Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Samples Collection and Examination
2.3. Molecular Analysis
2.4. Chemical Analysis for Measuring Heavy Metals
2.5. Statistical Analysis
3. Results
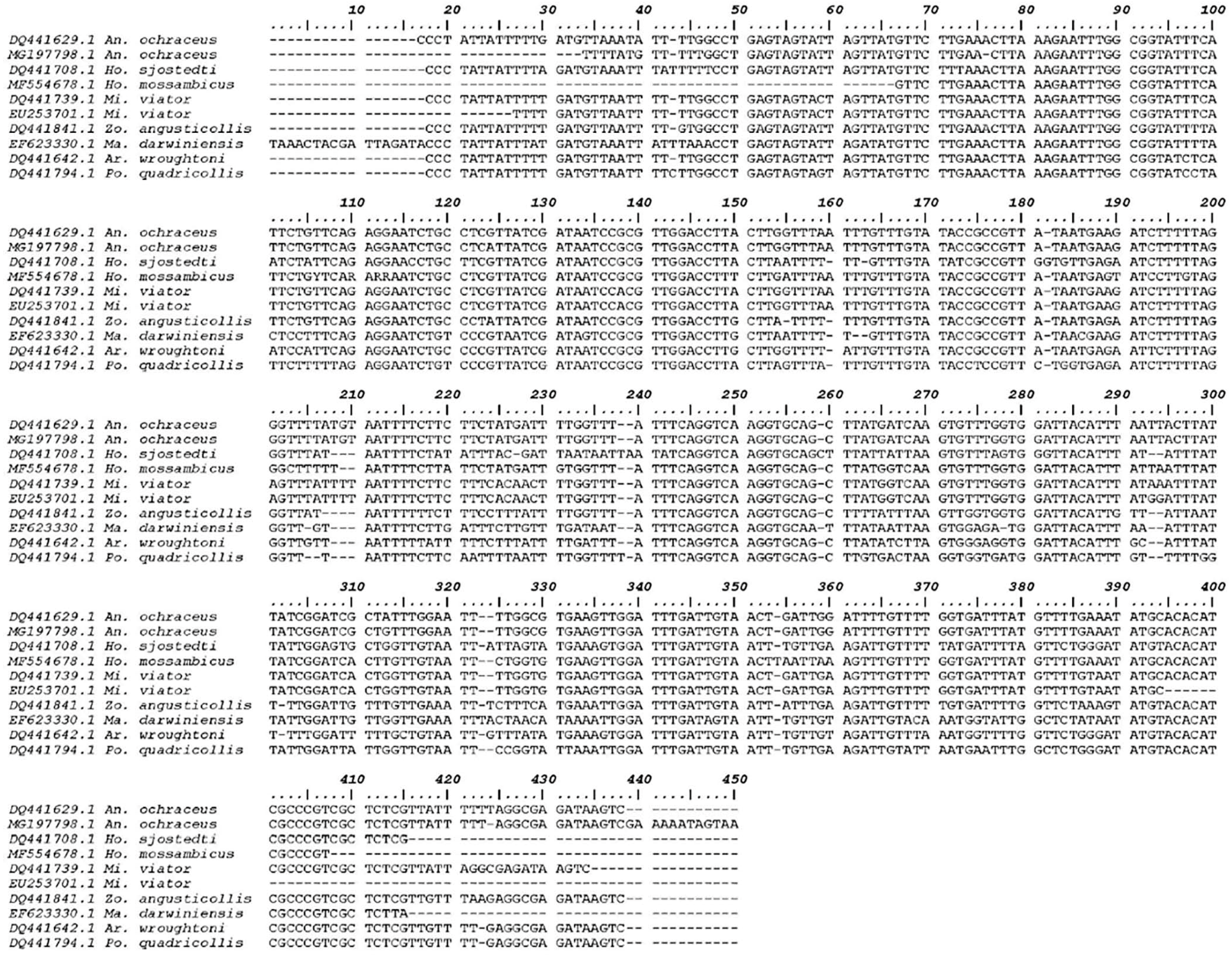
3.1. Molecular Analysis
3.2. Chemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eggleton, P.; Bignell, D.E.; Sands, W.A.; Mawdsley, N.A.; Lawton, J.H.; Wood, T.G.; Bignell, N.C. The diversity, abundance, and biomass of termites under differing levels of disturbance in the Mbalmayo Forest Reserve, southern Cameroon. Philos. Trans. R. Soc. Lond. Ser. B 1996, 351, 51–68. [Google Scholar]
- Krishna, K.; Grimaldi, D.A.; Krishna, V.; Engel, M.S. Treatise on the Isoptera of the world. Bull. Am. Mus. Nat. Hist. 2013, 377, 1–2704. [Google Scholar] [CrossRef]
- Kambhampati, S.; Eggleton, P. Phylogenetics and Taxonomy. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Higashi, M., Bignell, D.E., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2000; pp. 1–24. [Google Scholar]
- Ohkuma, M.; Yuzawa, H.; Amornsak, W.; Sornnuwat, Y.; Takematsu, Y.; Yamada, A.; Vongkaluang, C.; Sarnthoy, O.; Kirtibutr, N.; Noparatnaraporn, N.; et al. Molecular phylogeny of Asian termites (Isoptera) of the families Termitidae and Rhinotermitidae based on mitochondrial COII sequences. Mol. Phylogenet. Evol. 2004, 31, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Bignell, D.E.; Higashi, M. Termites: Evolution, Sociality, Symbioses, Ecology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; 488p. [Google Scholar]
- Bitsch, C.; Noirot, C. Gut characters and phylogeny of the higher termites (Isoptera: Termitidae). A cladistic analysis. Ann. Soc. Entomol. Fr. 2002, 38, 201–210. [Google Scholar]
- De Meyer, M. Phylogenetic relationships within the fruit fly genus Ceratitis MacLeay (Diptera: Tephritidae), derived from morphological and plant evidence. Insect Syst. Evol. 2005, 36, 459–480. [Google Scholar] [CrossRef]
- Kirton, L.G. The importance of accurate termite taxonomy in the broader perspective of termite management. In Proceedings of the Fifth International Conference on Urban Pests, Singapore, 10–13 July 2005; Chow-Yang, L., William, H.R., Eds.; Perniagaan Ph’ng@ P&Y Design Network: Penang, Malaysia, 2005. [Google Scholar]
- Ball, S.L.; Armstrong, K.F. DNA barcodes for insect pest identification: A test case with tussock moths (Lepidoptera: Lymantriidae). Can. J. Forensic Res. 2006, 36, 337–350. [Google Scholar] [CrossRef]
- Virgilio, M.; Backeljau, T.; Nevado, B.; De Meyer, M. Comparative performances of DNA barcoding across insect orders. BMC Bioinform. 2010, 11, 206. [Google Scholar] [CrossRef]
- Miura, T.; Maekwa, K.; Kitade, O.; Abe, T.; Matsumoto, T. Phylogenetic relationships among subfamilies in higher termites (Isoptera: Termitidae) based on mitochondrial COII gene sequences. Ann. Entomol. Soc. Am. 1998, 91, 515–523. [Google Scholar] [CrossRef]
- Kambhampati, S.; Blavk, W.C.; Rai, K.S. Random amplified polymorphic DNA of mosquito species and populations (Diptera: Culicidae): Techniques, statistical analysis, and applications. J. Med. Entomol. 1992, 29, 939–945. [Google Scholar] [CrossRef]
- Qadir, A.; Malik, R.N.; Husain, S.Z. Spatio-temporal variations in water quality of Nullah Aik-tributary of the river Chenab, Pakistan. Environ. Monit. Assess. 2008, 140, 43–59. [Google Scholar] [CrossRef]
- Atafar, Z.; Mesdaghinia, A.; Nouri, J.; Homaee, M.; Yunesian, M.; Ahmadimoghaddam, M.; Mahvi, A.H. Effect of fertilizer application on soil heavy metal concentration. Environ. Monit. Assess. 2010, 160, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.L.; Li, X.; Shi, W.; Cheung, S.C.N.; Thornton, I. Metal contamination in urban, suburban, and country park soils of Hong Kong: A study based on GIS and multivariate statistics. Sci. Total Environ. 2006, 356, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Eeva, T.; Rantala, L.M.; Rantala, M.J.; Hakkarainen, H.; Eeva, T. Heavy metal pollution disturbs immune response in wild and populations. Environ. Pollut. 2004, 145, 324–328. [Google Scholar]
- Warrington, S. Relationship between SO2 dose and growth of the pea aphid, Acyrthosiphon pisum, on peas. Environ. Pollut. 1987, 43, 155–162. [Google Scholar] [CrossRef]
- Mitterbock, F.; Fuhrer, E. Effects of fluoride-polluted spruce leaves on nun moth caterpillars (Lymantria monacha). J. Appl. Entomol. 1988, 105, 19–27. [Google Scholar]
