity
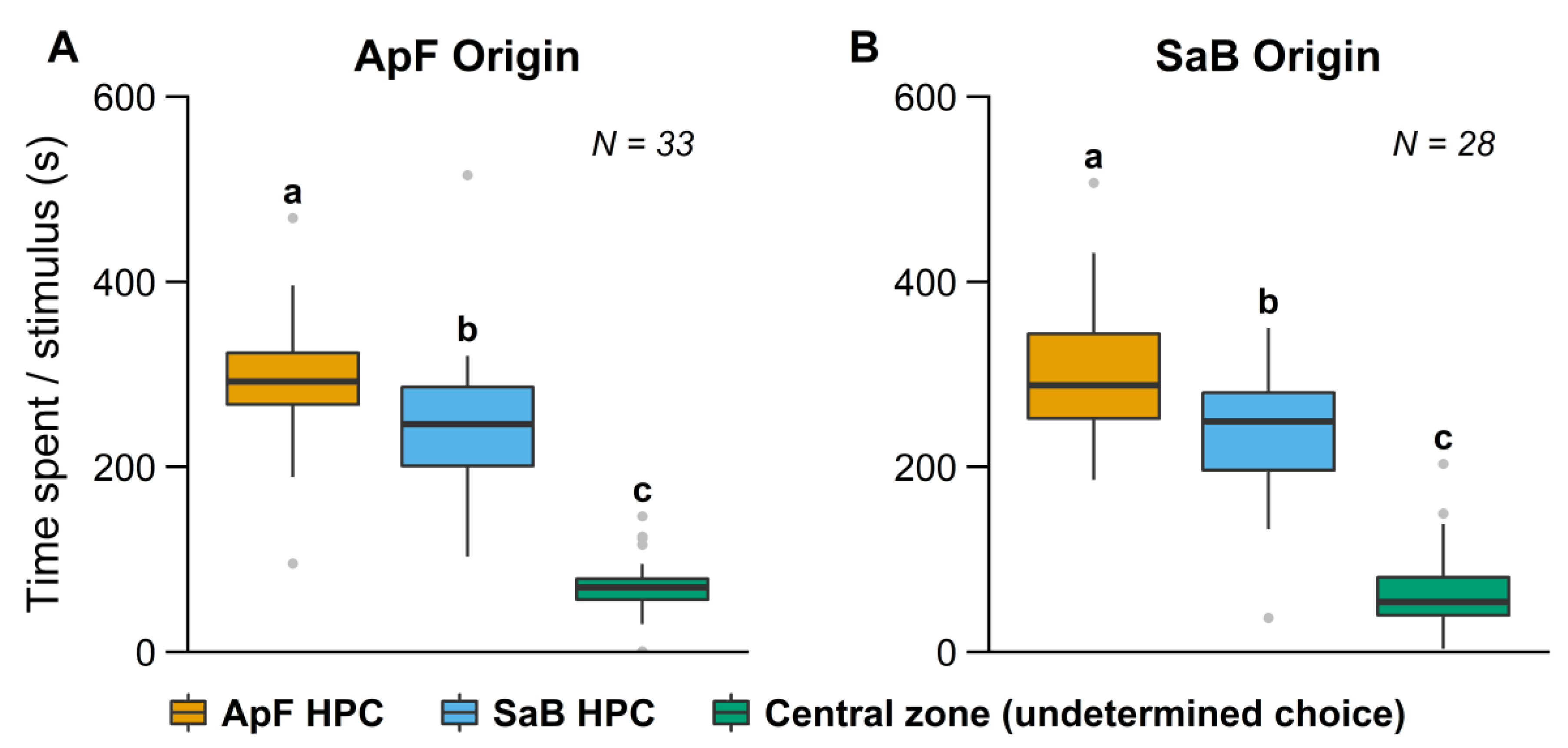
3.2. Influence of Early Experience in a Complex Environment
4. Discussion
4.1. Adaptive Implications of Experience-Modulated Host Choice
4.2. The Effect of Experience of Antennal Sensitivity to HPC-Emitted Volatiles
4.3. Influence of Environment Complexity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Powell, W.; Pennacchio, F.; Poppy, G.; Tremblay, E. Strategies involved in the location of hosts by the parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae: Aphidiinae). Biol. Control 1998, 11, 104–112. [Google Scholar] [CrossRef]
- Hare, J.D. Ecological Role of volatiles produced by plants in response to damage by herbivorous insects. Annu. Rev. Entomol. 2011, 56, 161–180. [Google Scholar] [CrossRef]
- Giunti, G.; Canale, A.; Messing, R.H.; Donati, E.; Stefanini, C.; Michaud, J.P.; Benelli, G. Parasitoid learning: Current knowledge and implications for biological control. Biol. Control 2015, 90, 208–219. [Google Scholar] [CrossRef]
- Chau, A.; Mackauer, M. Preference of the aphid parasitoid Monoctonus paulensis (Hymenoptera: Braconidae, Aphidiinae) for different aphid species: Female choice and offspring survival. Biol. Control 2001, 20, 30–38. [Google Scholar] [CrossRef]
- Poppy, G.M.; Powell, W.; Pennacchio, F. Aphid parasitoid responses to semiochemicals—Genetic, conditioned or learnt? Entomophaga 1997, 42, 193–199. [Google Scholar] [CrossRef]
- Henry, L.M.; Roitberg, B.D.; Gillespie, D.R. Host-Range Evolution in Aphidius Parasitoids: Fidelity, virulence and fitness trade-offs on an ancestral host. Evolution 2008, 62, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Raymond, L.; Plantegenest, M.; Gagic, V.; Navasse, Y.; Lavandero, B. Aphid parasitoid generalism: Development, assessment, and implications for biocontrol. J. Pest Sci. 2016, 89, 7–20. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Liburd, O.E. Behavioral evidence for host fidelity among populations of the parasitic wasp, Diachasma alloeum (Muesebeck). Naturwissenschaften 2005, 92, 65–68. [Google Scholar] [CrossRef]
- König, K.; Krimmer, E.; Brose, S.; Cornelia, G.; Ines, B.; Christian, K.; Seraina, K.; Ingo, W.; Hannes, B.; Lars, K.; et al. Does early learning drive ecological divergence during speciation processes in parasitoid wasps? Proc. R. Soc. B Biol. Sci. 2015, 282, 20141850. [Google Scholar] [CrossRef]
- Menzel, R. The honeybee as a model for understanding the basis of cognition. Nat. Rev. Neurosci. 2012, 13, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Arenas, A.; Giurfa, M.; Farina, W.M.; Sandoz, J.C. Early olfactory experience modifies neural activity in the antennal lobe of a social insect at the adult stage. Eur. J. Neurosci. 2009, 30, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, F.; Gemeno, C.; Monsempes, C.; Anton, S.; Jacquin-Joly, E.; Lucas, P.; Devaud, J.-M. Experience-dependent modulation of antennal sensitivity and input to antennal lobes in male moths (Spodoptera littoralis) pre-exposed to sex pheromone. J. Exp. Biol. 2012, 215, 2334–2341. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Paulo, F.A.; Ortiz-Martínez, S.A.; Figueroa, C.C.; Lavandero, B. Adaptive evolution of a generalist parasitoid: Implications for the effectiveness of biological control agents. Evol. Appl. 2013, 6, 983–999. [Google Scholar] [CrossRef]
