Heterologous Expression of Aedes aegypti Cation Chloride Cotransporter 2 (aeCCC2) in Xenopus laevis Oocytes Induces an Enigmatic Na+/Li+ Conductance
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning and cRNA Preparation
2.2. Heterologous Expression in Xenopus laevis Oocytes
2.3. Measurement of Ion Uptake in Xenopus Oocytes
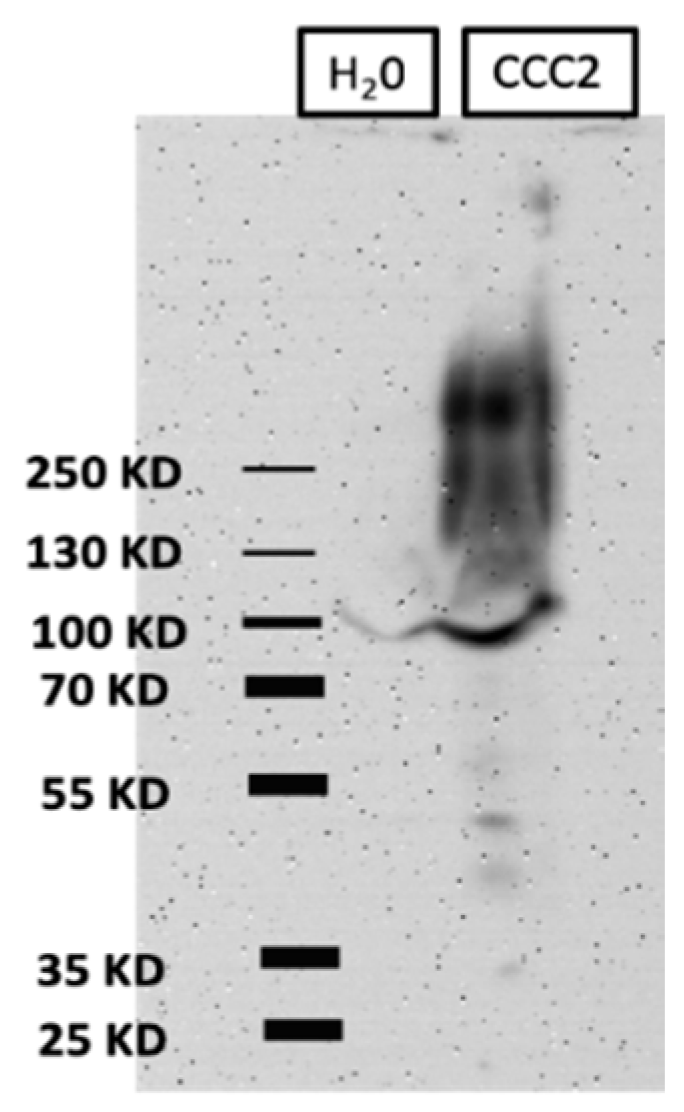
2.4. Western Blots of Total Membrane Protein
2.5. Xenopus Oocyte Electrophysiology
2.6. Statistics
3. Results and Discussion
3.1. Heterologous Expression of aeCCC2 in Xenopus Oocytes
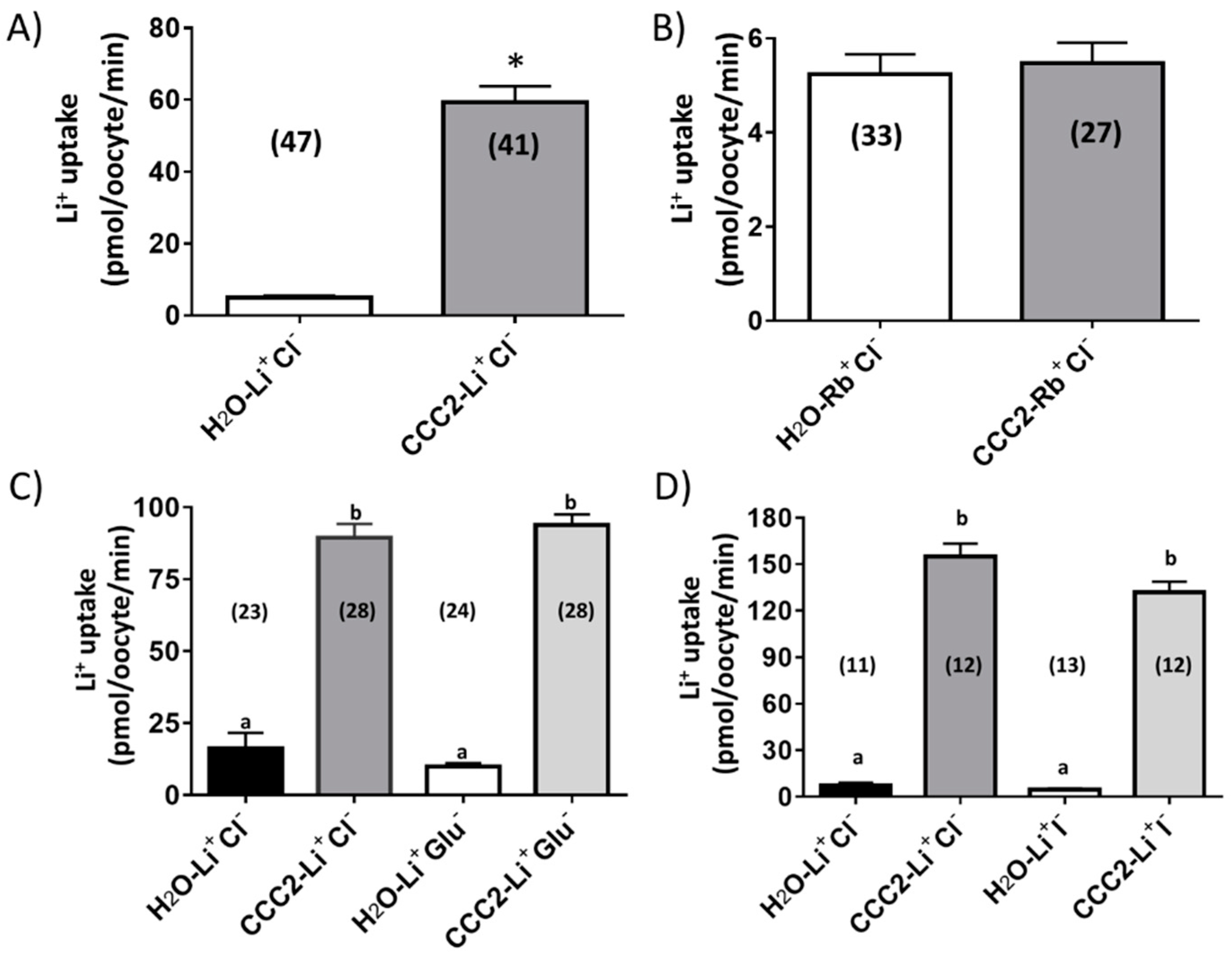
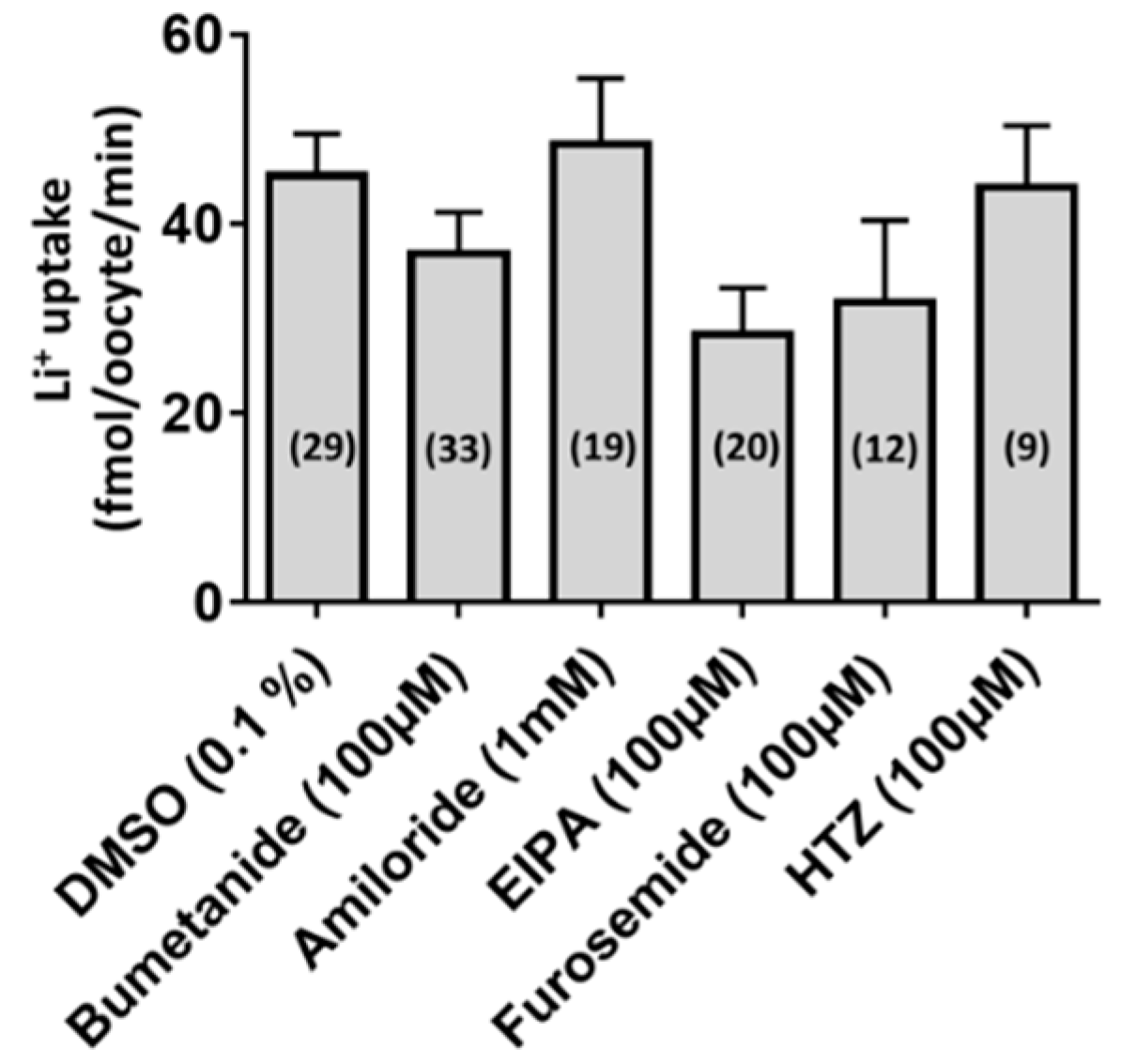
3.2. Functional Characterization of aeCCC2
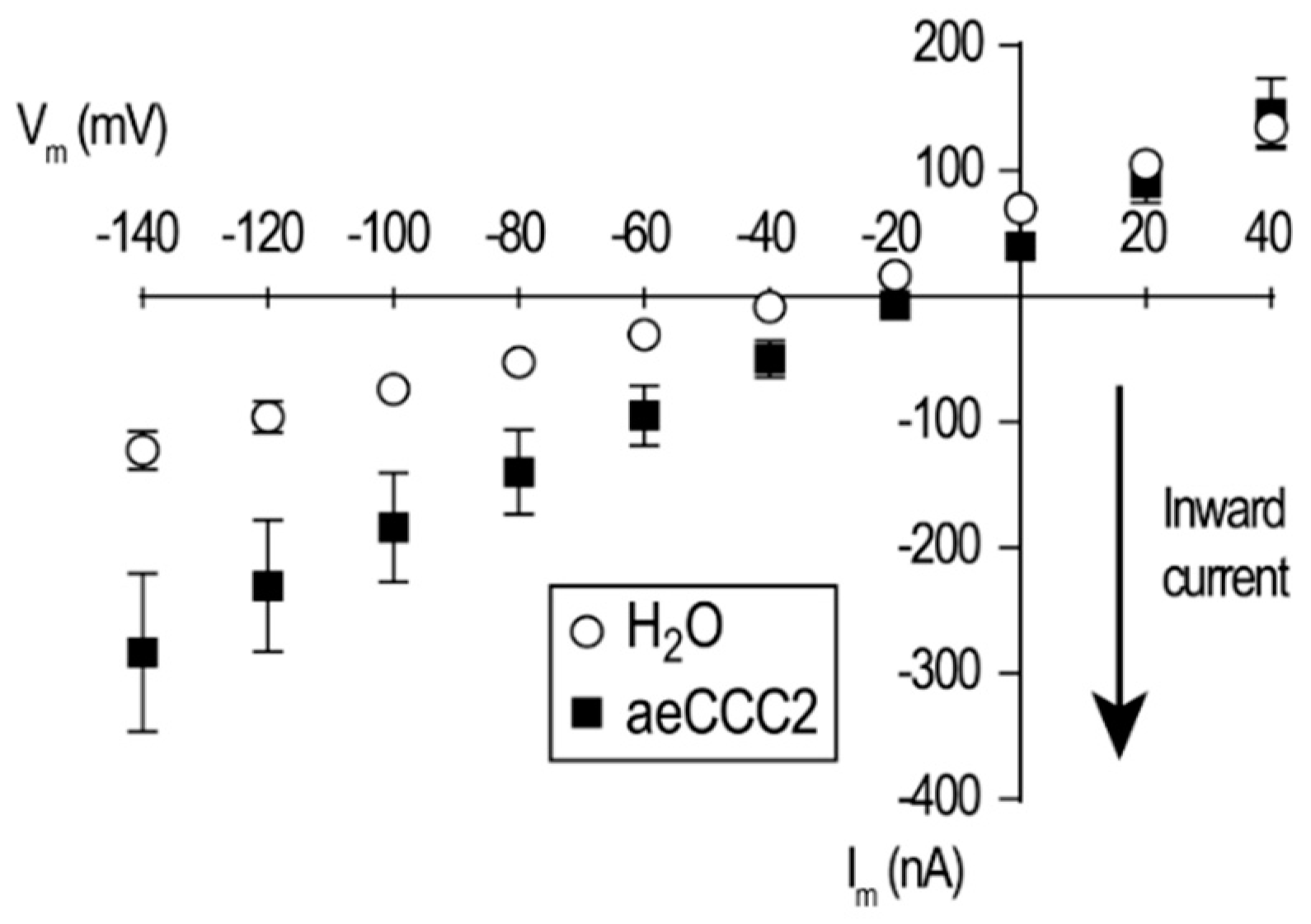
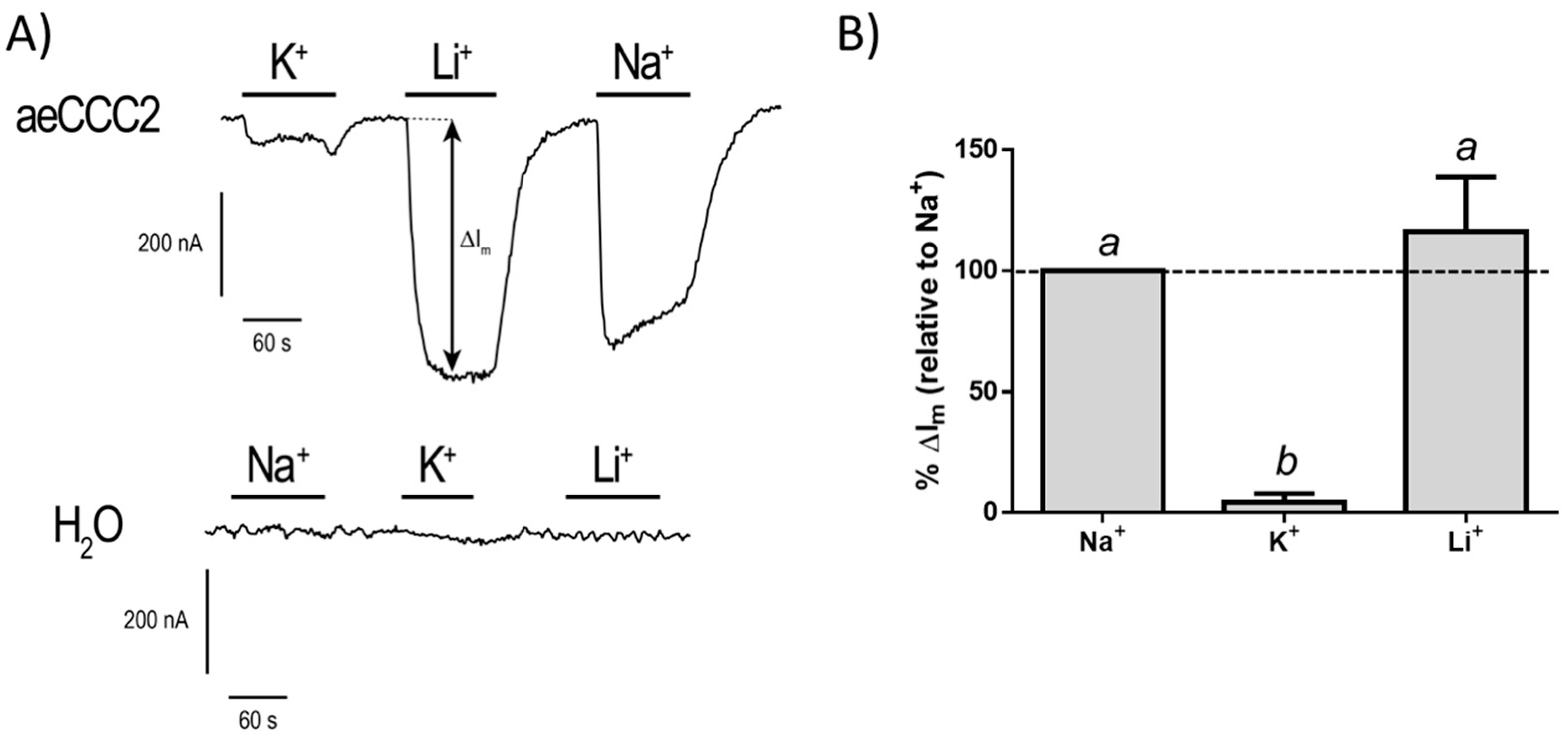
3.3. Electrophysiological Characterization of aeCCC2 in the Xenopus Oocytes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orlov, S.N.; Koltsova, S.V.; Kapilevich, L.V.; Dulin, N.O.; Gusakova, S.V. Cation-Chloride Cotransporters: Regulation, Physiological Significance, and Role in Pathogenesis of Arterial Hypertension. Biochem. Mosc. 2014, 79, 1546–1561. [Google Scholar] [CrossRef] [PubMed]
- Delpire, E.; Mount, D.B. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu. Rev. Physiol. 2002, 64, 803–843. [Google Scholar] [CrossRef]
- Pond, B.B.; Berglund, K.; Kuner, T.; Feng, G.P.; Augustine, G.J.; Schwartz-Bloom, R.D. The chloride transporter Na+-K+-Cl− cotransporter isoform-1 contributes to intracellular chloride increases after in vitro ischemia. J. Neurosci. 