The Association between Virus Prevalence and Intercolonial Aggression Levels in the Yellow Crazy Ant, Anoplolepis Gracilipes (Jerdon)
Abstract
:1. Introduction
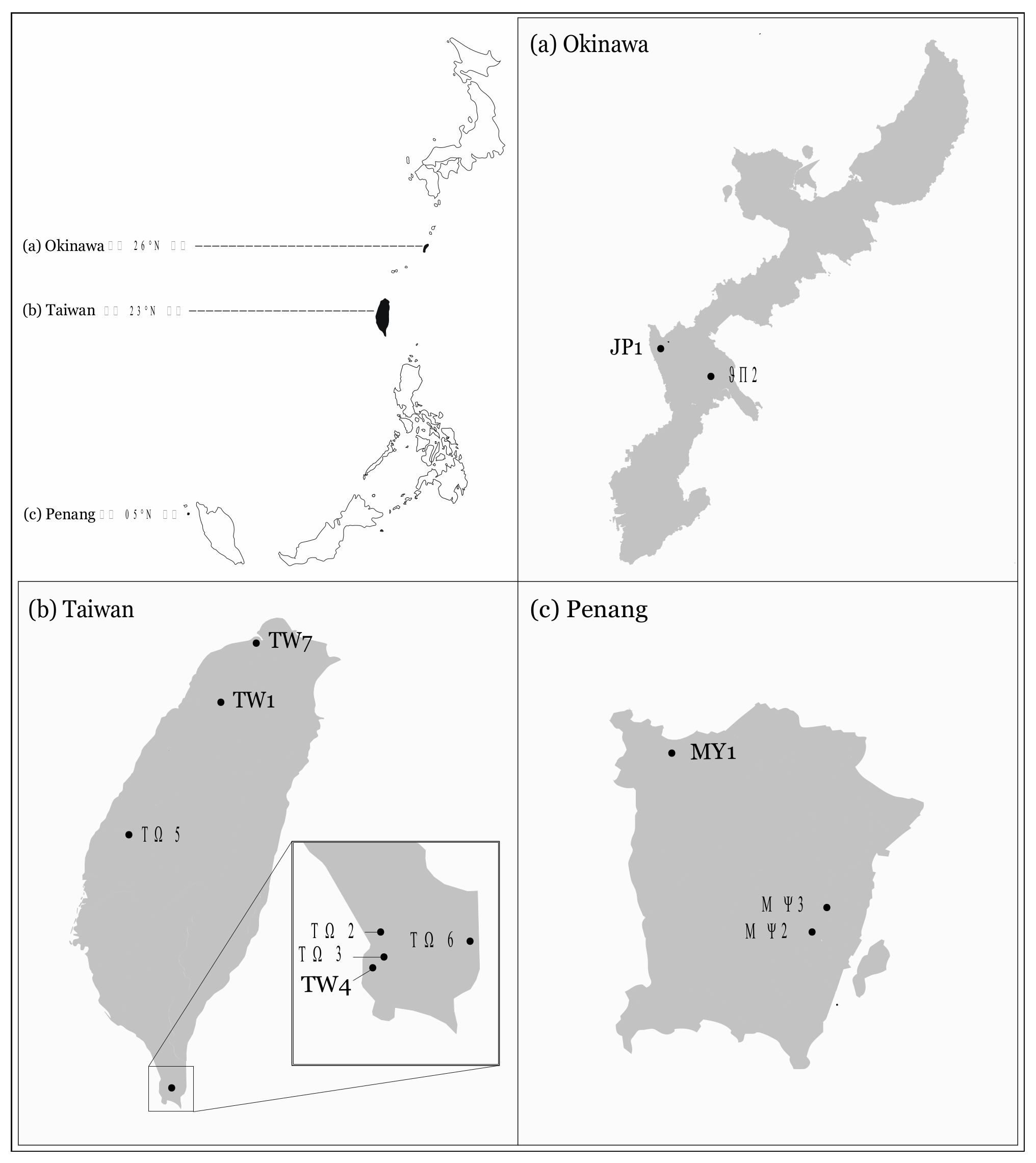
2. Materials and Methods
2.1. Virus Detection and Confirmation of Viral Replication
2.2. Aggression Assay
2.3. Correlation between Infection Status and Aggressive Behavior
3. Results
3.1. Horizontal Transimision, Replication and Prevalence of the TR44839 Virus
3.2. Colony Boundaries and Aggression Assay
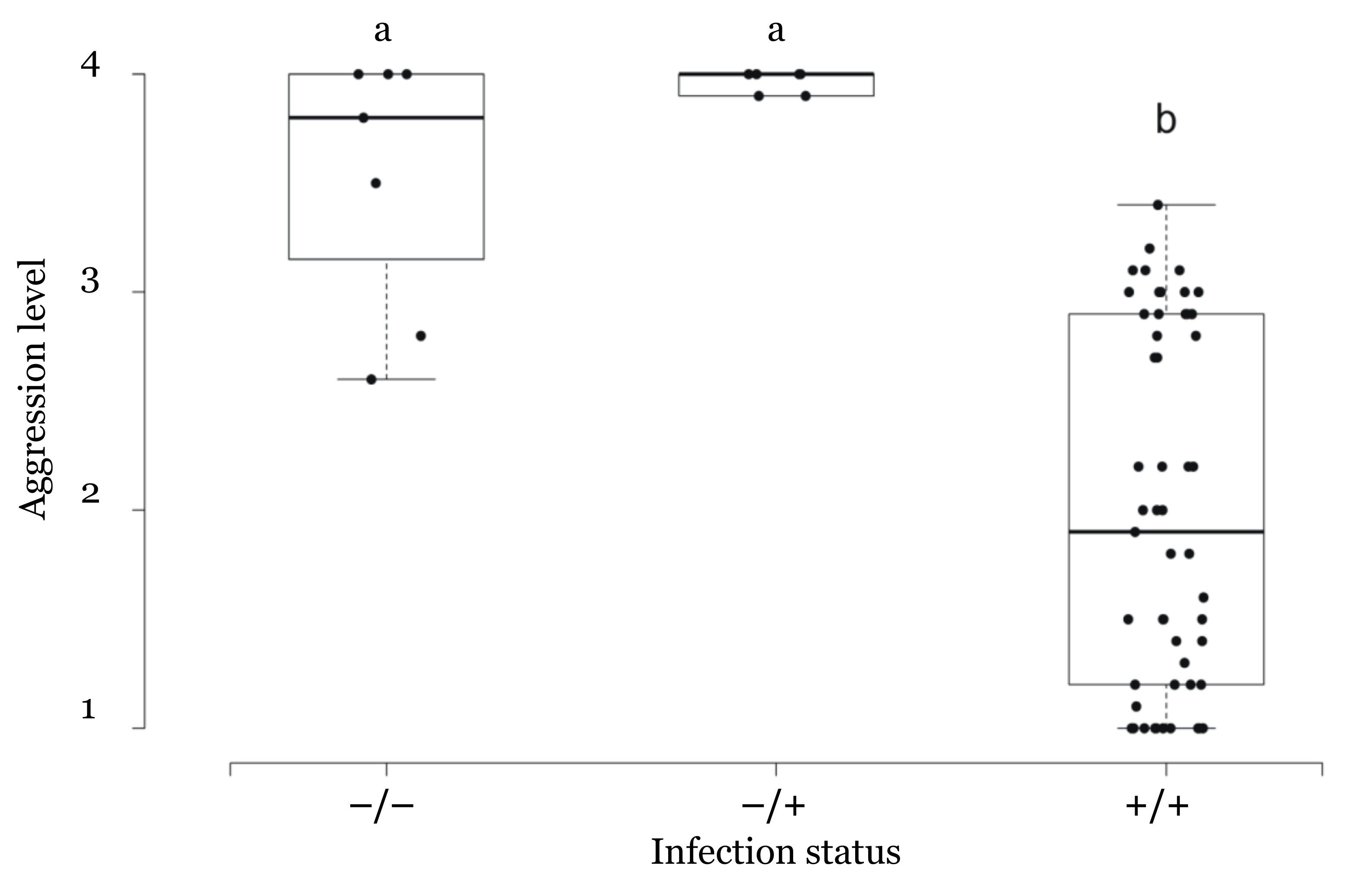
3.3. Correlation between Infection Status and Aggressive Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eyer, P.A.; Matsuura, K.; Vargo, E.L.; Kobayashi, K.; Yashiro, T.; Suehiro, W.; Himuro, C.; Yokoi, T.; Guenard, B.; Dunn, R.R.; et al. Inbreeding tolerance as a pre-adapted trait for invasion success in the invasive ant Brachyponera chinensis. Mol. Ecol. 2018, 27, 4711–4724. [Google Scholar]
- Eyer, P.A.; McDowell, B.; Johnson, L.N.L.; Calcaterra, L.A.; Fernandez, M.B.; Shoemaker, D.; Puckett, R.T.; Vargo, E.L. Supercolonial structure of invasive populations of the tawny crazy ant Nylanderia fulva in the US. BMC Evol. Biol. 2018, 18, 209. [Google Scholar] [CrossRef] [PubMed]
- King, J.R.; Tschinkel, W.R. Experimental evidence that dispersal drives ant community assembly in human-altered ecosystems. Ecology 2016, 97, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Van Wilgenburg, E.; Torres, C.W.; Tsutsui, N.D. The global expansion of a single ant supercolony. Evol. Appl. 2010, 3, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.D.; Hagedorn, H. Quantification of supercolonial traits in the yellow crazy ant, Anoplolepis gracilipes. J. Insect. Sci. 2014, 14, 1–21. [Google Scholar] [CrossRef]
- Fournier, D.; Tindo, M.; Kenne, M.; Masse, P.S.M.; Van Bossche, V.; De Coninck, E.; Aron, S. Genetic structure, nestmate recognition and behaviour of two cryptic species of the invasive big-headed ant Pheidole megacephala. PLoS ONE 2012, 7, e31480. [Google Scholar] [CrossRef] [Green Version]