- Gutiérrez-Ibáñez, C.; Villagra, C.A.; Niemeyer, H.M. Pre-pupation behaviour of the aphid parasitoid Aphidius ervi (Haliday) and its consequences for pre-imaginal learning. Naturwissenschaften 2007, 94, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, H.; Powell, W.; Pickett, J.; Kainoh, Y.; Takabayashi, J. Two-step learning involved in acquiring olfactory preferences for plant volatiles by parasitic wasps. Anim. Behav. 2012, 83, 1491–1496. [Google Scholar] [CrossRef]
- Daza-Bustamante, P.; Fuentes-Contreras, E.; Rodríguez, L.C.; Figueroa, C.C.; Niemeyer, H.M. Behavioural differences between Aphidius ervi populations from two tritrophic systems are due to phenotypic plasticity. Entomol. Exp. Appl. 2002, 104, 321–328. [Google Scholar] [CrossRef]
- Ballesteros, G.I.; Gadau, J.; Legeai, F.; Gonzalez-Gonzalez, A.; Lavandero, B.; Simon, J.-C.; Figueroa, C.C. Expression differences in Aphidius ervi (Hymenoptera: Braconidae) females reared on different aphid host species. PeerJ 2017, 5, e3640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amarawardana, L.; Bandara, P.; Kumar, V.; Pettersson, J.; Ninkovic, V.; Glinwood, R. Olfactory response of Myzus persicae (Homoptera: Aphididae) to volatiles from leek and chive: Potential for intercropping with sweet pepper. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2007, 57, 87–91. [Google Scholar] [CrossRef]
- Dardouri, T.; Gautier, H.; Issa, R.B.; Costagliola, G.; Gomez, L. Repellence of Myzus persicae (Sulzer): Evidence of two modes of action of volatiles from selected living aromatic plants. Pest Manag. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wäschke, N.; Meiners, T.; Rostás, M. Foraging strategies of parasitoids in complex chemical environments. In Chemical Ecology of Insect Parasitoids; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 37–63. ISBN 978-1-118-40958-9. [Google Scholar]
- Aartsma, Y.; Bianchi, F.J.J.A.; van der Werf, W.; Poelman, E.H.; Dicke, M. Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol. 2017, 216, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- van Emden, H.F.; Eletherianos, I.; Rose, J.; Douloumpaka, S.; Pettersson, J. Aphid parasitoids detect that an alien plant was present nearby during their development. Physiol. Entomol. 2002, 27, 199–205. [Google Scholar] [CrossRef]
- Pettersson, J. An Aphid sex attractant. Entomol. Scand. 1970, 1, 63–73. [Google Scholar] [CrossRef]
- Beyaert, I.; Wäschke, N.; Scholz, A.; Varama, M.; Reinecke, A.; Hilker, M. Relevance of resource-indicating key volatiles and habitat odour for insect orientation. Anim. Behav. 2010, 79, 1077–1086. [Google Scholar] [CrossRef]
- Hervé, M. sequenceR: Une Interface D’encodage de Séquences Comportementales 2013. Available online: https://drive.google.com/file/d/1GyYmbQhGZr_4UyFCGN34HKF7Sh5h6zLx/view (accessed on 5 June 2018).
