Do Grapholita funebrana Infestation Rely on Specific Plum Fruit Features?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Sampling Plane, and Infestation Level
2.2. Physical and Chemical Properties of Fruits
2.3. Laboratory Trials
2.4. Statistical Analysis
3. Results
3.1. Field Infestation and Physical-Chemical Properties of the Fruits
3.2. Laboratory Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Sharma, D.D.; Singh, N.; Shylla, B. Evaluation of newly introduced plum (Prunus salicina Lindl.) cultivars under mid-hills of Himachal Pradesh. Int. J. Chem. Stud. 2018, 6, 2925–2930. [Google Scholar]
- Sharma, D.D.; Kumar, M.; Singh, N.; Shylla, B. Plant growth and fruiting behavior of newly introduced plum (Prunus salicina Lindl.) cultivars under mid-hills conditions of Himachal Pradesh. Pharma Innov. J. 2018, 7, 408–413. [Google Scholar]
- Wu, W.; Chen, F.; Yeh, K.; Chen, J. ISSR analysis of genetic diversity and structure of plum varieties cultivated in southern China. Biology 2019, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agricultural Organization of United Nation). Global Fruit Production in 2017 by Variety; Statista; FAO: Rome, Italy, 2018; Available online: https://www.statista.com/statistics/264001/worldwide-production-of-fruit-by-variety/ (accessed on 4 May 2019).
- Wallace, T.C. Dried plums, prunes and bone health: A comprehensive review. Nutrients 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Stacewicz-Sapuntzakis, M. Dried plums and their products: Composition and health effects an updated review. Crit. Rev. Food Sci. Nutr. 2013, 53, 1277–1302. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Prajapati, P.M.; Solanki, A.S.; Sen, D.J. Nutrition value of plum tree for health. Int. Res. J. Pharm. 2012, 3, 53–56. [Google Scholar]
- García-Almodóvar, R.C.; Clemente-Moreno, M.J.; Díaz-Vivancos, P.; Petri, C.; Rubio, M.; Padilla, I.M.G.; Ilardi, V.; Burgos, L. Greenhouse evaluation confirms in vitro sharka resistance of genetically engineered h-UTR/P1 plum plants. Plant Cell Tiss. Org. 2015, 120, 791–796. [Google Scholar] [CrossRef]
- Głowacka, A.; Rozpara, E. Effect of rootstocks on the growth and yielding of sharkaresistant “Jojo” plum trees under organic orchard conditions. J. Res. Appl. Agric. Eng. 2018, 63, 16–19. [Google Scholar]
- Petri, C.; Alburquerque, N.; Faize, M.; Scorza, R.; Dardick, C. Current achievements and future directions in genetic engineering of European plum (Prunus domestica L.). Transgenic Res. 2018, 27, 225–240. [Google Scholar] [CrossRef]
- Polák, J.; Kundu, J.K.; Krška, B.; Beoni, E.; Komínek, P.; Pívalová, J.; Jarošová, J. Transgenic plum Prunus domestica L., clone C5 (cv. HoneySweet) for protection against sharka disease. J. Integr. Agric. 2017, 16, 516–522. [Google Scholar] [CrossRef]