- Tragust, S.; Feldhaar, H.; Espadaler, X.; Pedersen, J.S. Rapid increase of the parasitic fungus Laboulbenia formicarum in supercolonies of the invasive garden ant Lasius neglectus. Biol. Invasions 2015, 17, 2795–2801. [Google Scholar] [CrossRef]
- Ugelvig, L.V.; Cremer, S. Effects of social immunity and unicoloniality on host–parasite interactions in invasive insect societies. Funct. Ecol. 2012, 26, 1300–1312. [Google Scholar] [CrossRef]
- Valles, S.M.; Porter, S.D.; Choi, M.Y.; Oi, D.H. Successful transmission of Solenopsis invicta virus 3 to Solenopsis invicta fire ant colonies in oil, sugar, and cricket bait formulations. J. Invertebr. Pathol. 2013, 113, 198–204. [Google Scholar] [CrossRef]
- Dallas, T.A.; Krkosek, M.; Drake, J.M. Experimental evidence of a pathogen invasion threshold. R. Soc. Open Sci. 2018, 5, 171975. [Google Scholar] [CrossRef] [Green Version]
- Kavaliers, M.; Choleris, E. The role of social cognition in parasite and pathogen avoidance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altizer, S.; Nunn, C.L.; Thrall, P.H.; Gittleman, J.L.; Antonovics, J.; Cunningham, A.A.; Dobson, A.P.; Ezenwa, V.; Jones, K.E.; Pedersen, A.B.; et al. Social organization and parasite risk in mammals: Integrating theory and empirical studies. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 517–547. [Google Scholar] [CrossRef] [Green Version]
- Wetterer, J.K. World-wide distribution and potential spread of the long-legged ant, Anoplolepis gracilipes (Hymenoptera: Formicidae). Sociobiology 2005, 45, 77–97. [Google Scholar]
- Abbott, K.L. Supercolonies of the invasive yellow crazy ant, Anoplolepis gracilipes, on an oceanic island: Forager activity patterns, density and biomass. Insect. Soc. 2005, 52, 266–273. [Google Scholar] [CrossRef]
- Green, P.T.; O’Dowd, D.J.; Abbott, K.L.; Jeffery, M.; Retallick, K.; Mac, N.R. Invasional meltdown: Invader-invader mutualism facilitates a secondary invasion. Ecology 2011, 92, 1758–1768. [Google Scholar] [CrossRef]
- Chong, K.F.; Lee, C.Y. Inter-and intraspecific aggression in the invasive longlegged ant (Hymenoptera: Formicidae). J. Econ. Entomol. 2010, 103, 1775–1783. [Google Scholar] [CrossRef]