- Fountain, M.T.; Hopkin, S.P. Continuous monitoring of Folsomia candida (Insecta: Collembola) in a metal exposure test. Ecotoxicol. Environ. Saf. 2001, 48, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Sildanchandra, W.; Crane, M. Influence of sexual dimorphism in Chironomus riparius Meigen on toxic effects of cadmium. Environ. Toxicol. Chem. 2000, 19, 2309–2313. [Google Scholar] [CrossRef]
- Nascarella, M.A.; Stoffolano, J.G.; Stanek, E.J.; Kostecki, P.T.; Calabrese, E.J. Hormesis and stage specific toxicity induced by cadmium in an insect model, the queen blowfly, Phormia regina Meig. Environ. Pollut. 2003, 124, 257–262. [Google Scholar] [CrossRef]
- Musa, M.B.; Ajayi, F.A.; Abdulhadi, B.J. Denudation effect of termitaria and characterization of associated termite species in Laffia, Nasarawa State, Nigeria. Eur. Sci. J. 2014, 10, 186–195. [Google Scholar]
- Heckel, P.F.; Keener, T.C. Sex differences noted in mercury bioaccumulation in Magicicada cassini. Chemosphere 2007, 69, 78–81. [Google Scholar] [CrossRef]
- Zheng, D.M.; Wang, Q.C.; Zhang, Z.S.; Zhang, N.; Zhang, X.W. Bioaccumulation of total and methyl mercury by arthropods. Bull. Environ. Contam. Toxicol. 2008, 81, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.; Faregalla, A.A.; Dabbour, A.; Mostafa, S.A.S. Studies on the Termite’s Problem in Saudi Arabia; Science Research Division, King Abdel-Aziz University: Jeddah, Saudi Arabia, 1986; pp. 99–105. [Google Scholar]
- Murthy, S.; Rajeshwari, R.K.; Ramya, S.L.; Venkatesan, T.; Jalali, S.K.; Verghese, A. Genetic diversity among Indian termites based on mitochondrial 12S rRNA gene. Eur. J. Zool. Res. 2015, 4, 1–6. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schaffer, T.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acid Res. 1997, 25, 3389–3402. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL-X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6 molecular evolutionary genetics analysis version 60. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Håkanson, L. Ecological risk index for aquatic pollution control: Sediment logical approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Usero, J.; González-Regalado, E.; Gracia, I. Trace metals in the bivalve mollusc Chamelea gallina from the Atlantic Coast of Southern Spain. Mar. Pollut. Bull. 1996, 32, 305–310. [Google Scholar] [CrossRef]
- Sures, B.; Steiner, W.; Rydlo, M.; Taraschewski, H. Concentrations of 17 elements in the zebra mussel (Dreissena polymorpha), in different tissues of perch (Perca fluviatilis), and in perch intestinal parasites (Acanthocephalus lucii) from the subalpin lake Mondsee (Austria). Environ. Toxicol. Chem. 1999, 18, 2574–2579. [Google Scholar] [CrossRef]
- Kalleshwaraswamy, C.M.; Murthy, M.S.; Viraktamath, C.A.; Krishna Kumar, N.K. Occurrence of Tuta absoluta (Lepidoptera: Gelechiidae) in the Malnad and Hyderabad-Karnataka regions of Karnataka, India. Fla. Entomol. 2015, 98, 970–971. [Google Scholar] [CrossRef]
- Gentz, M.C.; Rubinoff, D.; Grace, J.K. Phylogenetic analysis of subrranean termites (Coptotermes spp., Isoptera, Rhinotermitidae) indicates the origins of Hawaiian and North American invasions: Potential implications for invasion biology. Proc. Hawaiian Entomol. Soc. 2008, 40, 1–9. [Google Scholar]
- Faragalla, A.A.; Alqhtani, M.H.; Abu Zeid, I.; Ahmed, M.M. DNA fingerprinting of some major genera of subterranean termites (Isoptera) (Anacanthotermes, Psammotermes and Microtermes) from Western Saudi Arabia. Acad. J. Entomol. 2015, 8, 104–109. [Google Scholar]
- Deligne, J. Functional morphology and evolution of a Carpenter’s plane-like tool in the mandibles of termite workers (Insecta, Isoptera). Belg. J. Zool. 1999, 129, 201–218. [Google Scholar]
- Ahmad, M. The phylogeny of termite genera based on imagoworker mandibles. Bull. Am. Mus. Nat. Hist. 1950, 95, 37–86. [Google Scholar]
- Roonwal, M.L.; Rathore, N.S. Evolution and systematic significance of wing micro-sculpturing in termites (Isoptera): New types in the Kalotermitidae and Hodotermitidae. Zool. Anz. 1978, 3, 219–232. [Google Scholar]
- Lynch, R.E. Resistance in peanut to major arthropod pests. Fla. Entomol. 1990, 73, 422–455. [Google Scholar] [CrossRef]