- Sestraş, R.; Botu, M.; Mitre, V.; Sestraş, A.; Roşu-Mareş, S. Comparative study on the response of several plum cultivars in central Transylvania conditions, Romania. Not. Bot. Hort. Agrobot. Cluj. 2007, 35, 69–75. [Google Scholar]
- Athanasiadis, I.; Nikoloudakis, N.; Hagidimitriou, M. Genetic relatedness among cultivars of the Greek plum germplasm. Not. Bot. Hort. Agrobot. Cluj. 2013, 41, 491–498. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Byrne, D.; Okie, W.R.; Cisneros-Zevallos, L. Selecting new peach and plum genotypes rich in phenolic compounds and enhanced functional properties. Food Chem. 2006, 96, 273–280. [Google Scholar] [CrossRef]
- Družić, J.; Voća, S.; Čmelik, Z.; Dobričević, N.; Duralija, B.; Skendrović Babojelić, M. Fruit quality of plum cultivars “Elena” and “Bistrica”. Agric. Conspec. Sci. 2007, 72, 307–310. [Google Scholar]
- Khadivi-Khub, A.; Barazandeh, M. A morphometric study of autochthonous plum genotypes based on multivariate analysis. Erwerbs Obstbau 2015, 57, 185–194. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N. Phenotypic diversity of autochthonous European (Prunus domestica L.) and Damson (Prunus insititia L.) plum accessions based on multivariate analysis. Hortic. Sci. 2012, 39, 8–20. [Google Scholar]
- Butturini, A.; Tiso, R.; Molinari, F. Phenological forecasting model for Cydia funebrana. EPPO Bull. 2000, 30, 131–136. [Google Scholar] [CrossRef]
- Molinari, F. Notes on biology and monitoring of Cydia funebrana (Treitschke). IOBC WPRS Bulletin. 1995, 18, 39–42. [Google Scholar]
- Rizzo, R.; Lo Verde, G. Primi studi sulla biologia e sul controllo di Cydia funebrana (Treitschke) in susineti biologici siciliani. In Progetto per lo Sviluppo dell’Agricoltura Biologica in Sicilia-Atti del Convegno; Palermo: Regione Siciliana, Italy, 2011; pp. 239–248. [Google Scholar]
- Beroza, M.; Bierl, B.A.; Moffitt, H.R. Sex pheromones: (E,E)-8,10-dodecadien-1-ol in the codling moth. Science 1974, 11, 89–90. [Google Scholar] [CrossRef]
- Löfstedt, C.; Vickers, N.J.; Baker, T.C. Courtship, pheromone titer and determination of the male mating success in the Oriental fruit moth, Grapholita molesta (Lepidoptera, Tortricidae). Entomol. Gen. 1990, 15, 121–125. [Google Scholar] [CrossRef]
- Roelofs, W.; Comeau, A.; Hill, A.; Milicevic, G. Sex attractant of the codling moth: Characterization and electroantennogram technique. Science 1971, 15, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.M. Searching behavior and survival of 1st-instar codling moth. Ann. Entomol. Soc. Am. 1982, 75, 284–289. [Google Scholar] [CrossRef]
- Alford, D.V. Farbatlas der Obstschädlinge; Ferdinand Enke: Stuttgart, Germany, 1987; pp. 168–169. [Google Scholar]