2006, 26, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.A.; Stevenson, T.J.; Donaldson, L.F. Molecular characterization of a putative K-Cl cotransporter in rat brain—A neuronal-specific isoform. J. Biol. Chem. 1996, 271, 16245–16252. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Carmosino, M.; Forbush, B. Intramolecular and intermolecular fluorescence resonance energy transfer in fluorescent protein-tagged Na-K-Cl cotransporter (NKCC1)—Sensitivity to regulatory conformational change and cell volume. J. Biol. Chem. 2008, 283, 2663–2674. [Google Scholar] [CrossRef] [PubMed]
- Blaesse, P.; Airaksinen, M.S.; Rivera, C.; Kaila, K. Cation-Chloride Cotransporters and Neuronal Function. Neuron 2009, 61, 820–838. [Google Scholar] [CrossRef]
- Kaila, K.; Price, T.J.; Payne, J.A.; Puskarjov, M.; Voipio, J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 2014, 15, 637–654. [Google Scholar] [CrossRef]
- Payne, J.A. Molecular Operation of the Cation Chloride Cotransporters: Ion Binding and Inhibitor Interaction. Curr. Top. Membr. 2012, 70, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Hekmat-Scafe, D.S.; Lundy, M.Y.; Ranga, R.; Tanouye, M.A. Mutations in the K+/Cl− cotransporter gene kazachoc (kcc) increase seizure susceptibility in Drosophila. J. Neurosci. 2006, 26, 8943–8954. [Google Scholar] [CrossRef] [PubMed]
- Ianowski, J.P.; O’Donnell, M.J. Basolateral ion transport mechanisms during fluid secretion by Drosophila Malpighian tubules: Na+ recycling, Na+: K+: 2Cl− cotransport and Cl− conductance. J. Exp. Biol. 2004, 207, 2599–2609. [Google Scholar] [CrossRef] [PubMed]