- Jenkins, B.N.; Dean, B.E.; Forschler, B.T. DNA technology, interstate commerce and the likely origin of the Formosan subterranean termites (Isoptera: Rhinotermitidae) infestation in Atlanta, Gerogia. J. Econ. Entomol. 2002, 95, 381–389. [Google Scholar] [CrossRef]
- Szalansky, A.L.; Austin, J.W.; Owens, C.B. Identification of Reticulotermes spp. (Isoptera: Reticulotermitidae) from the South Central United States by PCR-RFLP. J. Econ. Entomol. 2003, 96, 1514–1519. [Google Scholar] [CrossRef]
- Cameron, S.L.; Lo, N.; Bourguignon, T.; Svenson, G.J.; Evans, T.A. A mitochondrial genome phylogeny of termites (Blattodea: Termitoidae): Robust support for interfamilial relationship and molecular synapomorphies define major clades. Mol. Phylogenet. Evol. 2012, 65, 163–173. [Google Scholar] [CrossRef]
- Legendre, F.; Nel, A.; Svenson, G.J.; Robillard, T.; Pellens, R.; Grandcolas, P. Phylogeny of Dictyoptera: Dating the origin of cockroaches, praying mantises and termites with molecular data and controlled fossil evidence. PLoS ONE 2015, 10, e130127. [Google Scholar] [CrossRef]
- Nalepa, C.A.; Lenz, M. The ootheca of Mastotermes darwiniensis Froggatt (Isoptera: Mastotermitidae): Homology with cockroach oothecae. Proc. R. Soc. Lond. Ser. B 2000, 267, 1809–1813. [Google Scholar] [CrossRef] [PubMed]
- Legendre, F.; Whiting, M.F.; Bordereau, C.; Cancello, E.M.; Evans, T.A.; Grandcolas, P. The phylogeny of termites (Dictyoptera: Isoptera) based on mitochondrial and nuclear markers: Implications for the evolution of the worker and pseudergate castes, and foraging behaviors. Mol. Phylogenet. Evol. 2008, 48, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.J.; Kitade, O.; Lo, N.; Crozier, R.H. Phylogenetic evidence for a single ancestral origin of a ‘true’ worker caste in termites. J. Evol. Biol. 2000, 13, 869–881. [Google Scholar] [CrossRef]
- Inward, D.J.G.; Vogler, A.P.; Eggleton, P. A comprehensive phylogenetic analysis of termites (Isoptera) illuminates key aspects of their evolutionary biology. Mol. Phylogenet. Evol. 2007, 44, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.S.; Grimaldi, D.A.; Krishna, K. Termites (Isoptera): Their phylogeny, classification and rise to ecological dominance. Am. Mus. Novit. 2009, 3650, 1–27. [Google Scholar] [CrossRef]
- Lacey, M.J.; Lenz, M.; Evans, T.A. Cryoprotection in dampwood termites (Termopsidae: Isoptera). J. Insect Physiol. 2010, 56, 1–7. [Google Scholar] [CrossRef]
- Donovan, S.E.; Jones, D.T.; Sands, W.A.; Eggleton, P. Morphological phylogenetics of termites (Isoptera). Biol. J. Linn. Soc. 2000, 70, 467–513. [Google Scholar] [CrossRef]
- Ware, J.L.; Grimaldi, D.A.; Engel, M.S. The effects of fossil placement and calibration on divergence times and rates: An example from the termites (Insecta: Isoptera). Arthropod Struct. Dev. 2010, 39, 204–219. [Google Scholar] [CrossRef]
- Lo, N.; Eggleton, P. Termite phylogenetics and co-cladogenesis with symbionts. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 27–50. [Google Scholar]
- Kambhampati, S.; Kjer, K.M.; Thorne, B.L. Phylogenetic relationship among termite families based on DNA sequence of mito 16S ribosomal RNA gene. Insect Mol. Biol. 1996, 5, 229–238. [Google Scholar] [CrossRef]
- Lo, N.; Kitade, O.; Miurua, T.; Constantino, R.; Matsumoto, T. Molecular phylogeny of the Rhinotermitidae. Insectes Sociaux 2004, 51, 365–371. [Google Scholar] [CrossRef]
- Grassé, P.P. Termitologia: Comportement, Socialité, Ecologie, Evolution, Systématique; Masson: Paris, France, 1986; 716p. [Google Scholar]
- Hladun, K.R.; Parker, D.R.; Trumble, J.T. Cadmium, copper, and lead accumulation and bioconcentration in the vegetative and reproductive organs of Raphanus sativus: Implications for plant performance and pollution. J. Chem. Ecol. 2015, 41, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Egyptian Higher Committee of Water (EHCW). Egyptian Standards for Drinking and Domestic Water According to the Act 27/1978 in Regulating of the Public Water Supplies; Egyptian Governmental Press: Cairo, Egypt, 1995. [Google Scholar]
- SASO (Saudi Arabian Standards Organization). Maximum Limits of Contaminating Metallic Elements in Foods; Saudi Standards, Metrology and Quality Organization: Riyadh, Saudi Arabia, 1997. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Trace Elements in Water, Solids, and Biosolids by Inductively Coupled Plasma-Atomic Emission Spectrometry; EPA Method: Washington, DC, USA, 1998.