- Rizzo, R.; Lo Verde, G.; Lombardo, A. Effectiveness of spinosad and mineral oil for control of Grapholita funebrana Treitschke in organic plum orchards. New Medit. 2012, 11, 70–72. [Google Scholar]
- Myers, C.T.; Hull, L.A.; Krawczyk, G. Seasonal and cultivar associated variation in oviposition preference of oriental fruit moth (Lepitoptera: Tortricidae) adults and feeding behavior of neonate larvae in apples. J. Econom. Entomol. 2006, 99, 349–358. [Google Scholar] [CrossRef]
- Sharon, R.; Zahavi, T.; Soroker, V.; Harari, A.R. The effect of grape vine cultivars on Lobesia botrana (Lepidoptera: Tortricidae) population levels. J. Pest Sci. 2009, 82, 187–193. [Google Scholar] [CrossRef]
- Wearing, C.H. Distribution characteristics of eggs and neonate larvae of codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae). Int. J. Insect Sci. 2016, 8, IJIS-S38587. [Google Scholar] [CrossRef]
- Pavan, F.; Stefanelli, G.; Villani, A.; Cargnus, E. Influence of Grapevine Cultivar on the Second Generations of Lobesia botrana and Eupoecilia ambiguella. Insects 2018, 9, 8. [Google Scholar] [CrossRef]
- Davis, T.S.; Garczynski, S.F.; Stevens-Rumann, C.; Landolt, P.J. A test of fruit varieties on entry rate and development by neonate larvae of the codling moth, Cydia pomonella. Entomol. Exp. Appl. 2013, 148, 259–266. [Google Scholar] [CrossRef]
- Markheiser, A.; Rid, M.; Biancu, S.; Gross, J.; Hoffmann, C. Physical factors influencing the oviposition behaviour of European grapevine moths Lobesia botrana and Eupoecilia ambiguella. J. Appl. Entomol. 2018, 142, 201–210. [Google Scholar] [CrossRef]
- Moreau, J.; Benrey, B.; Thiery, D. Grape variety affects larval performance and also female reproductive performance of the European grapevine moth Lobesia botrana (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2006, 96, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Thiéry, D.; Troussard, J.P.; Benrey, B. Grape variety affects female but also male reproductive success in wild European grapevine moths. Ecol. Entomol. 2007, 32, 747–753. [Google Scholar] [CrossRef]
- Thiéry, D.; Moreau, J. Grape cultivar affects larval and female fitness of the European grapevine moth, Lobesia botrana (Lepidoptera: Tortricidae). IOBC WPRS Bull. 2006, 29, 131–138. [Google Scholar]
- Cha, D.H.; Hesler, S.P.; Linn, J.C.E.; Zhang, A.; Teal, P.E.A.; Knight, A.L.; Roelofs, W.L.; Loeb, G.M. Influence of trap design on upwind flight behaviour and capture of female grape berry moth (Lepidoptera: Tortricidae) with a kairomone lure. Environ. Entomol. 2013, 42, 150–157. [Google Scholar] [CrossRef][Green Version]
- Gilbert, C.; Kuenen, L.P. Multimodal integration: Visual cues help odor-seeking fruit flies. Curr. Biol. 2008, 18, 295–297. [Google Scholar] [CrossRef][Green Version]