- Beyenbach, K.W.; Piermarini, P.M. Transcellular and paracellular pathways of transepithelial fluid secretion in Malpighian (renal) tubules of the yellow fever mosquito Aedes aegypti. Acta Physiol. 2011, 202, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Prael, F.J.; Chen, R.; Li, Z.L.; Reed, C.W.; Lindsley, C.W.; Weaver, C.D.; Swale, D.R. Use of chemical probes to explore the toxicological potential of the K+/Cl− cotransporter (KCC) as a novel insecticide target to control the primary vector of dengue and Zika virus, Aedes aegypti. Pestic Biochem. Phys. 2018, 151, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rodan, A.R.; Baum, M.; Huang, C.L. The Drosophila NKCC Ncc69 is required for normal renal tubule function. Am. J. Physiol.-Cell Physiol. 2012, 303, C883–C894. [Google Scholar] [CrossRef] [PubMed]
- Leiserson, W.M.; Forbush, B.; Keshishian, H. Drosophila Glia Use a Conserved Cotransporter Mechanism to Regulate Extracellular Volume. Glia 2011, 59, 320–332. [Google Scholar] [CrossRef]
- Sun, Q.F.; Tian, E.; Turner, R.J.; Ten Hagen, K.G. Developmental and functional studies of the SLC12 gene family members from Drosophila melanogaster. Am. J. Physiol.-Cell Physiol. 2010, 298, C26–C37. [Google Scholar] [CrossRef] [PubMed]
- Piermarini, P.M.; Akuma, D.C.; Crow, J.C.; Jamil, T.L.; Kerkhoff, W.G.; Viel, K.; Gillen, C.M. Differential expression of putative sodium-dependent cation-chloride cotransporters in Aedes aegypti. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 214, 40–49. [Google Scholar] [CrossRef]
- Durr, K.L.; Tavraz, N.N.; Spiller, S.; Friedrich, T. Measuring cation transport by Na,K- and H,K-ATPase in Xenopus oocytes by atomic absorption spectrophotometry: An alternative to radioisotope assays. J. Vis. Exp. 2013, e50201. [Google Scholar] [CrossRef]