- Cooling, M.; Gruber, M.A.M.; Hoffmann, B.D.; Sébastien, A.; Lester, P.J. A metatranscriptomic survey of the invasive yellow crazy ant, Anoplolepis gracilipes, identifies several potential viral and bacterial pathogens and mutualists. Insect. Soc. 2017, 64, 197–207. [Google Scholar] [CrossRef]
- Bonning, B.C. The Dicistroviridae: An emerging family of invertebrate viruses. Virol. Sin. 2009, 24, 415–427. [Google Scholar] [CrossRef]
- Suarez, A.V.; Tsutsui, N.D.; Holway, D.A.; Case, T.J. Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biol. Invasions 1999, 1, 43–53. [Google Scholar] [CrossRef]
- Berville, L.; Blight, O.; Renucci, M.; Hefetz, A.; Provost, E. A peaceful zone bordering two Argentine ant (Linepithema humile) supercolonies. Chemoecology 2013, 23, 213–218. [Google Scholar] [CrossRef]
- Naug, D.; Camazine, S. The role of colony organization on pathogen transmission in social insects. J. Theor. Biol. 2002, 215, 427–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konrad, M.; Pull, C.D.; Metzler, S.; Seif, K.; Naderlinger, E.; Grasse, A.V.; Cremer, S. Ants avoid superinfections by performing risk-adjusted sanitary care. Proc. Natl. Acad. Sci. USA 2018, 115, 2782–2787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 20 December 2018).
- Giraud, T.; Pedersen, J.S.; Keller, L. Evolution of supercolonies: The Argentine ants of southern Europe. Proc. Natl. Acad. Sci. USA 2002, 99, 6075–6079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krapf, P.; Russo, L.; Arthofer, W.; Most, M.; Steiner, F.M.; Schlick-Steiner, B.C. An Alpine ant’s behavioural polymorphism: Monogyny with and without internest aggression in Tetramorium alpestre. Ethol. Ecol. Evol. 2018, 30, 220–234. [Google Scholar] [CrossRef]
- Oi, D.H. Effect of mono-and polygyne social forms on transmission and spread of a microsporidium in fire ant populations. J. Invertebr. Pathol. 2006, 92, 146–151. [Google Scholar] [CrossRef]
- Valles, S.M.; Oi, D.H.; Porter, S.D. Seasonal variation and the co-occurrence of four pathogens and a group of parasites among monogyne and polygyne fire ant colonies. Biol. Control 2010, 54, 342–348. [Google Scholar] [CrossRef]