- Gadd, G.M. Interaction of fungi with toxic metals. New Phytol. 1993, 124, 25–60. [Google Scholar] [CrossRef]
- Bååth, E.; Diaz-Ravina, M.; Frostegard, A.; Campbell, C.D. Effect of metal-rich sludge amended on the soil microbial community. Appl. Environ. Microbiol. 1998, 64, 238–245. [Google Scholar] [PubMed]
- Pennanen, T.; Fostegråd, Å.; Fritze, H.; Bååth, E. Phospholipid fatty acid composition and heavy metal-polluted gradients in coniferous forests. Appl. Environ. Microbiol. 1996, 62, 420–428. [Google Scholar] [PubMed]
- Pennanen, T.; Perkiömäki, J.; Kiikkilä, O.; Vanhala, P.; Neuvonen, S.; Fritze, H. Prolonged, simulated acid rain and heavy metal deposition: separated and combined effects on forest soil microbial community structure. FEMS Microbiol. Ecol. 1998, 27, 291–300. [Google Scholar] [CrossRef]
- Zafar, S.; Aqil, F.; Ahmad, I. Metal tolerance and biosorption potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour. Technol. 2007, 98, 2557–2561. [Google Scholar] [CrossRef] [PubMed]
- Jop, K.M. Concentration of metals in various larval stages of four Ephemeroptera species. Bull. Environ. Contam. Toxicol. 1991, 46, 901–905. [Google Scholar] [CrossRef]
- Giguère, A.; Campbell, P.G.C.; Hare, L.; Couture, P. Sub-cellular partitioning of cadmium, copper, nickel and zinc in indigenous yellow perch (Perca flavescens) sampled along a pollymetallic gradient. Aquat. Toxicol. 2006, 77, 178–189. [Google Scholar] [CrossRef]
- Maavara, V.; Martin, A.J.; Oja, A.; Nuorteva, P. Sampling of different social categories of red wood ants (Formica s. str.) for biomonitoring. In Environmental Sampling for Trace Metal; Market, B., Ed.; VCH: Weinheim, Germany, 1994; pp. 466–489. [Google Scholar]
- Rabitsch, W.B. Metal accumulation in arthropods near e lead/zinc smelter in Arnoldstein, Austria. II. Formicidae. Environ. Pollut. 1995, 90, 239–247. [Google Scholar] [CrossRef]
- Rabitsch, W.B. Tissue-specific accumulation patterns of Pb, Cd, Cu, Zn, Fe, and Mn in workers of three ant species (Formicidae: Hymenoptera) from a metal-polluted site. Arch. Environ. Contam. Toxicol. 1997, 32, 172–177. [Google Scholar] [CrossRef]
- Hackman, R. Chemistry of the insect cuticle. In The Physiology of Insecta, 2nd ed.; Rockstein, M., Ed.; Academic Press, Inc.: London, UK, 1974; pp. 215–270. [Google Scholar]
- Bagatto, G.; Shorthouse, J.D. Accumulation of Cu and Ni in successive stages of Lymantria dispar L. (Lymantriidae: Lepidoptera) near ore smelters at Sudbury, Ontario, Canada. Environ. Pollut. 1996, 92, 7–12. [Google Scholar] [CrossRef]
- Lu, G.; Di, S.; Xueping, Z. Analysis on the current pollution situation of Cu, Pb and Zn in the cultivated black soil of songnen plain. Chin. Agric. Sci. Bull. 2011, 27, 261–265. [Google Scholar]
- Zan, S.T.; Yang, R.Y.; Wang, W.W. The correlation of soil physicochemical properties and copper-zinc accumulation of Elsholtzia splendens. J. Biol. 2011, 28, 46–49. [Google Scholar]
- Cheruiyot, D.J.; Boyd, R.S.; Coudron, T.A.; Cobine, P.A. Bioaccumulation and effects of herbivore dietary Co, Cu, Ni, and Zn on growth and development of the insect predator Podisus maculiventris (Say). J. Chem. Ecol. 2013, 39, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Roth-Holzapfel, M. Multi-element analysis of invertebrate animals in a forest ecosystem (Picea abies L.). In Element Concentration Cadasters in Ecosystems; Lieth, H., Markert, B., Eds.; VCH Verlagsgesellschaft: Weinheim, Germany, 1990; pp. 281–295. [Google Scholar]
- Barajas-Aceves, M.; Grace, C.; Ansorena, J.; Dendooven, L.; Brookes, P.C. Soil microbial biomass and organic C in a gradient of zinc concentrations in soils around a mine spoil tip. Soil Biol. Biochem. 1999, 31, 867–876. [Google Scholar] [CrossRef]