- Lytle, C.; Forbush, B., 3rd. Regulatory phosphorylation of the secretory Na-K-Cl cotransporter: Modulation by cytoplasmic Cl. Am. J. Physiol. 1996, 270, C437–C448. [Google Scholar] [CrossRef]
- Darman, R.B.; Forbush, B. A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J. Biol. Chem. 2002, 277, 37542–37550. [Google Scholar] [CrossRef]
- Ponce-Coria, J.; San-Cristobal, P.; Kahle, K.T.; Vazquez, N.; Pacheco-Alvarez, D.; de Los Heros, P.; Juarez, P.; Munoz, E.; Michel, G.; Bobadilla, N.A.; et al. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc. Natl. Acad. Sci. USA 2008, 105, 8458–8463. [Google Scholar] [CrossRef]
- Breitwieser, G.E.; Altamirano, A.A.; Russell, J.M. Osmotic Stimulation of Na+, K+, Cl− Cotransport in Squid Giant-Axon Is [Cl−]I-Dependent. Biophys. J. 1990, 57, A183. [Google Scholar] [CrossRef] [PubMed]
- Bowles, D.W.; Gillen, C.M. Characterization of Rb uptake into Sf9 cells using cation chromatography: Evidence for a K-Cl cotransporter. J. Insect Physiol. 2001, 47, 523–532. [Google Scholar] [CrossRef]
- Piermarini, P.M.; Weihrauch, D.; Meyer, H.; Huss, M.; Beyenbach, K.W. NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow fever mosquito Aedes aegypti. Am. J. Physiol.-Renal. 2009, 296, F730–F750. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Piermarini, P.M.; Hine, R.M.; Schepel, M.; Miyauchi, J.; Beyenbach, K.W. Role of an apical K,Cl cotransporter in urine formation by renal tubules of the yellow fever mosquito (Aedes aegypti). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1318–R1337. [Google Scholar] [CrossRef]
