A Comparison of Three Approaches for Larval Instar Separation in Insects—A Case Study of Dendrolimus pini
Abstract
:1. Introduction
2. Materials and Methods
2.1. Head Capsule Measurements
2.2. Data Analysis
3. Results and Discussion
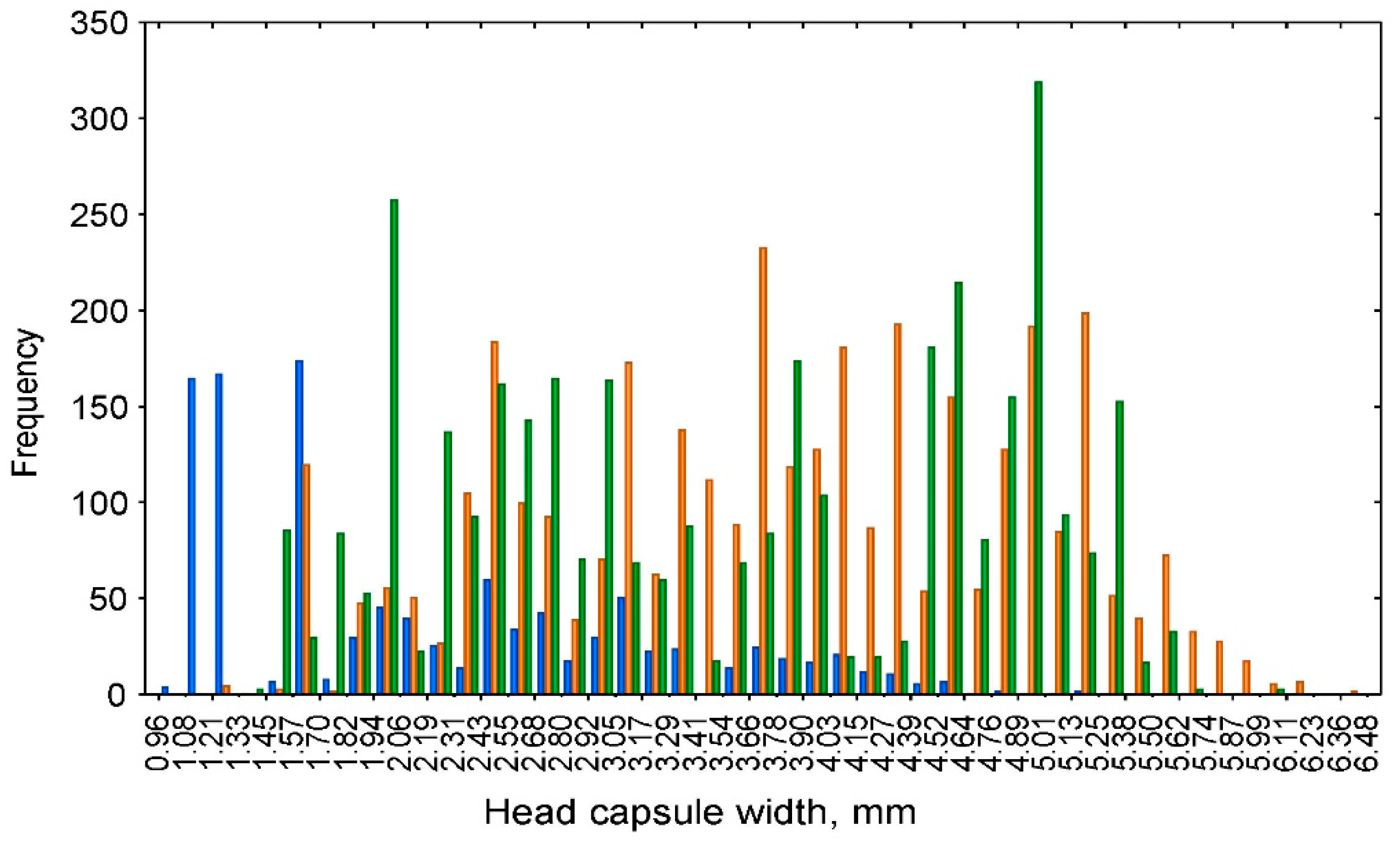
3.1. Visual Analysis of the Frequency Distribution and NLLS Estimation
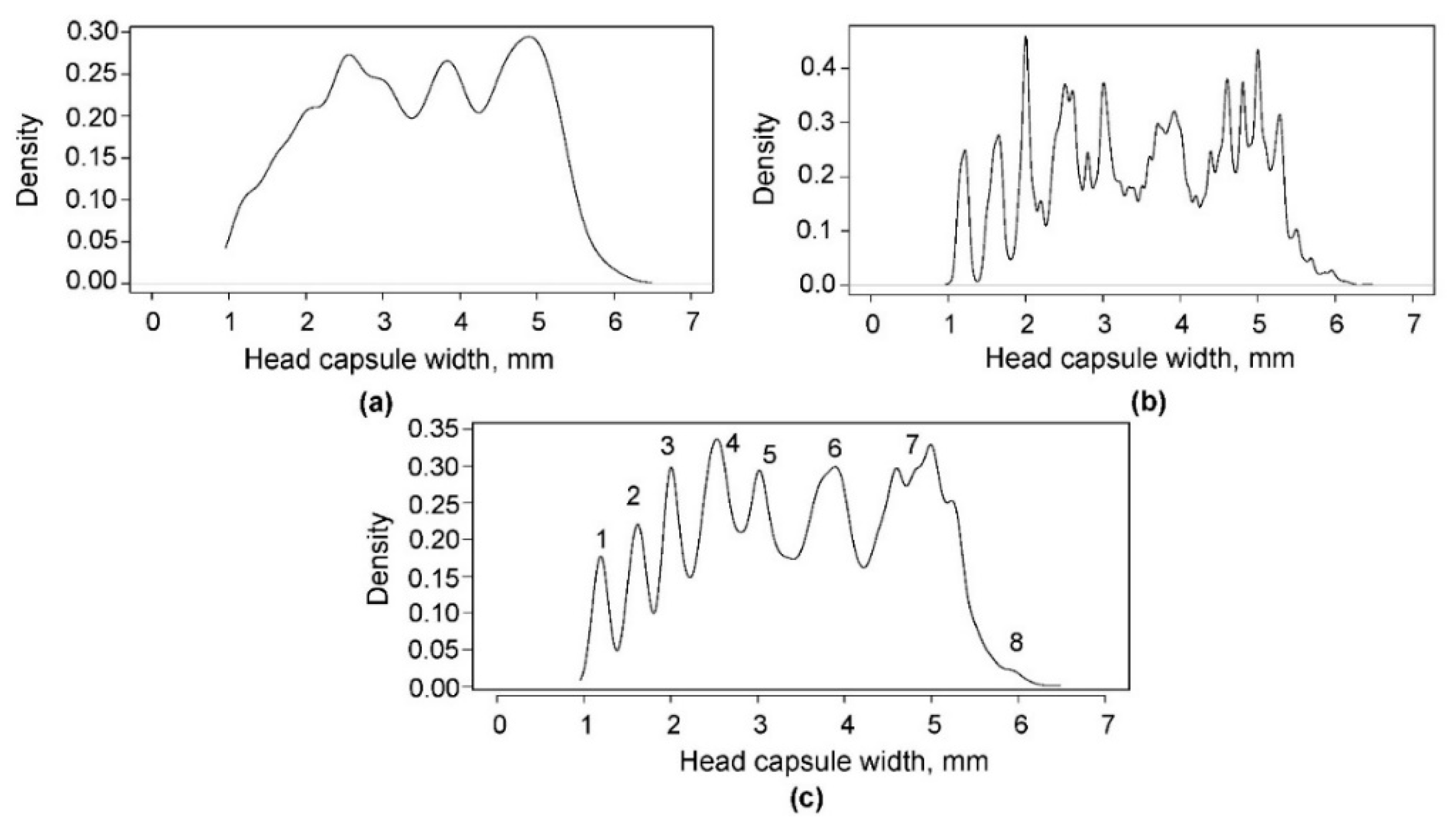