- Bose, S.; Bhattacharyya, A.K. Heavy metal accumulation in wheat plant grown in soil amended with industrial sludge. Chemosphere 2008, 70, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Toth, T.; Tomas, J.; Lazor, P.; Bajcan, D.; Jomova, D. The transfer of metals from contaminated soils into agricultural plants in high Tatars region, Czech. J. Food Sci. 2009, 27, 390–393. [Google Scholar]
- Grzesiak, M.; Sieradzki, Z. Environment. Information and Statistical Papers; Statistical Publications House: Warsaw, Poland, 2000. [Google Scholar]
- Rogival, D.; Scheirs, J.; Blust, R. Transfer and accumulation of metals in a soil-diet-wood mouse food chain along a metal pollution gradient. Environ. Pollut. 2007, 145, 516–528. [Google Scholar] [CrossRef]
- Flannagan, J.F.; Lockhart, W.L.; Cobb, D.G.; Metner, D. Stonefly (Plecoptera) head cholinesterase as an indicator of exposure to fenitrothion. Manit. Entomol. 1978, 12, 42–48. [Google Scholar]
- Ibrahim, H.; Kheir, R.; Helmi, S.; Lewis, J.; Crane, M. Effects of organophosphorus, carbamate, pyrethroid and organochlorine pesticides, and a heavy metal on survival and cholinesterase activity of Chironomus riparius Meigen. Bull. Environ. Contam. Toxicol. 1998, 60, 448–455. [Google Scholar] [CrossRef]
- Iyaka, Y.A.; Kakulu, S.E. Copper and zinc contents in urban agricultural soils of Niger State, Nigeria. Int. Multi-Discip. J. Ethiop. 2009, 3, 23. [Google Scholar] [CrossRef]
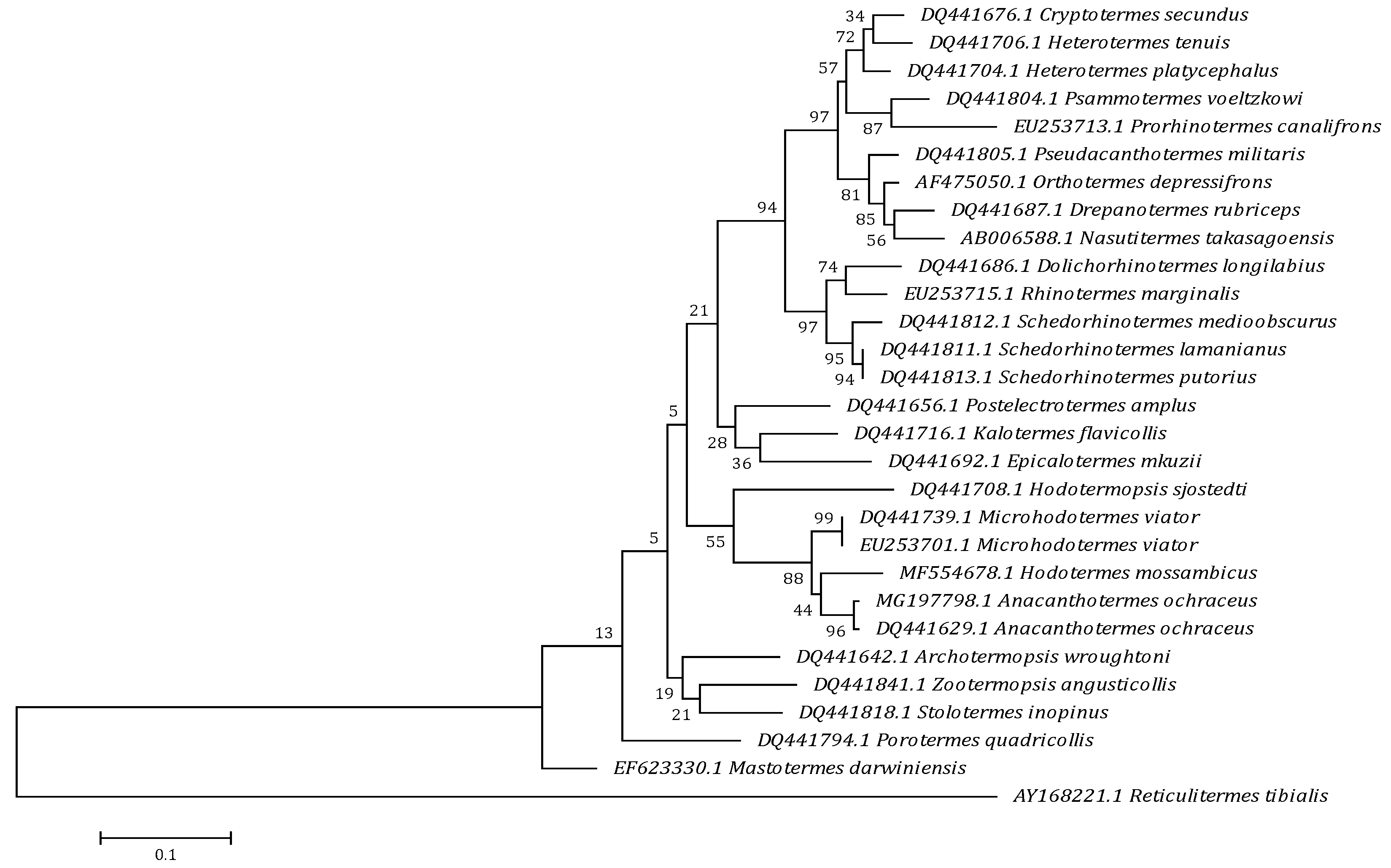
| Parasite Species | Order/Family | Source | Accession No. | Sequence Length (bp) | Percent Identity (%) |
|---|---|---|---|---|---|
| Cryptotermes secundus | Blattodea/Kalotermitidae | GenBank | DQ441676.1 | 407 | 80 |
| Heterotermes tenuis | Blattodea/Rhinotermitidae | GenBank | DQ441706.1 | 416 | 81 |
| Heterotermes platycephalus | Blattodea/Rhinotermitidae | GenBank | DQ441704.1 | 413 | 81 |
| Psammotermes voeltzkowi | Blattodea/Rhinotermitidae | GenBank | DQ441804.1 | 417 | 83 |
| Prorhinotermes canalifrons | Blattodea/Rhinotermitidae | GenBank | EU253713.1 | 365 | 83 |
| Pseudacanthotermes militaris | Blattodea/Termitidae | GenBank | DQ441805.1 | 413 | 84 |
| Orthotermes depressifrons | Blattodea/Termitidae | GenBank | AF475050.1 | 438 | 82 |
| Drepanotermes rubriceps | Blattodea/Termitidae | GenBank | DQ441687.1 | 412 | 82 |
| Nasutitermes takasagoensis | Blattodea/Termitidae | GenBank | AB006588.1 | 404 | 82 |
| Dolichorhinotermes longilabius | Blattodea/Rhinotermitidae | GenBank | DQ441686.1 | 415 | 83 |
| Rhinotermes marginalis | Blattodea/Rhinotermitidae | GenBank | EU253715.1 | 359 | 83 |
| Schedorhinotermes medioobscurus | Blattodea/Rhinotermitidae | GenBank | DQ441812.1 | 414 | 83 |
| Schedorhinotermes lamanianus | Blattodea/Rhinotermitidae | GenBank | DQ441811.1 | 413 | 83 |
| Schedorhinotermes putorius | Blattodea/Rhinotermitidae | GenBank | DQ441813.1 | 392 | 83 |
| Postelectrotermes amplus | Blattodea/Kalotermitidae | GenBank | DQ441656.1 | 407 | 83 |
| Kalotermes flavicollis | Blattodea/Kalotermitidae | GenBank | DQ441716.1 | 407 | 84 |
| Epicalotermes mkuzii | Blattodea/Kalotermitidae | GenBank | DQ441692.1 | 402 | 82 |
| Hodotermopsis sjostedti | Blattodea/Termopsidae | GenBank | DQ441708.1 | 388 | 90 |
| Microhodotermes viator | Blattodea/Hodotermitidae | GenBank | DQ441739.1 | 409 | 93 |
| Microhodotermes viator | Blattodea/Hodotermitidae | GenBank | EU253701.1 | 360 | 93 |
| Hodotermes mossambicus | Blattodea/Hodotermitidae | GenBank | MF554678.1 | 333 | 90 |
| Anacanthotermes ochraceus | Blattodea/Hodotermitidae | GenBank | DQ441629.1 | 414 | 99 |