- Nessler, S.; Friedrich, O.; Bakouh, N.; Fink, R.H.A.; Sanchez, C.P.; Planelles, G.; Lanzer, M. Evidence for activation of endogenous transporters in Xenopus laevis oocytes expressing the Plasmodium falciparum chloroquine resistance transporter, PfCRT. J. Biol. Chem. 2004, 279, 39438–39446. [Google Scholar] [CrossRef]
- Walters, Z.S.; Haworth, K.E.; Latinkic, B.V. NKCC1 (SLC12a2) induces a secondary axis in Xenopus laevis embryos independently of its co-transporter function. J. Physiol. 2009, 587, 521–529. [Google Scholar] [CrossRef]
- Fiumelli, H.; Briner, A.; Puskarjov, M.; Blaesse, P.; Belem, B.J.T.; Dayer, A.G.; Kaila, K.; Martin, J.L.; Vutskits, L. An Ion Transport-Independent Role for the Cation-Chloride Cotransporter KCC2 in Dendritic Spinogenesis In Vivo. Cereb Cortex 2013, 23, 378–388. [Google Scholar] [CrossRef]
- Horn, Z.; Ringstedt, T.; Blaesse, P.; Kaila, K.; Herlenius, E. Premature expression of KCC2 in embryonic mice perturbs neural development by an ion transport-independent mechanism. Eur. J. Neurosci. 2010, 31, 2142–2155. [Google Scholar] [CrossRef]
- Worrell, R.T.; Oghene, J.; Matthews, J.B. Ammonium effects on colonic Cl− secretion: Anomalous mole fraction behavior. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G14–G22. [Google Scholar] [CrossRef]
- Kinne, R.; Kinne-Saffran, E.; Schutz, H.; Scholermann, B. Ammonium transport in medullary thick ascending limb of rabbit kidney: Involvement of the Na+,K+,Cl−-cotransporter. J. Membr. Biol. 1986, 94, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, D.C. Ion channels versus ion pumps: The principal difference, in principle. Nat. Rev. Mol. Cell Biol. 2009, 10, 344–352. [Google Scholar] [CrossRef]