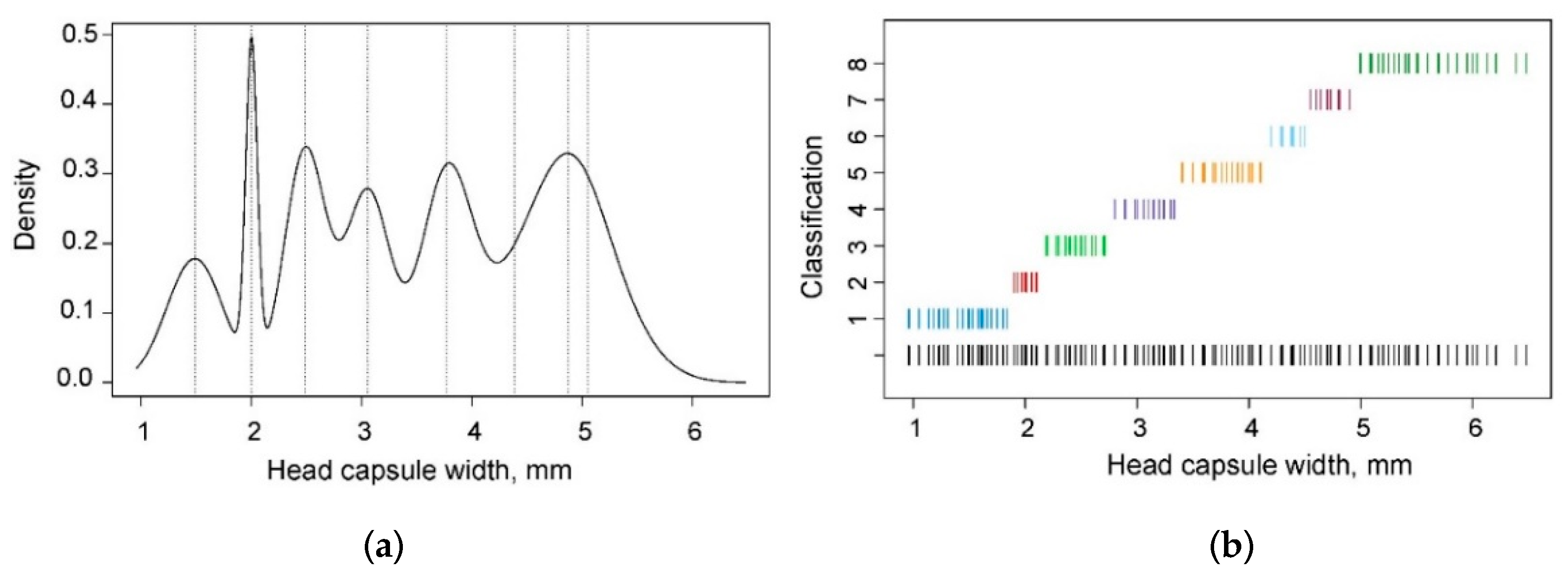
3.2. Kernel Density and NLLS Estimation
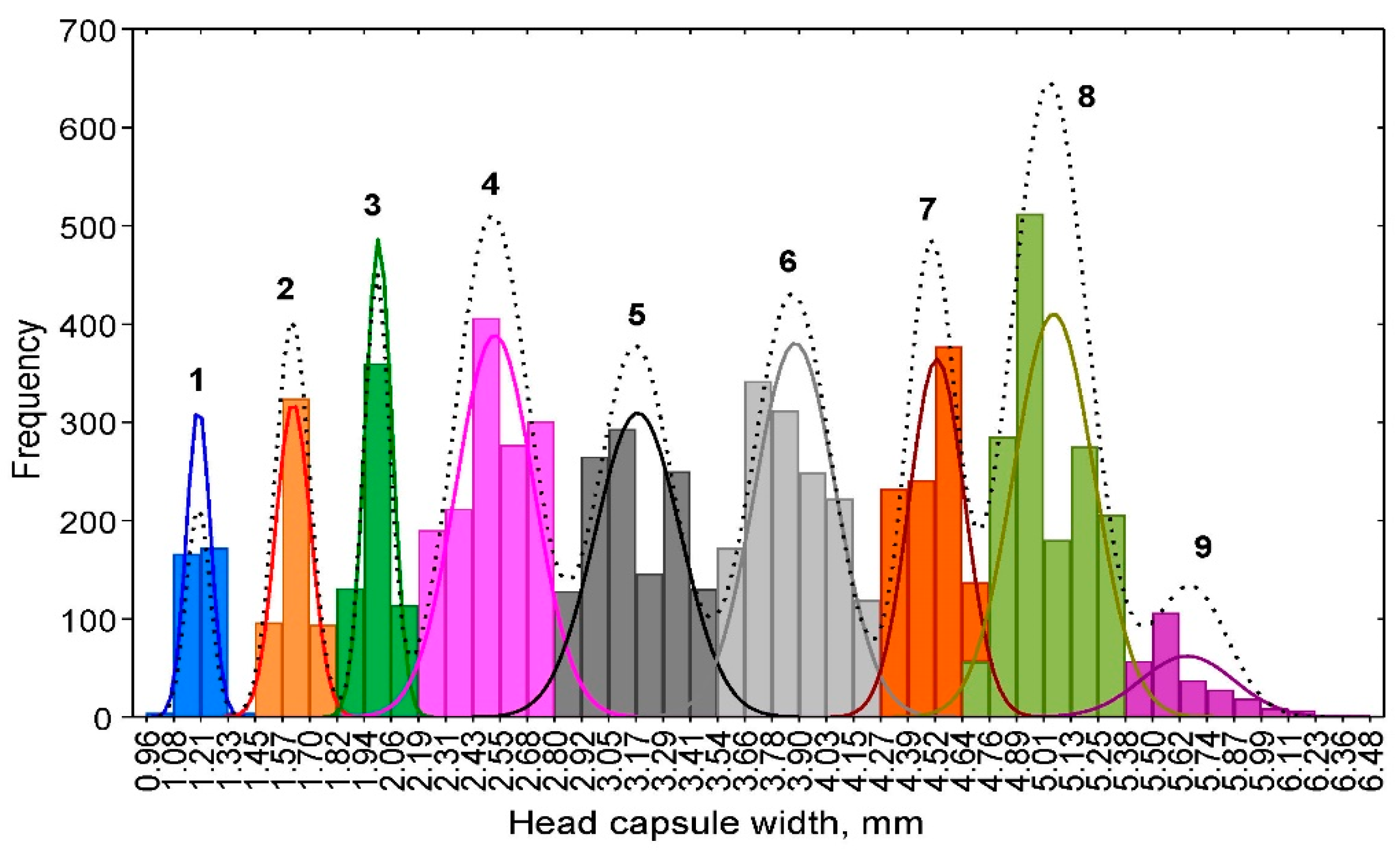
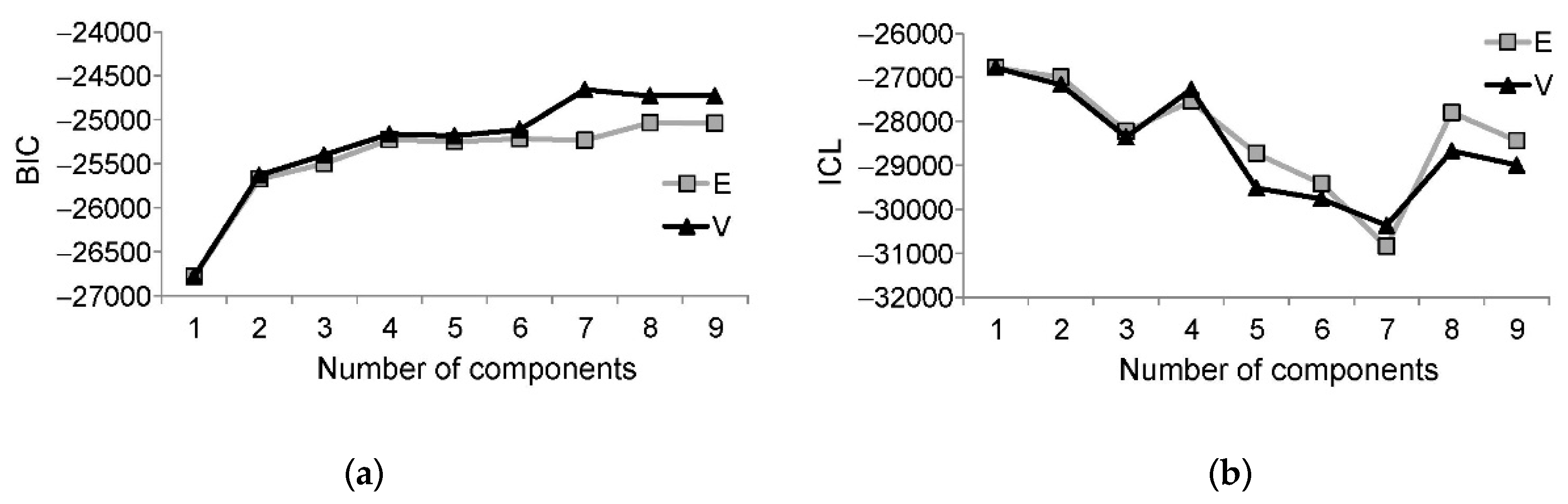