- Yang, C.C.; Yu, Y.C.; Valles, S.M.; Oi, D.H.; Chen, Y.C.; Shoemaker, D.; Wu, W.J.; Shih, C.J. Loss of microbial (pathogen) infections associated with recent invasions of the red imported fire ant Solenopsis invicta. Biol. Invasions 2010, 12, 3307–3318. [Google Scholar] [CrossRef]
- Sébastien, A.; Lester, P.J.; Hall, R.J.; Wang, J.; Moore, N.E.; Gruber, M.A.M. Invasive ants carry novel viruses in their new range and form reservoirs for a honeybee pathogen. Biol. Lett. 2015, 11, 20150610. [Google Scholar] [CrossRef]
- Vogel, V.; Pedersen, J.S.; d’Ettorre, P.; Lehmann, L.; Keller, L. Dynamics and genetic structure of Argentine ant supercolonies in their native range. Evolution 2009, 63, 1627–1639. [Google Scholar] [CrossRef] [Green Version]
- Ito, F.; Asfiya, W.; Kojima, J. Discovery of independent-founding solitary queens in the yellow crazy ant Anoplolepis gracilipes in East Java, Indonesia (Hymenoptera: Formicidae). Entomol. Sci. 2016, 19, 312–314. [Google Scholar] [CrossRef]
- Valles, S.M. Positive-strand RNA viruses infecting the red imported fire ant, Solenopsis invicta. Psyche 2012, 2012, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Viljakainen, L.; Holmberg, I.; Abril, S.; Jurvansuu, J. Viruses of invasive Argentine ants from the European Main supercolony: Characterization, interactions and evolution. J. Gen. Virol. 2018, 99, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.W.; Chiu, M.C.; Shoemaker, D.; Yang, C.S. Viral infections in fire ants lead to reduced foraging activity and dietary changes. Sci. Rep. 2018, 8, 13498. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Shoemaker, D.; Grozinger, C.M. Dynamic changes in host-virus interactions associated with colony founding and social environment in fire ant queens (Solenopsis invicta). Ecol. Evol. 2016, 6, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Valles, S.M.; Hashimoto, Y. Isolation and characterization of Solenopsis invicta virus 3, a new positive-strand RNA virus infecting the red imported fire ant, Solenopsis