- Cater, R.; Ryan, R.; Vandenberg, R. The Split Personality of Glutamate Transporters: A Chloride Channel and a Transporter. Neurochem. Res. 2016, 41, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Aalkjaer, C.; Boulpaep, E.L.; Boron, W.F. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature 2000, 405, 571–575. [Google Scholar] [PubMed]
- Linsdell, P. Cystic fibrosis transmembrane conductance regulator (CFTR): Making an ion channel out of an active transporter structure. Channels 2018, 12, 284–290. [Google Scholar] [CrossRef]
- Beyenbach, K.W.; Masia, R. Membrane conductances of principal cells in Malpighian tubules of Aedes aegypti. J. Insect Physiol. 2002, 48, 375–386. [Google Scholar] [CrossRef]
- Sawyer, D.B.; Beyenbach, K.W. Dibutyryl-cAMP increases basolateral sodium conductance of mosquito Malpighian tubules. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1985, 248, R339–R345. [Google Scholar] [CrossRef]
- Petzel, D.H.; Hagedorn, H.H.; Beyenbach, K.W. Preliminary isolation of mosquito natriuretic factor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1985, 249, R379–R386. [Google Scholar] [CrossRef]
- Coast, G.M.; Garside, C.S.; Webster, S.G.; Schegg, K.M.; Schooley, D.A. Mosquito natriuretic peptide identified as a calcitonin-like diuretic hormone in Anopheles gambiae (Giles). J. Exp. Biol. 2005, 208, 3281–3291. [Google Scholar] [CrossRef]
- Petzel, D.H.; Berg, M.M.; Beyenbach, K.W. Hormone-controlled cAMP-mediated fluid secretion in yellow-fever mosquito. Am. J. Physiol. 1987, 253, R701–R711. [Google Scholar] [CrossRef]