- Takemoto, H.; Takabayashi, J. Parasitic wasps Aphidius ervi are more attracted to a blend of host-induced plant volatiles than to the independent compounds. J. Chem. Ecol. 2015, 41, 801–807. [Google Scholar] [CrossRef]
- Quiroz, A.; Pettersson, J.; Pickett, J.A.; Wadhams, L.J.; Niemeyer, H.M. Semiochemicals mediating spacing behavior of bird cherry-oat aphid, Rhopalosiphum padi feeding on cereals. J. Chem. Ecol. 1997, 23, 2599–2607. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.-L.; Guo, G.-X.; Ji, X.-L. Volatile emission in wheat and parasitism by Aphidius avenae after exogenous application of salivary enzymes of Sitobion avenae. Entomol. Exp. Appl. 2009, 130, 215–221. [Google Scholar] [CrossRef]
- Dong, L.; Hou, Y.; Li, F.; Piao, Y.; Zhang, X.; Zhang, X.; Li, C.; Zhao, C. Characterization of volatile aroma compounds in different brewing barley cultivars. J. Sci. Food Agric. 2015, 95, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, V.; Ahmed, E.; Glinwood, R.; Pettersson, J. Effects of two types of semiochemical on population development of the bird cherry oat aphid Rhopalosiphum padi in a barley crop. Agric. For. Entomol. 2003, 5, 27–34. [Google Scholar] [CrossRef]
- Bjostad, L. Electrophysiological Methods. In Methods in Chemical Ecology—Volume 1, Chemical Methods; Kluwer Academic Publishing: Dordrecht, The Netherlands, 1998; pp. 339–375. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using Ime4. J. Stat. Softw. 2016, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Rodriguez, L.C.; Niemeyer, H.M.; Fuentes-Contreras, E. Effect of innate preferences, conditioning and adult experience on the attraction of Aphidius ervi (Hymenoptera: Braconidae) toward plant volatiles. Eur. J. Entomol. Czech Repub. 2002, 99, 285–288. [Google Scholar] [CrossRef]
- Starý, P.; Gerding, I.M.; Norambuena, I.H.; Remaudière, G. Environmental research on aphid parasitoid biocontrol agents in Chile (Hym., Aphidiidae; Hom., Aphidoidea). J. Appl. Entomol. 1993, 115, 292–306. [Google Scholar] [CrossRef]
- Zepeda-Paulo, F.; Dion, E.; Lavandero, B.; Mahéo, F.; Outreman, Y.; Simon, J.C.; Figueroa, C.C. Signatures of genetic bottleneck and differentiation after the introduction of an exotic parasitoid for classical biological control. Biol. Invasions 2016, 18, 565–581. [Google Scholar] [CrossRef]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; ISBN 978-0-691-00047-3. [Google Scholar]
- Jaenike, J. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 1978, 14, 350–356. [Google Scholar] [CrossRef]
- Henry, L.M.; Gillespie, D.R.; Roitberg, B.D. Does mother really know best? Oviposition preference reduces reproductive performance in the generalist parasitoid Aphidius ervi. Entomol. Exp. Appl. 2005, 116, 167–174. [Google Scholar] [CrossRef]
- Daza-Bustamante, P.; Fuentes-Contreras, E.; Niemeyer, H.M. Acceptance and suitability of Acyrthosiphon pisum and Sitobion avenae as hosts of the aphid parasitoid Aphidius ervi (Hymenoptera: Braconidae). EJE Eur. J. Entomol. 2003, 100, 49–53. [Google Scholar] [CrossRef]