invicta. Virology 2009, 388, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valles, S.M.; Strong, C.A.; Dang, P.M.; Hunter, W.B.; Pereira, R.M.; Oi, D.H.; Shapiro, A.M.; Williams, D.F. A picorna-like virus from the red imported fire ant, Solenopsis invicta: Initial discovery, genome sequence, and characterization. Virology 2004, 328, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Oi, D.; Valles, S.; Porter, S.; Cavanaugh, C.; White, G.; Henke, J. Introduction of fire ant biological control agents into the Coachella Valley of California. Fla. Entomol. 2019, 102, 284–286. [Google Scholar]
- Hashimoto, Y.; Valles, S.M. Solenopsis invicta virus-1 tissue tropism and intra-colony infection rate in the red imported fire ant: A quantitative PCR-based study. J. Invertebr. Pathol. 2007, 96, 156–161. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.; Feldlaufer, M. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 2006, 92, 152–159. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, H.-W.; Chiu, M.-C.; Lee, C.-C.; Lee, C.-Y.; Yang, C.-C.S. The Association between Virus Prevalence and Intercolonial Aggression Levels in the Yellow Crazy Ant, Anoplolepis Gracilipes (Jerdon). Insects 2019, 10, 436. https://doi.org/10.3390/insects10120436
Hsu H-W, Chiu M-C, Lee C-C, Lee C-Y, Yang C-CS. The Association between Virus Prevalence and Intercolonial Aggression Levels in the Yellow Crazy Ant, Anoplolepis Gracilipes (Jerdon). Insects. 2019; 10(12):436. https://doi.org/10.3390/insects10120436
Chicago/Turabian StyleHsu, Hung-Wei, Ming-Chung Chiu, Ching-Chen Lee, Chow-Yang Lee, and Chin-Cheng Scotty Yang. 2019. "The Association between Virus Prevalence and Intercolonial Aggression Levels in the Yellow Crazy Ant, Anoplolepis Gracilipes (Jerdon)" Insects 10, no. 12: 436. https://doi.org/10.3390/insects10120436
APA StyleHsu, H.-W., Chiu, M.-C., Lee, C.-C., Lee, C.-Y., & Yang, C.-C. S. (2019). The Association between Virus Prevalence and Intercolonial Aggression Levels in the Yellow Crazy Ant, Anoplolepis Gracilipes (Jerdon). Insects, 10(12), 436. https://doi.org/10.3390/insects10120436