3.3. Model-Based Clustering
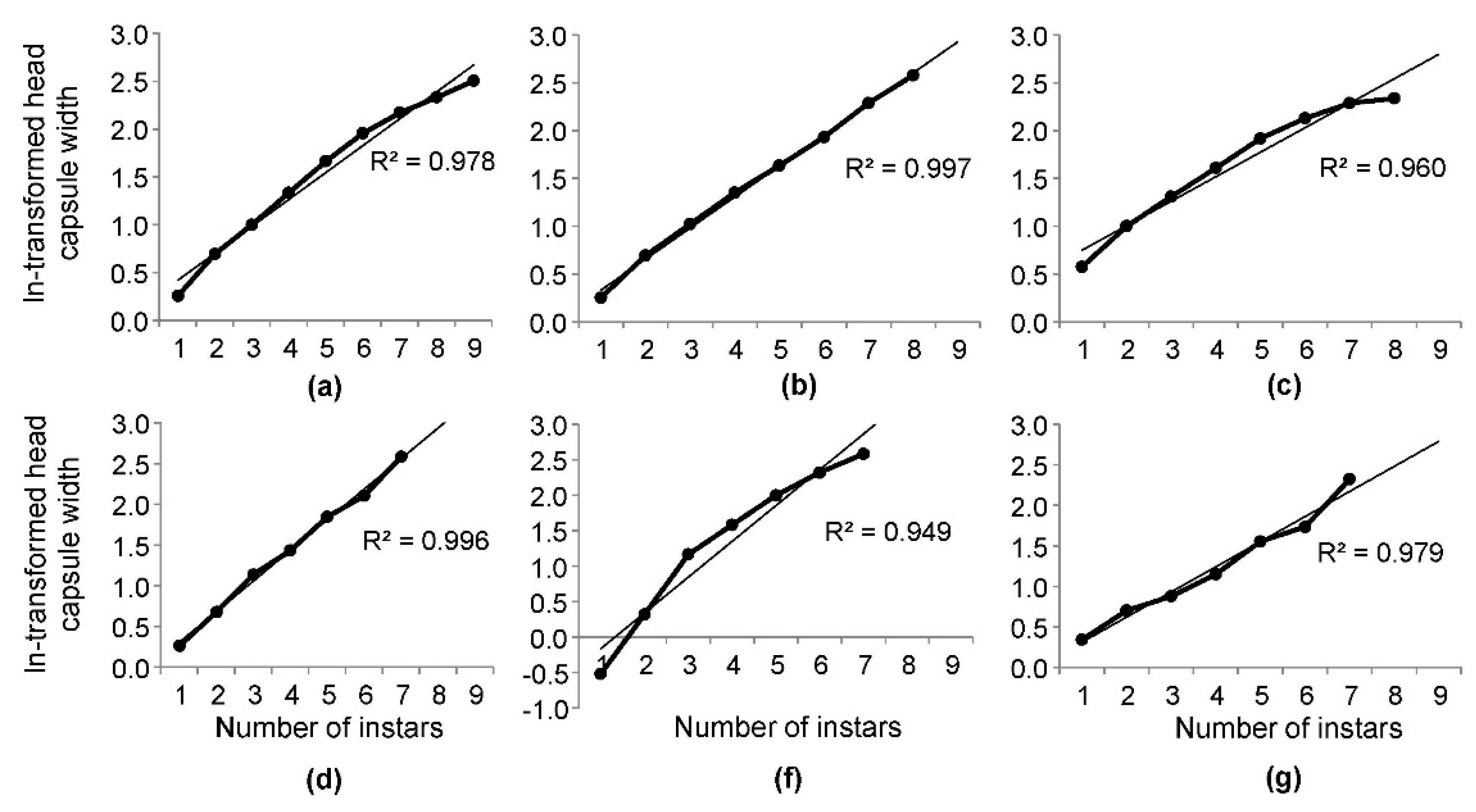
3.4. Adherence of the Mean Head Capsule Widths to Brooks-Dyar’s Rule
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- EPPO. PQR Database; European and Mediterranean Plant Protection Organization: Paris, France, 2014; Available online: http://www.eppo.int/DATABASES/pqr/pqr.htm (accessed on 17 October 2019).