- Beyenbach, K.W.; Petzel, D.H. Diuresis in Mosquitoes Role of a Natriuretic Factor. News Physiol. Sci. 1987, 2, 171–175. [Google Scholar] [CrossRef]
- Kwon, H.; Lu, H.L.; Longnecker, M.T.; Pietrantonio, P.V. Role in diuresis of a calcitonin receptor (GPRCAL1) expressed in a distal-proximal gradient in renal organs of the mosquito Aedes aegypti (L.). PLoS ONE 2012, 7, e50374. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Beyenbach, K.W. Differential effects of secretagogues on Na and K secretion in the Malpighian tubules of Aedes aegypti. J. Comp. Physiol. 1983, 149, 511–518. [Google Scholar] [CrossRef]
- Williams, J.C.; Hagedorn, H.H.; Beyenbach, K.W. Dynamic changes in flow rate and composition of urine during the post blood meal diuresis in Aedes aegypti. J. Comp. Physiol. 1983, 153, 257–266. [Google Scholar] [CrossRef]
- Coast, G.M. Neuroendocrine control of ionic homeostasis in blood-sucking insects. J. Exp. Biol. 2009, 212, 378–386. [Google Scholar] [CrossRef]
| Buffer | ND96 | I | II | III | IV | V | VI | VII | VIII | IX | Manufacturer |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | 96 | 0.5 | 0.5 | 25 | 0.5 | Thermo Fisher Scientific | |||||
| KCl | 2 | 0.5 | 25 | 0.5 | 0.5 | Thermo Fisher Scientific | |||||
| LiCl | 25 | 25 | 25 | Acros Organics (Waltham, MA, USA) | |||||||
| RbCl | 25 | Acros Organics | |||||||||
| MgCl2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Thermo Fisher Scientific | |||
| CaCl2 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | Thermo Fisher Scientific | |||