- Cameron, P.J.; Powell, W.; Loxdale, H.D. Reservoirs for Aphidius ervi Haliday (Hymenoptera: Aphidiidae), a polyphagous parasitoid of cereal aphids (Hemiptera: Aphididae). Bull. Entomol. Res. 1984, 74, 647–656. [Google Scholar] [CrossRef]
- Pungerl, N.B. Host preferences of Aphidius (Hymenoptera: Aphidiidae) populations parasitising pea and cereal aphids (Hemiptera: Aphididae). Bull. Entomol. Res. 1984, 74, 153–162. [Google Scholar] [CrossRef]
- Chesnais, Q.; Ameline, A.; Doury, G.; Le Roux, V.; Couty, A. Aphid parasitoid mothers don’t always know best through the whole host selection process. PLoS ONE 2015, 10, e0135661. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Anton, S. Experience-based modulation of behavioural responses to plant volatiles and other sensory cues in insect herbivores. Plant Cell Environ. 2014, 37, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; McNeil, J. Chemical and behavioral ecology in insect parasitoids: How to behave optimally in a complex odorous environment. In Behavioral Ecology of Insect Parasitoids; Wajnberg, É., Bernstein, C., van Alphen, J., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; pp. 92–112. ISBN 978-0-470-69620-0. [Google Scholar]
- Xu, Q.; Hatt, S.; Han, Z.; Francis, F.; Chen, J. Combining E-β-farnesene and methyl salicylate release with wheat-pea intercropping enhances biological control of aphids in North China. Biocontrol Sci. Technol. 2018, 28, 883–894. [Google Scholar] [CrossRef]
| Environment | Treatment | Friedman’s Test (df = 2) | Pairwise Comparisons (p-value) 1 | |||
|---|---|---|---|---|---|---|
| χ2 | p | ApF vs. SaB | ApF vs. Central | SaB vs. Central | ||
| Simple | ApF (no host switching) | 44 | <0.01 | 0.005 | <0.01 | <0.01 |
| ApF (host switching) | 46 | <0.01 | 0.034 | <0.01 | <0.01 | |
| SaB (no host switching) | 48.3 | <0.01 | 0.68 | <0.01 | <0.01 | |
| SaB (host switching) | 34.9 | <0.01 | 0.22 | <0.01 | <0.01 | |
| Complex | ApF (no host switching) | 48 | <0.01 | 0.011 | <0.01 | <0.01 |
| SaB (no host switching) | 36.5 | <0.01 | 0.048 | <0.01 | <0.01 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luquet, M.; Tritto, O.; Cortesero, A.-M.; Jaloux, B.; Anton, S. Early Olfactory Environment Influences Antennal Sensitivity and Choice of the Host-Plant Complex in a Parasitoid Wasp. Insects 2019, 10, 127. https://doi.org/10.3390/insects10050127
Luquet M, Tritto O, Cortesero A-M, Jaloux B, Anton S. Early Olfactory Environment Influences Antennal Sensitivity and Choice of the Host-Plant Complex in a Parasitoid Wasp. Insects. 2019; 10(5):127. https://doi.org/10.3390/insects10050127
Chicago/Turabian StyleLuquet, Martin, Olympe Tritto, Anne-Marie Cortesero, Bruno Jaloux, and Sylvia Anton. 2019. "Early Olfactory Environment Influences Antennal Sensitivity and Choice of the Host-Plant Complex in a Parasitoid Wasp" Insects 10, no. 5: 127. https://doi.org/10.3390/insects10050127
APA StyleLuquet, M., Tritto, O., Cortesero, A.-M., Jaloux, B., & Anton, S. (2019). Early Olfactory Environment Influences Antennal Sensitivity and Choice of the Host-Plant Complex in a Parasitoid Wasp. Insects, 10(5), 127. https://doi.org/10.3390/insects10050127