- Jabłoński, T. Barczatka sosnówka—Dendrolimus pini L. In Krótkoterminowa Prognoza Występowania Ważniejszych Szkodników i Chorób Infekcyjnych Drzew Leśnych w Polsce w 2014 r; Analizy i Raporty 22; Instytut Badawczy Leśnictwa: Sękocin Stary, Poland, 2014; pp. 45–49. ISBN 978-83-62830-30-5. [Google Scholar]
- Ray, D.; Peace, A.; Moore, R.; Petr, M.; Grieve, Y.; Convery, C.; Ziesche, T. Improved prediction of the climate-driven outbreaks of Dendrolimus pini in Pinus sylvestris forests. Forestry 2016, 89, 230–244. [Google Scholar] [CrossRef]
- Björkman, C.; Lindelöw, Å.; Eklund, K.; Kyrk, S.; Klapwijk, M.J.; Fedderwitz, F.; Nordlander, G. A rare event—An isolated outbreak of the pine-tree lappet moth (Dendrolimus pini) in the Stockholm archipelago. Entomol. Tidskr. 2013, 134, 1–9. [Google Scholar]
- Altermatt, F. Climatic warming increases voltinism in European butterflies and moths. Proc. Biol. Sci. 2010, 277, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Ziter, C.; Robinson, E.A.; Newman, J.A. Climate change and voltinism in Californian insect pest species: Sensitivity to location, scenario and climate model choice. Glob. Chang. Biol. 2012, 18, 2771–2780. [Google Scholar] [CrossRef]
- Choi, W.I.; Park, Y.-K.; Park, Y.-S.; Ryoo, M.I.; Lee, H.-P. Changes in voltinism in a pine moth Dendrolimus spectabilis (Lepidoptera: Lasiocampidae) population: Implications of climate change. Appl. Entomol. Zool. 2011, 46, 319–325. [Google Scholar] [CrossRef]
- Billings, R.F. The pine caterpillar Dendrolimus punctatus in Viet Nam; recommendations for integrated pest management. For. Ecol. Manag. 1991, 39, 97–106. [Google Scholar] [CrossRef]
- Zhang, A.-B.; Wang, Z.-J.; Tan, S.-J.; Li, D.-M. Monitoring the masson pine moth, Dendrolimus punctatus (Walker) (Lepidoptera: Lasiocampidae) with synthetic sex pheromone-baited traps in Qianshan County, China. Appl. Entomol. Zool. 2003, 38, 177–186. [Google Scholar] [CrossRef]
- Kolk, A.; Starzyk, J.R. Barczatka sosnówka Dendrolimus pini L. (= Bombyx mori L.). In Atlas Szkodliwych Owadów Leśnych; Multico O.W.: Warszawa, Poland, 1996; p. 259. ISBN 83-7073-095-7. [Google Scholar]
- Śliwa, E. Barczatka Sosnówka (Dendrolimus pini L.); Dyrekcja Generalna Lasów Państwowych: Warszawa, Poland, 1992.
- Moore, R.; Cottrell, J.; A’Hara, S.; Ray, D. Pine-tree lappet moth (Dendrolimus pini) in Scotland: Discovery, timber movement controls and assessment of risk. Scott. For. 2017, 71, 34–43. [Google Scholar]
- Il’inskij, A.I.; Tropin, I.V. Nadzor, Uchet i Prognoz Massovyh Razmnozhenij Hvoe- i Listogryzushhih Nasekomyh v Lesah SSSR.; Lesnaja Promyshlennost’: Moskva, Russia, 1965. [Google Scholar]
- Pszczolkowski, M.A.; Smagghe, G. Effect of 20-hydroxyecdysone agonist, tebufenozide, on pre- and post-diapause larvae of Dendrolimus pini (L.) (Lep., Lasiocampidae). J. Appl. Entomol. 1999, 123, 151–157. [Google Scholar] [CrossRef]
- McClellan, Q.C.; Logan, J.A. Instar determination for the gypsy moth (Lepidoptera: Lymantriidae) based on the frequency distribution of head capsule widths. Environ. Entomol. 1994, 23, 248–253. [Google Scholar] [CrossRef]
- Chen, Y.; Seybold, S.J. Application of frequency distribution method for determining instars of the beet armyworm (Lepidoptera: Noctuidae) from widths of cast head capsules. J. Econ. Entomol. 2013, 106, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Jia, R.; Xun, H.; Yang, J.; Chen, Q.; Zeng, X.G.; Yang, M. Determining the number of instars in Simulium quinquestriatum (Diptera: Simuliidae) using k-means clustering via the Canberra distance. J. Med. Entomol. 2018, 55, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Appel, A.G.; Hu, X.P. Instar determination of Blaptica dubia (Blattodea: Blaberidae) using Gaussian mixture models. Ann. Entomol. Soc. Am. 2013, 106, 323–328. [Google Scholar] [CrossRef]