- Nava, D.E.; Parra, J.R.P.; Diez-Rodríguez, G.I.; Bento, J.M.S. Oviposition behavior of Stenoma catenifer (Lepidoptera: Elachistidae): Chemical and physical stimuli and diel pattern of egg laying. Ann. Entomol. Soc. Am. 2005, 98, 409–414. [Google Scholar] [CrossRef]
- Raguso, R.R.; Willis, M.A. Synergy between visual and olfactory cues in nectar feeding by naive hawk moths, Manduca sexta. Anim. Behav. 2002, 63, 685–695. [Google Scholar] [CrossRef]
- Rausher, M.D. Larval habitat suitability and oviposition preference in three related butterflies. Ecology 1979, 60, 503–511. [Google Scholar] [CrossRef]
- Sohrabi, F.; Nooryazdan, H.; Gharati, B.; Saeidi, Z. Evaluation of ten tomato cultivars for resistance against tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) under field infestation conditions. Entomol. Gen. 2016, 36, 163–175. [Google Scholar] [CrossRef]
- Thompson, J.N.; Pellmyr, O. Evolution of oviposition behaviour and host preference in Lepidoptera. Ann. Rev. Entomol. 1991, 36, 65–89. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Mazomenos, B.E. Effect of trap type, trap color, trapping location, and pheromone dispenser on captures of male Palpita unionalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2004, 97, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.L. Increased catch of female codling moth (Lepidoptera: Tortricidae) in kairomone-baited clear delta traps. Environ. Entomol. 2010, 39, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.L.; Fisher, J. Increased catch of codling moth (Lepidoptera: Tortricidae) in semiochemical baited orange plastic delta-shaped traps. Environ. Entomol. 2006, 35, 1597–1602. [Google Scholar] [CrossRef]
- Knight, A.L.; Miliczky, E. Influence of trap colour on the capture of codling moth (Lepidoptera: Tortricidae), honeybees, and non-target flies. J. Entomol. Soc. Br. Columbia 2003, 100, 65–70. [Google Scholar]
- Leskey, T.C. Visual cues and capture mechanisms associated with traps for plum curculio (Coleoptera: Curculiondae). J. Entomol. Sci. 2006, 41, 97–106. [Google Scholar] [CrossRef]
- Rizzo, R.; Caleca, V.; Lombardo, A.; Lo Verde, G. Effectiveness of spinosad and mineral oil based commercial products on oviposition and egg hatching of Grapholita funebrana Treitschke. Redia 2018, 101, 161–166. [Google Scholar] [CrossRef]
- Farina, V.; Barone, F.; Mazzaglia, A.; Lanza, C.M. Evaluation of fruit quality in loquat using both chemical and sensory analyses. Acta Hortic. 2010, 887, 345–349. [Google Scholar] [CrossRef]
- Francaviglia, D.; Farina, V.; Avellone, G.; Lo Bianco, R. Fruit yield and quality responses of apple cvars Gala and Fuji to partial rootzone drying under Mediterranean conditions. J. Agric. Sci. 2013, 151, 556–569. [Google Scholar] [CrossRef]