| Archotermopsis wroughtoni | Blattodea/Termopsidae | GenBank | DQ441642.1 | 406 | 82 |
| Zootermopsis angusticollis | Blattodea/Termopsidae | GenBank | DQ441841.1 | 405 | 88 |
| Stolotermes inopinus | Blattodea/Termopsidae | GenBank | DQ441818.1 | 414 | 82 |
| Porotermes quadricollis | Blattodea/Termopsidae | GenBank | DQ441794.1 | 406 | 87 |
| Mastotermes darwiniensis | Blattodea/Mastotermitidae | GenBank | EF623330.1 | 400 | 85 |
| Reticulitermes tibialis | Blattodea/Rhinotermitidae | GenBank | AY168221.1 | 441 | 83 |
| Heavy Metal | Soil Samples from Site I (Riyadh) | Soil Samples from Site II (Taif) | Anacanthotermes ochraceus | |
|---|---|---|---|---|
| Site I (Riyadh) | Site II (Taif) | |||
| Al | 196.0533 ± 32.90069 c | 378.4967 ± 57.72633 b c | 961.9387 ± 75.84870 a b | 1514.1020 ± 406.63030 a |
| Be | 0.4422 ± 0.07197 b | 0.3940 ± 0.11393 b | 12.1700 ± 3.09869 a b | 6.3833 ± 1.88832 a |
| Ba | 2.4420 ± 1.08289 b | 4.1214 ± 1.45522 a b | 6.8607 ± 1.92336 a b | 8.9527 ± 2.59292 a |
| Ca | 883.5467 ± 101.56333 b | 455.5233 ± 88.38769 b | 9625.6327 ± 2672.88999 a | 4835.3907 ± 1277.84243 a b |
| Cd | 0.1060 ± 0.2784 b | 0.0847 ± 0.2207 b | 0.4533 ± 0.10056 a | 0.3529 ± 0.09159 a |
| Co | 0.519 ± 0.01354 b | 0.0264 ± 0.00968 b | 0.2793 ± 0.09498 b | 0.4573 ± 0.18576 a |
| Cr | 0.6619 ± 0.29671 b | 0.2162 ± 0.09240 b | 2.1513 ± 0.58658 a b | 2.8853 ± 0.94224 a |
| Cu | 3.0313 ± 0.84077 b | 2.8978 ± 0.61136 b | 42.7720 ± 14.69708 a b | 23.2357 ± 5.94914 a |
| Fe | 26.2683 ± 6.97426 b | 32.3260 ± 8.31177 b | 441.7793 ± 138.77551 b | 940.9813 ± 250.35349 a |
| Mg | 65.3030 ± 8.54049 b | 26.6207 ± 6.22629 b | 2009.0593 ± 523.75384 a | 1656.1273 ± 428.84791 a |
| Mn | 2.8454 ± 1.15156 b | 6.3062 ± 1.31417 b | 14.6213 ± 3.97113 a b | 11.5678 ± 2.76065 a |
| Mo | 0.0383 ± 0.01091 b | 0.475 ± 0.01586 b | 0.4327 ± 0.18244 a | 0.4313 ± 0.13122 a |
| Ni | 0.2883 ± 0.07573 b | 0.1363 ± 0.04804 b | 1.5987 ± 0.41954 a | 2.0727 ± 0.57519 a |
| Pb | 0.1553 ± 0.07672 b | 0.2074 ± 0.07582 b | 3.0707 ± 0.78007 a | 2.6593 ± 0.66487 a |
| V | 0.2692 ± 0.07972 b | 0.3537 ± 0.11612 b | 1.6913 ± 0.42388 a | 2.2733 ± 0.59009 a |
| Zn | 42.4307 ± 7.21991 b | 89.7970 ± 9.78533 a b | 124.9000 ± 31.53582 a b | 185.1847 ± 48.74656 a |
| Heavy Metals | Contamination Factor (CF) | Pollution Load Index (PLI) | Degree of Contamination (DC) | |||
|---|---|---|---|---|---|---|
| Site I | Site II | Site I | Site II | Site I | Site II | |
| Al | 1633.7 | 3154.1 | 0.7648 | 1.0632 | 4876.3 | 9765.2 |
| Be | 2.2113 | 1.9701 | 0.2778 | 0.3923 | 6.2453 | 5.7873 |
| Ba | 15.262 | 25.758 | 0.8440 | 0.1756 | 45.785 | 76.256 |
| Ca | 883.35 | 455.52 | 0.4402 | 0.3170 | 2576.2 | 1298.6 |
| Cd | 2.6501 | 2.1175 | 0.1610 | 0.3159 | 7.9343 | 6.0549 |
| Co | 25.951 | 1.3242 | 0.1717 | 1.2919 | 75.987 | 3.6757 |
| Cr | 16.547 | 5.4052 | 0.6619 | 0.1899 | 43.765 | 13.657 |
| Cu | 60.626 | 57.956 | 0.1346 | 0.1541 | 175.76 | 169.65 |
| Fe | 2626.8 | 3232.6 | 1.6551 | 8.8810 | 7545.6 | 9654.7 |
| Mg | 6530.3 | 2662.1 | 1.0747 | 1.5895 | 18545.2 | 7644.5 |
| Mn | 56.908 | 126.12 | 0.1628 | 0.1495 | 165.87 | 325.54 |
| Mo | 4.7661 | 9.5202 | 0.0277 | 0.0347 | 2.0857 | 26.549 |
| Ni | 288.32 | 136.30 | 0.1252 | 0.1183 | 765.98 | 389.98 |
| Pb | 1.1946 | 1.5953 | 1.7578 | 0.7389 | 3.4598 | 4.2983 |
| V | 5.3842 | 7.0743 | 0.0192 | 0.0847 | 15.986 | 20.765 |
| Zn | 1060.7 | 2244.9 | 1.2509 | 2.6525 | 3058.4 | 6375.6 |
| Heavy Metals | C[A. ochraceus]/C[Site I] | C[A. ochraceus]/C[Site II] | C[A. ochraceus Site I]/C[A. ochraceus Site II] |
|---|---|---|---|
| Al | 5.1183 | 3.4995 | 0.5222 |