- Craig, D.A. The larvae of Tahitian Simuliidae (Diptera: Nematocera). J. Med. Entomol. 1975, 12, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.A.; Bentz, B.J.; Vandygriff, J.C.; Turner, D.L. General program for determining instar distributions from head capsule widths: Example analysis of mountain pine beetle (Coleoptera: Scolytidae) data. Environ. Entomol. 1998, 27, 555–563. [Google Scholar] [CrossRef]
- Chen, Y.-C. A tutorial on kernel density estimation and recent advances. Biostat. Epidemiol. 2017, 1, 161–187. [Google Scholar] [CrossRef]
- Silverman, B.W. Density Estimation; Chapman and Hall: London, UK; New York, NY, USA, 1986. [Google Scholar]
- Sheather, S.J.; Jones, M.C. A reliable data-based bandwidth selection method for kernel density estimation. J. R. Stat. Soc. B 1991, 53, 683–690. [Google Scholar] [CrossRef]
- Wand, M.P.; Jones, M.C. Kernel Smoothing, 1st ed.; Chapman and Hall/CRC: New York, NY, USA, 1994; 224p. [Google Scholar] [CrossRef]
- Fraley, C.; Raftery, A.E. Model-based methods of classification: Using the mclust sofware in chemometrics. J. Stat. Softw. 2007, 18, 1–13. [Google Scholar] [CrossRef]
- Fraley, C.; Raftery, A.E. How many clusters? Which clustering method? Answers via model-based cluster analysis. Comput. J. 1998, 41, 578–588. [Google Scholar] [CrossRef]
- Scrucca, L.; Fop, M.; Murphy, T.B.; Raftery, A.E. mclust 5: Clustering, classification and density estimation using Gaussian finite mixture models. R J. 2016, 8, 289–317. [Google Scholar] [CrossRef]
- Flaherty, L.; Régnière, J.; Sweeney, J. Number of instars and sexual dimorphism of Tetropium fuscum (Coleoptera: Cerambycidae) larvae determined by maximum likelihood. Can. Entomol. 2012, 144, 720–726. [Google Scholar] [CrossRef]
- Schmidt, F.H.; Campbell, R.K.; Trotter, S.J., Jr. Errors in determining instar numbers through head capsule measurements of a Lepidopteran—A laboratory study and critique. Ann. Entomol. Soc. Am. 1977, 70, 750–756. [Google Scholar] [CrossRef]
- Esperk, T.; Tammatu, T.; Nylin, S. Intraspecific variability in number of larval instars in insects. J. Econ. Entomol. 2007, 100, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Penz, C.; Bueno, R. Supernumerary larval molts are male-biased and lead to larger pupae size in Papilio hectorides (Papilionidae). J. Lepid. Soc. 2018, 72, 185–191. [Google Scholar] [CrossRef]
- Salgado-Ugarte, I.H.; Pérez-Hernández, M.A. Exploring the use of variable bandwidth kernel density estimators. Stata J. 2003, 3, 133–147. [Google Scholar] [CrossRef]
- Liao, J.G.; Wu, Y.; Lin, Y. Improving Sheater and Jones’ bandwidth selector for difficult densities in kernel density estimation. J. Nonparametr. Stat. 2010, 22, 105–114. [Google Scholar] [CrossRef]
- Cole, B.J. Growth ratios in holometabolous and hemimetabolous insects. Ann. Entomol. Soc. Am. 1980, 73, 489–491. [Google Scholar] [CrossRef]
| Instar | Head Capsule Width (mm) According to | ||
|---|---|---|---|
| Il’inskij and Tropin [13] | Śliwa [11] | Pszczolkowski and Smagghe [14] | |
| 1 | 1.2 | 0.7 | 1.27 |
| 2 | 1.6 | 1–1.5 | 1.63 |
| 3 | 2.2 | 2–2.5 | 1.84 |
| 4 | 2.7 | ~3 | 2.22 |
| 5 | 3.6 | ~4 | 2.94 |
| 6 | 4.3 | ~5 | 3.33 |
| 7 | 6.0 | 6 | 5.01 |