- Beeke, H.; De Jong, D.J. Identification of larvae and pupae. In World Crop Pests. Tortricid Pests: Their Biology, Natural Enemies and Control; van der Geest, L.P.S., Evenhuis, H.H., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1991; pp. 65–76. [Google Scholar]
- Dorn, S.; Hughes, J.; Molinari, F.; Cravedi, P. Cydia molesta and Cydia pomonella: Comparison of adult behaviour. IOBC WPRS Bulletin. 2001, 24, 133–137. [Google Scholar]
- Nicholls, E.; de Ibarra, N.H. Bees associate colour cues with differences in pollen rewards. J. Exp. Biol. 2014, 217, 2783–2788. [Google Scholar] [CrossRef]
- Tang, Y.C.; Zhou, C.L.; Chen, X.M.; Zheng, H. Visual and olfactory responses of seven butterfly species during foraging. J. Insect Behav. 2013, 26, 387–401. [Google Scholar] [CrossRef]
- Cornelius, M.L.; Duan, J.J.; Messing, R.H. Visual stimuli and the response of female cues, which only attracted males. To prevent damage in spruce seed oriental fruit flies (Diptera: Tephritidae) to fruit-mimicking traps. J. Econ. Entomol. 1999, 92, 121–129. [Google Scholar] [CrossRef]
- Owens, E.D.; Prokopy, R.J. Habitat background characteristics influencing Rhagoletis pomonella (Walsh) (Dipt., Tephritidae) fly response to foliar and fruit mimic traps. Z. für Angew. Entomol. 1984, 98, 98–103. [Google Scholar] [CrossRef]
- Owens, E.D.; Prokopy, R.J. Relationship between reflectance spectra of host plant surfaces and visual detection of host fruit by Rhagoletis pomonella flies. Physiol. Entomol. 1986, 11, 297–307. [Google Scholar] [CrossRef]
- Feron, M. L’instinct de reproduction chez la mouche méditerranéenne des fruits Ceratitis capitata Wied. (Dipt. Trypetidae). Comportement sexuel. Comportement de ponte. Rev. Pat. Veg. Entomol. Veg. 1962, 41, 1–129. [Google Scholar]
- Prokopy, R.J. Artificial oviposition devices for apple maggot. J. Econ. Entomol. 1966, 59, 231–232. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Boller, E.F. Stimuli eliciting oviposition of European cherry fruit flies Rhagoletis cerasi (Diptera: Tephritidae), into inanimate objects. Entomol. Exp. Appl. 1971, 14, 1–14. [Google Scholar] [CrossRef]
- Rizzo, R.; Caleca, V.; Lombardo, A. Relation of fruit color, elongation, hardness, and volume to the infestation of olive cultivars by the olive fruit fly, Bactrocera oleae. Entomol. Exp. Appl. 2012, 145, 15–22. [Google Scholar] [CrossRef]
- Sanders, W. Das Verhalten der Mittelmeerfruchtfliege Ceratitis capitata Wied. bei der Eiablage. Zeitschrift für Tier- zuchtung und Zuchtungsbiologie. J. Anim. Breed. Genet. 1962, 19, 1–28. [Google Scholar]
- Wiesmann, R. Die Orientierung der Kirschfliege, Rhagoletis cerasi L., bei der Eiablage. (Eine sinnesphysio logische Untersuchung). Landwirtsch. Jahrb. Schweiz 1937, 51, 1080–1109. [Google Scholar]