| Be | 28.290 | 19.088 | 1.9065 |
| Ba | 3.3319 | 2.5774 | 0.7663 |
| Ca | 10.611 | 8.9935 | 1.9906 |
| Cd | 2.7169 | 2.5480 | 1.2519 |
| Co | 6.4979 | 26.032 | 0.5249 |
| Cr | 2.5215 | 18.137 | 0.7456 |
| Cu | 22.586 | 7.3969 | 1.8408 |
| Fe | 17.825 | 27.608 | 0.5694 |
| Mg | 27.394 | 50.377 | 1.2131 |
| Mn | 4.5726 | 3.0160 | 0.6498 |
| Mo | 15.919 | 11.677 | 1.4667 |
| Ni | 5.9092 | 19.347 | 0.7713 |
| Pb | 19.774 | 11.263 | 1.1546 |
| V | 4.6089 | 6.1567 | 0.7439 |
| Zn | 2.0687 | 1.6891 | 0.6744 |
| Variables | Concentrations of Different Heavy Metals in Site I | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Be | Ba | Ca | Cd | Co | Cr | Cu | Fe | Mg | Mn | Mo | Ni | Pb | V | Zn | |
| Al | 1 | |||||||||||||||
| Be | 0.968 | 1 | ||||||||||||||
| Ba | 0.935 | 0.994 | 1 | |||||||||||||
| Ca | 0.929 | 0.992 | 1.000 * | 1 | ||||||||||||
| Cd | 0.996 | 0.941 | 0.899 | 0.891 | 1 | |||||||||||
| Co | 0.406 | 0.623 | 0.704 | 0.716 | 0.321 | 1 | ||||||||||
| Cr | −0.572 | −0.348 | −0.244 | −0.228 | −0.645 | 0.517 | 1 | |||||||||
| Cu | 0.987 | 0.996 | 0.980 | 0.976 | 0.968 | 0.547 | −0.434 | 1 | ||||||||
| Fe | 0.966 | 1.000 ** | 0.995 | 0.993 | 0.938 | 0.629 | −0.340 | 0.995 | 1 | |||||||
| Mg | 0.636 | 0.422 | 0.321 | 0.305 | 0.704 | −0.446 | −0.997 | 0.505 | 0.415 | 1 | ||||||
| Mn | 0.997 | 0.985 | 0.961 | 0.959 | 0.985 | 0.480 | −0.503 | 0.997 * | 0.984 | 0.570 | 1 | |||||
| Mo | 0.999 * | 0.977 | 0.948 | 0.942 | 0.992 | 0.440 | −0.541 | 0.992 | 0.975 | 0.607 | 0.999 * | 1 | ||||
| Ni | 0.996 | 0.986 | 0.962 | 0.958 | 0.984 | 0.484 | −0.499 | 0.997 * | 0.985 | 0.567 | 1.000 ** | 0.999 * | 1 | |||
| Pb | 1.000 ** | 0.968 | 0.935 | 0.929 | 0.996 | 0.407 | −0.572 | 0.987 | 0.966 | 0.636 | 0.997 | 0.999 * | 0.996 | 1 | ||
| V | 0.604 | 0.384 | 0.281 | 0.266 | 0.674 | −0.483 | 0.999 * | 0.468 | 0.376 | 0.999 * | 0.536 | 0.573 | 0.532 | 0.603 | 1 | |
| Zn | 0.574 | 0.761 | 0.827 | 0.836 | 0.497 | 0.981 | 0.343 | 0.697 | 0.766 | −0.267 | 0.639 | 0.604 | 0.642 | 0.574 | −0.307 | 1 |
| Variables | Concentrations of Different Heavy Metals in Site I | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Be | Ba | Ca | Cd | Co | Cr | Cu | Fe | Mg | Mn | Mo | Ni | Pb | V | Zn | |
| Al | 1 | |||||||||||||||
| Be | 0.965 | 1 | ||||||||||||||
| Ba | 0.664 | 0.446 | 1 | |||||||||||||
| Ca | 0.961 | 1.000 * | 0.432 | 1 | ||||||||||||
| Cd | −0.875 | −0.971 | −0.219 | −0.975 | 1 | |||||||||||
| Co | 0.902 | 0.984 | 0.277 | 0.986 | −0.998 * | 1 | ||||||||||
| Cr | 0.783 | 0.593 | 0.985 | 0.580 | −0.383 | 0.438 | 1 | |||||||||
| Cu | 0.998 * | 0.981 | 0.613 | 0.977 | −0.905 | 0.929 | 0.739 | 1 | ||||||||
| Fe | 0.976 | 0.999* | 0.487 | 0.998* | −0.959 | 0.974 | 0.630 | 0.989 | 1 | |||||||
| Mg | 0.914 | 0.988 | 0.304 | 0.991 | −0.996 | 1.000 * | 0.463 | 0.939 | 0.980 | 1 | ||||||
| Mn | 0.843 | 0.954 | 0.159 | 0.959 | −0.998 * | 0.993 | 0.326 | 0.877 | 0.939 | 0.989 | 1 | |||||
| Mo | 0.867 | 0.967 | 0.203 | 0.971 | −1.000 * | 0.997 * | 0.368 | 0.898 | 0.954 | 0.995 | 0.999 * | 1 | ||||
| Ni | 0.900 | 0.982 | 0.272 | 0.985 | −0.999 * | 1.000 ** | 0.433 | 0.927 | 0.973 | 0.999 * | 0.993 | 0.998 * | 1 | |||
| Pb | 0.925 | 0.992 | 0.331 | 0.994 | −0.993 | 0.998 * | 0.487 | 0.948 | 0.985 | 1.000 * | 0.984 | 0.991 | 0.998 * | 1 | ||
| V | 0.997 | 0.941 | 0.724 | 0.935 | −0.832 | 0.864 | 0.831 | 0.989 | 0.955 | 0.877 | 0.796 | 0.823 | 0.861 | 0.891 | 1 | |
| Zn | −0.598 | −0.786 | 0.202 | −0.796 | 0.911 | −0.885 | 0.031 | −0.650 | −0.757 | −0.872 | −0.935 | −0.918 | −0.888 | −0.858 | −0.530 | 1 |
| Variables | Concentrations of Different Heavy Metals in Site I | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Be | Ba | Ca | Cd | Co | Cr | Cu | Fe | Mg | Mn | Mo | Ni | Pb | V | Zn | |