| Instar | Observed | Estimated | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Limits | Mean | SD | Limits | |
| Visual approach followed by NLLS estimation (nine instars) | ||||||
| 1 | 1.19 | 0.053 | <1.45 | 1.19 | 0.053 | <1.36 |
| 2 | 1.62 | 0.078 | 1.45–1.82 | 1.62 | 0.078 | 1.36–1.83 |
| 3 | 2.00 | 0.061 | 1.83–2.18 | 2.00 | 0.059 | 1.84–2.13 |
| 4 | 2.52 | 0.174 | 2.19–2.80 | 2.52 | 0.167 | 2.14–2.85 |
| 5 | 3.17 | 0.191 | 2.81–3.53 | 3.17 | 0.170 | 2.86–3.50 |
| 6 | 3.87 | 0.182 | 3.54–4.21 | 3.87 | 0.176 | 3.51–4.25 |
| 7 | 4.51 | 0.125 | 4.22–4.70 | 4.50 | 0.110 | 4.26–4.69 |
| 8 | 5.03 | 0.180 | 4.71–5.37 | 5.03 | 0.174 | 4.70–5.43 |
| 9 | 5.63 | 0.209 | >5.37 | 5.67 | 0.167 | >5.43 |
| KDE approach followed by NLLS estimation (eight instars) | ||||||
| 1 | 1.19 | 0.048 | <1.39 | 1.19 | 0.048 | <1.36 |
| 2 | 1.62 | 0.080 | 1.39–1.80 | 1.62 | 0.080 | 1.36–1.80 |
| 3 | 2.03 | 0.091 | 1.81–2.22 | 2.03 | 0.089 | 1.81–2.23 |
| 4 | 2.56 | 0.150 | 2.23–2.81 | 2.55 | 0.143 | 2.24–2.83 |
| 5 | 3.11 | 0.148 | 2.82–3.41 | 3.11 | 0.135 | 2.84–3.39 |
| 6 | 3.82 | 0.216 | 3.42–4.22 | 3.81 | 0.196 | 3.40–4.18 |
| 7 | 4.88 | 0.345 | 4.23–5.70 | 4.89 | 0.339 | 4.19–5.80 |
| 8 | 5.94 | 0.143 | >5.70 | 5.97 | 0.116 | >5.80 |
| Clustering approach (eight instars, limits are based on the classification of the observed values) | ||||||
| 1 | - | - | - | 1.49 | 0.255 | 0.96–1.84 |
| 2 | - | - | - | 2.00 | 0.052 | 1.90–2.10 |
| 3 | - | - | - | 2.48 | 0.183 | 2.19–2.71 |
| 4 | - | - | - | 3.05 | 0.212 | 2.80–3.33 |
| 5 | - | - | - | 3.78 | 0.226 | 3.40–4.11 |
| 6 | - | - | - | 4.37 | 0.321 | 4.20–4.50 |
| 7 | - | - | - | 4.88 | 0.312 | 4.55–5.08 |
| 8 | - | - | - | 5.05 | 0.417 | 5.10–6.48 |
| Instar | Visual Approach and NLLS (Nine Instars) | KDE Approach and NLLS (Eight Instars) | Clustering Approach (Eight Instars) | Il’inskij and Tropin [13] (Seven Instars) | Śliwa [11] (Seven Instars) | Pszczolkowski and Smagghe [14] (Seven Instars) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | C | g | C | g | C | g | C | g | C | g | C | |
| 1 | ||||||||||||
| 2 | 1.36 | 1.36 | 1.34 | 1.33 | 1.79 | 1.28 | ||||||
| 3 | 1.24 | −8.83 | 1.26 | −7.44 | 1.24 | −7.62 | 1.38 | 3.12 | 1.80 | 0.80 | 1.13 | −12.05 |
| 4 | 1.26 | 2.04 | 1.26 | −0.10 | 1.23 | −0.82 | 1.23 | −10.74 | 1.33 | −25.93 | 1.21 | 6.88 |
| 5 | 1.26 | −0.31 | 1.22 | −3.02 | 1.24 | 0.77 | 1.33 | 8.64 | 1.33 | 0.00 | 1.32 | 9.76 |
| 6 | 1.22 | −2.67 | 1.23 | 0.80 | 1.16 | −6.72 | 1.20 | −10.42 | 1.25 | −6.25 | 1.13 | −14.47 |
| 7 | 1.16 | −5.04 | 1.28 | 4.46 | 1.12 | −3.41 | 1.40 | 16.82 | 1.20 | −4.00 | 1.50 | 32.83 |
| 8 | 1.12 | −3.70 | 1.22 | −4.70 | 1.03 | −7.33 | - | - | - | - | - | - |
| 9 | 1.13 | 0.69 | - | - | - | - | - | - | - | - | - | - |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukovata, L. A Comparison of Three Approaches for Larval Instar Separation in Insects—A Case Study of Dendrolimus pini. Insects 2019, 10, 384. https://doi.org/10.3390/insects10110384
Sukovata L. A Comparison of Three Approaches for Larval Instar Separation in Insects—A Case Study of Dendrolimus pini. Insects. 2019; 10(11):384. https://doi.org/10.3390/insects10110384
Chicago/Turabian StyleSukovata, Lidia. 2019. "A Comparison of Three Approaches for Larval Instar Separation in Insects—A Case Study of Dendrolimus pini" Insects 10, no. 11: 384. https://doi.org/10.3390/insects10110384
APA StyleSukovata, L. (2019). A Comparison of Three Approaches for Larval Instar Separation in Insects—A Case Study of Dendrolimus pini. Insects, 10(11), 384. https://doi.org/10.3390/insects10110384