- Zhao, Z.G.; Rong, E.H.; Li, S.C.; Zhang, L.J.; Kong, W.N.; Hu, R.S.; Zhang, J.T.; Ma, R.Y. Research on the practical parameters of sex pheromone traps for the oriental fruit moth. Pest. Manag. Sci. 2013, 69, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.W.; Liu, W.X.; Zhang, G.F.; Wan, F.H.; Xu, H.F.; Pu, C.J. Affecting factors on capture efficacy of sex pheromone traps for Cydia pomonella L. Chin. J. Appl. Ecol. 2010, 21, 801–806. [Google Scholar]
- Jakobsson, J.; Henze, M.J.; Svensson, G.P.; Lind, O.; Anderbrant, O. Visual cues of oviposition sites and spectral sensitivity of Cydia strobilella L. J. Insect Physiol. 2017, 101, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Barreto, O.; Martínez, A.M.; Viñuela, E.; Figueroa, J.I.; Rebollar, Á.; Chavarrieta, J.M.; Lobit, P.; Pineda, S. Biological Parameters of Argyrotaenia montezumae (Lepidoptera: Tortricidae) and Influence of the Oviposition Substrate Color on Fecundity. Ann. Entomol. Soc. Am. 2016, 109, 671–677. [Google Scholar] [CrossRef]
- Städler, E. Host plant stimuli affecting oviposition behavior of the eastern spruce budworm. Entomol. Exp. Appl. 1974, 17, 176–188. [Google Scholar] [CrossRef]
- Maher, N.; Thiéry, D. A bioassay to evaluate the activity of chemical stimuli from grape berries on the oviposition of Lobesia botrana (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2004, 94, 27–33. [Google Scholar] [CrossRef]
- Savopoulou-Soultani, M.; Nikolaou, N.; Milonas, P.G. Influence of maturity stage of grape berries on the development of Lobesia botrana (Lepidoptera: Tortricidae) larvae. J. Econ. Entomol. 1999, 92, 551–556. [Google Scholar] [CrossRef]
- Harvey, G.T. Nutritional studies of eastern spruce budworm (Lepidoptera: Tortricidae): I. Soluble sugars. Can. Entomol. 1974, 106, 353–365. [Google Scholar] [CrossRef]
- Savopoulou-Soultani, M.; Stavridis, D.G.; Vassiliou, A.; Stafilidis, J.E.; Iraklidis, I. Response of Lobesia botrana (Lepidoptera: Tortricidae) to levels of sugar and protein in artificial diets. J. Econ. Entomol. 1994, 87, 84–90. [Google Scholar] [CrossRef]
- Liverani, A.; Sirri, S.; Nencetti, V.; Missere, D.; Etiopi, C. A list of plum cultivars in Italy. Inf. Agrar. 2014, 70, 44–47. [Google Scholar]
- Joshi, N.K.; Rajotte, E.G.; Myers, C.T.; Krawczyk, G.; Hull, L.A. Development of a susceptibility index of apple cultivars for codling moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae) oviposition. Front. Plant Sci. 2015, 6, 992. [Google Scholar] [CrossRef] [PubMed]