| K-Gluc | 2 | Acros Organics | |||||||||
| Li-Gluc | 25 | BOC Sciences (Shirley, NY, USA) | |||||||||
| LiI | 25 | Thermo Fisher Scientific | |||||||||
| Mg-Gluc | 1 | 1 | 1 | Sigma-Aldrich (St. Louis, MO, USA) | |||||||
| Ca-Gluc | 1.8 | 1.8 | 1.8 | Sigma-Aldrich | |||||||
| NMDG | 75 | 50 | 48 | 75 | 75 | 97.5 | 75 | 75 | 75 | Acros Organics | |
| Sucrose | 77 | Thermo Fisher Scientific | |||||||||
| HEPES | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | Thermo Fisher Scientific |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalsi, M.; Gillen, C.; Piermarini, P.M. Heterologous Expression of Aedes aegypti Cation Chloride Cotransporter 2 (aeCCC2) in Xenopus laevis Oocytes Induces an Enigmatic Na+/Li+ Conductance. Insects 2019, 10, 71. https://doi.org/10.3390/insects10030071
Kalsi M, Gillen C, Piermarini PM. Heterologous Expression of Aedes aegypti Cation Chloride Cotransporter 2 (aeCCC2) in Xenopus laevis Oocytes Induces an Enigmatic Na+/Li+ Conductance. Insects. 2019; 10(3):71. https://doi.org/10.3390/insects10030071
Chicago/Turabian StyleKalsi, Megha, Christopher Gillen, and Peter M. Piermarini. 2019. "Heterologous Expression of Aedes aegypti Cation Chloride Cotransporter 2 (aeCCC2) in Xenopus laevis Oocytes Induces an Enigmatic Na+/Li+ Conductance" Insects 10, no. 3: 71. https://doi.org/10.3390/insects10030071
APA StyleKalsi, M., Gillen, C., & Piermarini, P. M. (2019). Heterologous Expression of Aedes aegypti Cation Chloride Cotransporter 2 (aeCCC2) in Xenopus laevis Oocytes Induces an Enigmatic Na+/Li+ Conductance. Insects, 10(3), 71. https://doi.org/10.3390/insects10030071