| Al | 1 | |||||||||||||||
| Be | 0.848 | 1 | ||||||||||||||
| Ba | 0.751 | 0.987 | 1 | |||||||||||||
| Ca | 0.928 | 0.985 | 0.943 | 1 | ||||||||||||
| Cd | 0.868 | 0.999 * | 0.980 | 0.991 | 1 | |||||||||||
| Co | 0.654 | 0.955 | 0.991 | 0.889 | 0.944 | 1 | ||||||||||
| Cr | 0.069 | −0.469 | −0.607 | −0.307 | −0.435 | −0.709 | 1 | |||||||||
| Cu | 1.000 * | 0.832 | 0.730 | 0.916 | 0.853 | 0.631 | 0.100 | 1 | ||||||||
| Fe | 0.943 | 0.623 | 0.487 | 0.751 | 0.653 | 0.365 | 0.398 | 0.952 | 1 | |||||||
| Mg | 0.900 | 0.994 | 0.964 | 0.998 * | 0.998 * | 0.918 | −0.372 | 0.886 | 0.703 | 1 | ||||||
| Mn | 0.847 | 1.000 ** | 0.987 | 0.984 | 0.999 * | 0.956 | −0.471 | 0.831 | 0.622 | 0.994 | 1 | |||||
| Mo | 0.935 | 0.607 | 0.469 | 0.736 | 0.636 | 0.345 | 0.417 | 0.946 | 1.000 * | 0.688 | 0.605 | 1 | ||||
| Ni | −0.856 | −1.000 ** | −0.984 | −0.987 | −1.000 * | −0.951 | 0.456 | −0.840 | −0.635 | −0.996 | −1.000 * | −0.618 | 1 | |||
| Pb | 0.948 | 0.973 | 0.922 | 0.998 * | 0.981 | 0.861 | −0.251 | 0.938 | 0.788 | 0.992 | 0.972 | 0.775 | −0.976 | 1 | ||
| V | 0.947 | 0.974 | 0.924 | 0.999 * | 0.982 | 0.863 | −0.256 | 0.936 | 0.785 | 0.993 | 0.973 | 0.772 | −0.977 | 1.000 ** | 1 | |
| Zn | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1.000 ** | 1 |
| Variables | Concentrations of Different Heavy Metals in Site I | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Be | Ba | Ca | Cd | Co | Cr | Cu | Fe | Mg | Mn | Mo | Ni | Pb | V | Zn | |
| Al | 1 | |||||||||||||||
| Be | 0.957 | 1 | ||||||||||||||
| Ba | 0.566 | 0.781 | 1 | |||||||||||||
| Ca | 0.995 | 0.980 | 0.642 | 1 | ||||||||||||
| Cd | 0.956 | 1.000 ** | 0.782 | 0.980 | 1 | |||||||||||
| Co | 0.978 | 0.996 | 0.725 | 0.994 | 0.996 | 1 | ||||||||||
| Cr | 0.911 | 0.752 | 0.176 | 0.867 | 0.750 | 0.805 | 1 | |||||||||
| Cu | 0.994 | 0.983 | 0.654 | 1.000 ** | 0.983 | 0.995 | 0.860 | 1 | ||||||||
| Fe | −0.893 | −0.724 | −0.135 | −0.846 | −0.723 | −0.780 | −0.999 * | −0.838 | 1 | |||||||
| Mg | 0.996 | 0.979 | 0.638 | 1.000 ** | 0.979 | 0.993 | 0.870 | 1.000 * | −0.849 | 1 | ||||||
| Mn | 0.994 | 0.983 | 0.652 | 1.000 ** | 0.982 | 0.995 | 0.861 | 1.000 ** | −0.839 | 1.000 * | 1 | |||||
| Mo | 0.989 | 0.989 | 0.680 | 0.999 * | 0.989 | 0.998 * | 0.841 | 0.999 * | −0.818 | 0.998 * | 0.999 * | 1 | ||||
| Ni | 0.577 | 0.315 | −0.347 | 0.497 | 0.313 | 0.395 | 0.863 | 0.483 | −0.883 | 0.501 | 0.485 | 0.452 | 1 | |||
| Pb | −0.519 | −0.249 | 0.410 | −0.436 | −0.247 | −0.331 | −0.826 | −0.422 | 0.848 | −0.441 | −0.423 | −0.389 | −0.998 * | 1 | ||
| V | −0.429 | −0.148 | 0.502 | −0.341 | −0.146 | −0.232 | −0.764 | −0.327 | 0.790 | −0.346 | −0.328 | −0.293 | −0.985 | 0.995 | 1 | |
| Zn | −0.173 | 0.120 | 0.714 | −0.079 | 0.122 | 0.035 | −0.564 | −0.063 | 0.598 | −0.084 | −0.065 | −0.028 | −0.904 | 0.932 | 0.964 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alajmi, R.; Abdel-Gaber, R.; AlOtaibi, N. Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia. Insects 2019, 10, 51. https://doi.org/10.3390/insects10020051
Alajmi R, Abdel-Gaber R, AlOtaibi N. Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia. Insects. 2019; 10(2):51. https://doi.org/10.3390/insects10020051
Chicago/Turabian StyleAlajmi, Reem, Rewaida Abdel-Gaber, and Noura AlOtaibi. 2019. "Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia" Insects 10, no. 2: 51. https://doi.org/10.3390/insects10020051
APA StyleAlajmi, R., Abdel-Gaber, R., & AlOtaibi, N. (2019). Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia. Insects, 10(2), 51. https://doi.org/10.3390/insects10020051