- Bawin, T.; De Backer, L.; Dujeu, D.; Legrand, P.; Megido, R.; Francis, F.; Verheggen, F. Infestation level influences oviposition site selection in the tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae). Insects 2014, 5, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Chew, F.S.; Robbins, K.L. Egg-laying in butterflies. Biol. Butterflies 1984, 11, 65–79. [Google Scholar]
- Renwick, J.A.A. Chemical ecology of oviposition in phytophagous insects. Experimentia 1989, 45, 223–228. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference–performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Kogan, M. Integrated pest management: Historical perspectives and contemporary developments. Annu. Rev. Entomol. 1998, 43, 243–270. [Google Scholar] [CrossRef]
- Arioli, C.J.; Bottom, M.; Carvalho, G.A. Controle quimico da Grapholita molesta (Busck) (Lepidoptera: Tortricidae) na cultura do pesseguiero. Ciência Rural 2004, 34, 1695–1700. [Google Scholar] [CrossRef][Green Version]
- Chaves, C.C.; Baronio, C.A.; Botton, M.; Garcia, M.S. Efeito de inseticidas em diferentes fases de desenvolvimento de Grapholita molesta (Busck, 1916) (Lepidoptera: Tortricidae) e estruturas vegetais da macieira e do pessegueiro. Rev. Bras. Frutic. 2014, 36, 842–852. [Google Scholar] [CrossRef]
- Arioli, C.J.; Pastori, P.L.; Botton, M.; Garcia, M.S.; Borges, R.; Mafra-Neto, A. Assessment of SPLAT formulations to control Grapholita molesta (Lepidoptera: Tortricidae) in a Brazilian apple orchard. Chil. J. Agric. Res. 2014, 74, 184–190. [Google Scholar] [CrossRef]
- Pastori, P.L.; Arioli, C.J.; Botton, M.; Monteiro, L.B.; Stoltman, L.; Mafra-Neto, A. Integrated control of two tortricid (Lepidoptera) pests in apple orchards with sex pheromones and insecticides. Rev. Colomb. Entomol. 2012, 38, 224–230. [Google Scholar]
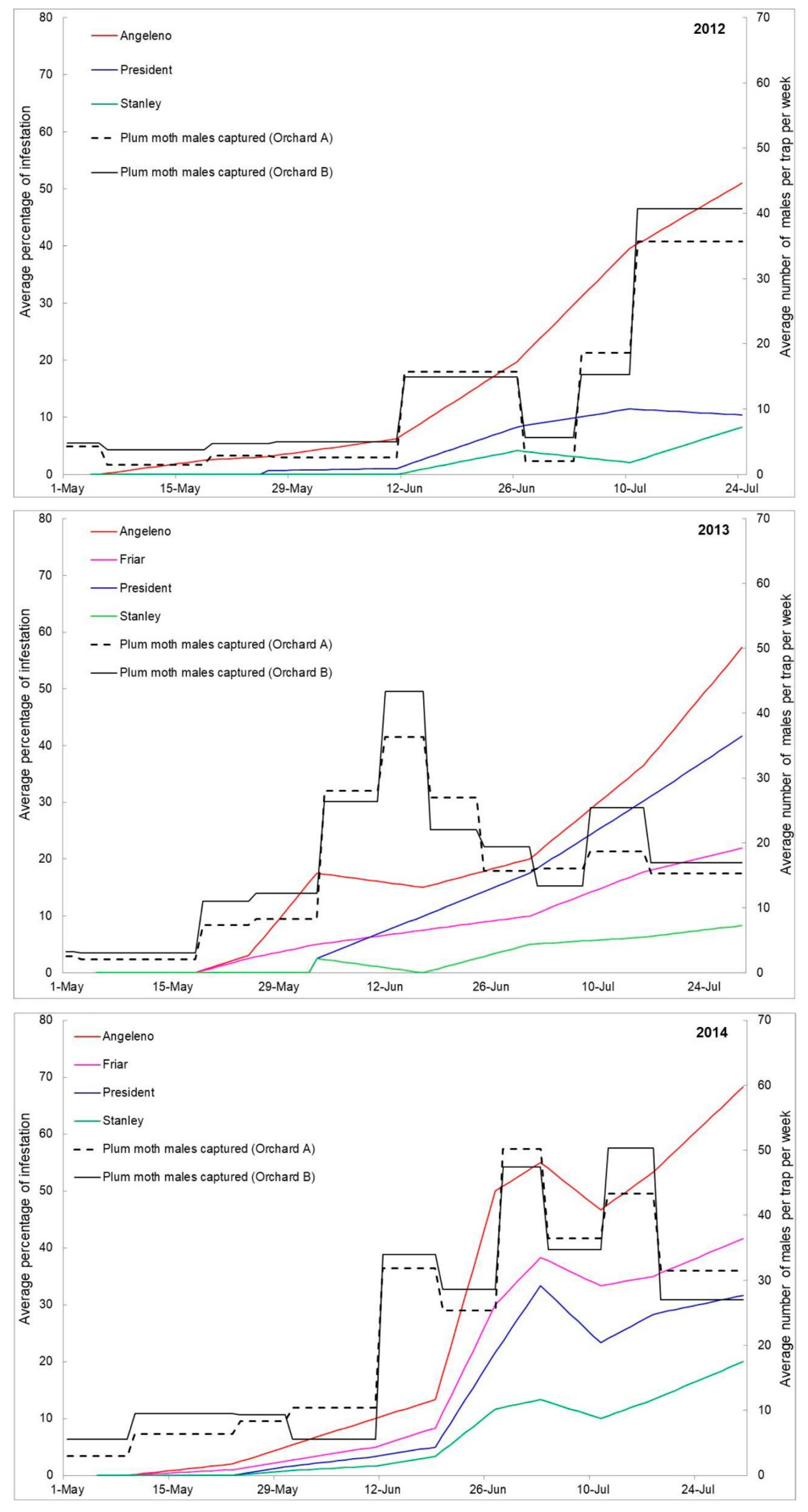
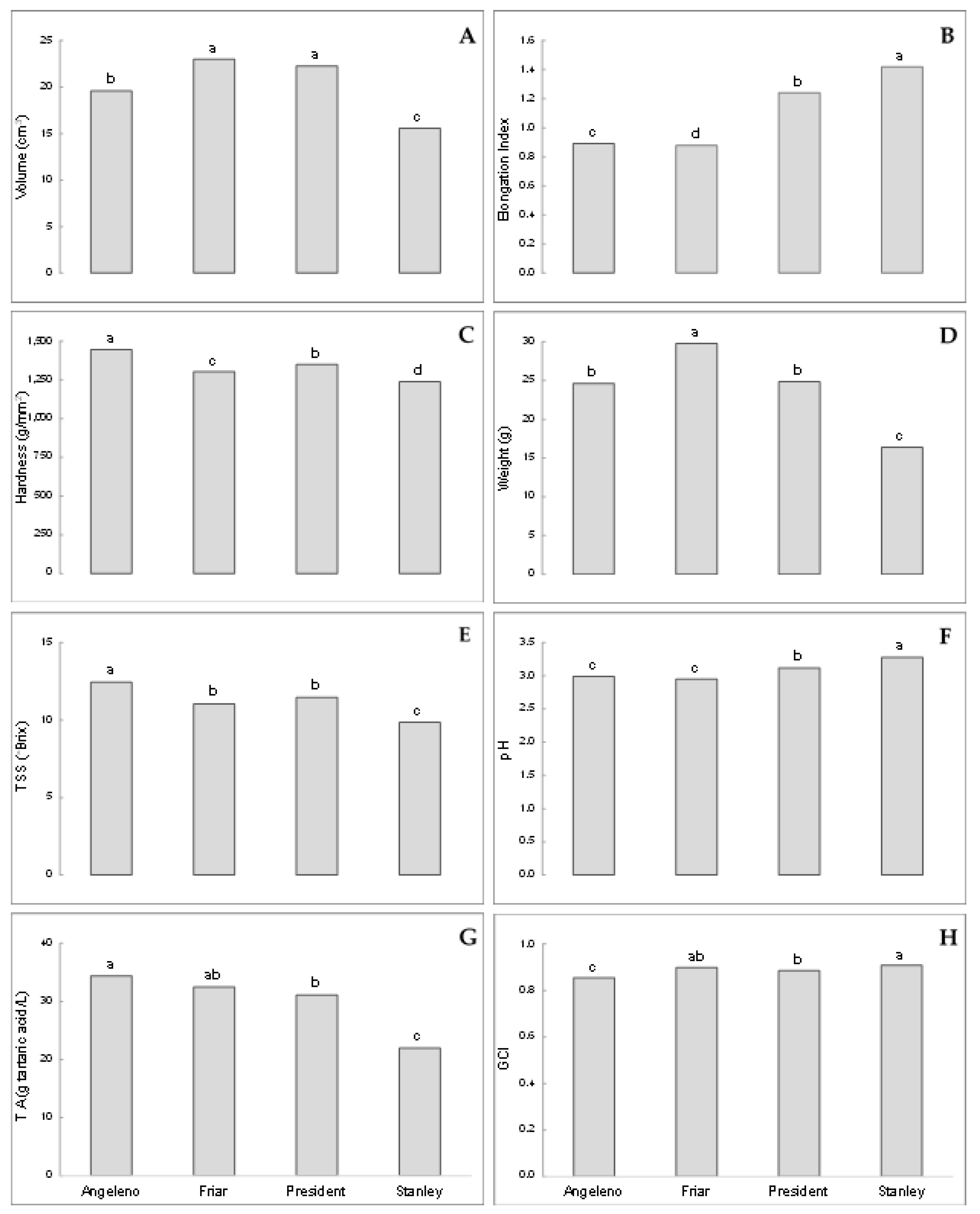
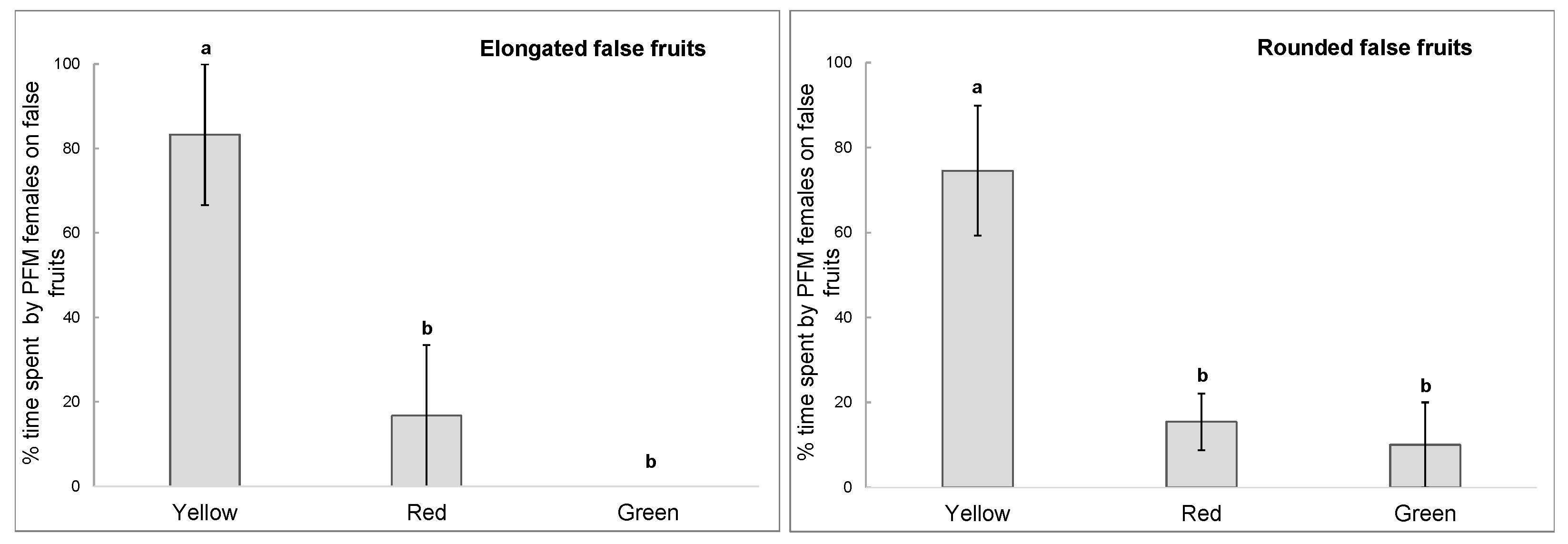

| Cultivar | 2012 | 2013 | 2014 |
|---|---|---|---|
| Angeleno | 29.17 a | 35.58 a | 42.38 a |
| Friar | - | 15.06 b | 27.38 b |
| President | 7.81 b | 25.96 c | 20.95 b |
| Stanley | 3.65 c | 5.45 d | 10.48 c |
| Binary logistic regression results | |||
| Date | χ2 = 77.19 ** (DF 3) | χ2 = 84.28 ** (DF 4) | χ2 = 179.54 ** (DF 7) |
| Cultivar | χ2 = 126.83 ** (DF 2) | χ2 = 113.07 ** (DF 3) | χ2 = 133.73 ** (DF 3) |
| Goodness-of-fit tests | χ2 = 717.17 NS (DF 1146) | χ2 = 1075.70 NS (DF 1240) | χ2 = 1598.95 NS (DF 1670) |
| Factors | DF | F-Values | |||
|---|---|---|---|---|---|
| Volume | Weight | Hardness | Elongation Index | ||
| Cultivar | 3 | 56.97 | 115.23 | 78.89 | 3983.68 |
| Year | 1 | 26.84 | 19.34 | 16.27 | 67.77 |
| Cultivar * Year | 3 | 15.24 | 10.86 | 33.16 | 12.92 |
| Date (Year) | 10 | 351.92 | 422.61 | 309.50 | 72.45 |
| Tree (Cultivar, Year) | 76 | 5.18 | 5.75 | 2.11 | 3.34 |
| Total DF | 2927 | ||||
| Put into Group | True Group | |||
| Angeleno | Friar | President | Stanley | |
| Angeleno | 46 | 6 | 0 | 0 |
| Friar | 2 | 42 | 0 | 0 |
| President | 0 | 0 | 47 | 0 |
| Stanley | 0 | 0 | 1 | 48 |
| Total No. | 48 | 48 | 48 | 48 |
| No. correct | 46 | 42 | 47 | 48 |
| Proportion | 0.96 | 0.88 | 0.98 | 1.00 |
| Linear Discriminant Function for Cultivar | ||||
| Angeleno | Friar | President | Stanley | |
| Constant | −19.15 | −25.79 | −8.67 | −42.48 |
| Volume | −8.29 | −15.73 | 9.23 | 14.80 |
| Elongation Index | −41.91 | −49.12 | 28.19 | 62.84 |
| Weight | 8.66 | 16.56 | −8.28 | −16.94 |
| Hardness | 2.36 | 1.70 | −0.90 | −3.16 |
| Titratable Acidity | −2.72 | −4.73 | 3.08 | 4.37 |
| pH | −1.59 | −1.53 | 0.76 | 2.37 |
| Total Soluble Solid | 0.21 | 1.03 | −0.12 | −1.12 |
| Ground Color Index | 1.39 | 3.94 | −1.73 | −3.60 |
| Cover Color Index | −4.25 | −5.02 | 2.07 | 7.19 |
| Cover Color Percentage | 1.26 | −0.03 | −1.09 | −0.15 |
| Source | DF | Adj Dev | Adj Mean | Chi-Square | p-Value |
|---|---|---|---|---|---|
| Regression | 21 | 424.67 | 20.22 | 424.67 | <0.001 |
| Titratable Acidity | 1 | 15.26 | 15.26 | 15.26 | <0.001 |
| Elongation Index * Cultivar | 3 | 30.29 | 10.10 | 30.29 | <0.001 |
| Total Soluble Solid * Cultivar | 3 | 27.29 | 9.10 | 27.29 | <0.001 |
| Titratable Acidity * Date | 11 | 166.21 | 15.11 | 166.21 | <0.001 |
| Cover Colour Percentage * Cultivar | 3 | 16.06 | 5.35 | 16.06 | <0.001 |
| Error | 170 | 134.91 | 0.79 | ||
| Total | 191 | 559.58 |
| Source | DF | Adj Dev | Adj Mean | Chi-Square | p-Value |
|---|---|---|---|---|---|
| Regression | 14 | 387.90 | 27.71 | 387.87 | <0.001 |
| Cultivar | 3 | 176.60 | 58.85 | 176.56 | <0.001 |
| Date | 11 | 229.30 | 20.84 | 229.27 | <0.001 |
| Error | 177 | 171.70 | 0.97 | ||
| Total | 191 | 559.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, R.; Farina, V.; Saiano, F.; Lombardo, A.; Ragusa, E.; Lo Verde, G. Do Grapholita funebrana Infestation Rely on Specific Plum Fruit Features? Insects 2019, 10, 444. https://doi.org/10.3390/insects10120444
Rizzo R, Farina V, Saiano F, Lombardo A, Ragusa E, Lo Verde G. Do Grapholita funebrana Infestation Rely on Specific Plum Fruit Features? Insects. 2019; 10(12):444. https://doi.org/10.3390/insects10120444
Chicago/Turabian StyleRizzo, Roberto, Vittorio Farina, Filippo Saiano, Alberto Lombardo, Ernesto Ragusa, and Gabriella Lo Verde. 2019. "Do Grapholita funebrana Infestation Rely on Specific Plum Fruit Features?" Insects 10, no. 12: 444. https://doi.org/10.3390/insects10120444
APA StyleRizzo, R., Farina, V., Saiano, F., Lombardo, A., Ragusa, E., & Lo Verde, G. (2019). Do Grapholita funebrana Infestation Rely on Specific Plum Fruit Features? Insects, 10(12), 444. https://doi.org/10.3390/insects10120444







