Transcriptomic Analysis Reveals Insect Hormone Biosynthesis Pathway Involved in Desynchronized Development Phenomenon in Hybridized Sibling Species of Tea Geometrids (Ectropis grisescens and Ectropis obliqua)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Identification
2.3. Hybridization Experiment
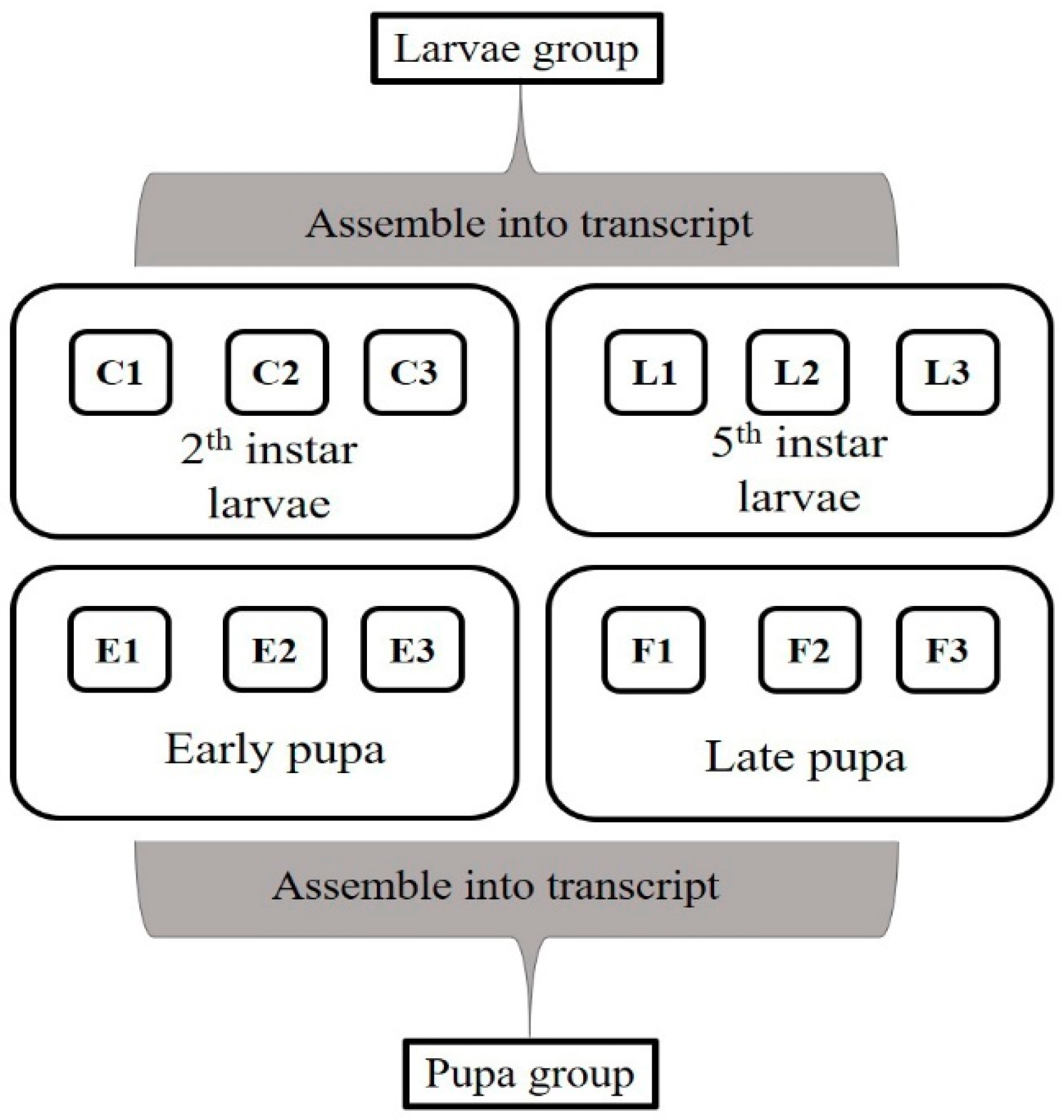
2.4. Sample Preparation and Collection for Transcriptomic Analysis
2.5. Total RNA Extraction, cDNA Library Construction and Illumina RNA-Seq
2.6. Transcriptome Assembly and Functional Annotation
2.7. Differentially Expressed Genes (DEGs) Analyzes and Gene Ontology (GO), KEGG Enrichment of DEGs
2.8. Analyzes of Genes Related to Developmental Patterns
2.9. Statistical Analysis
3. Results
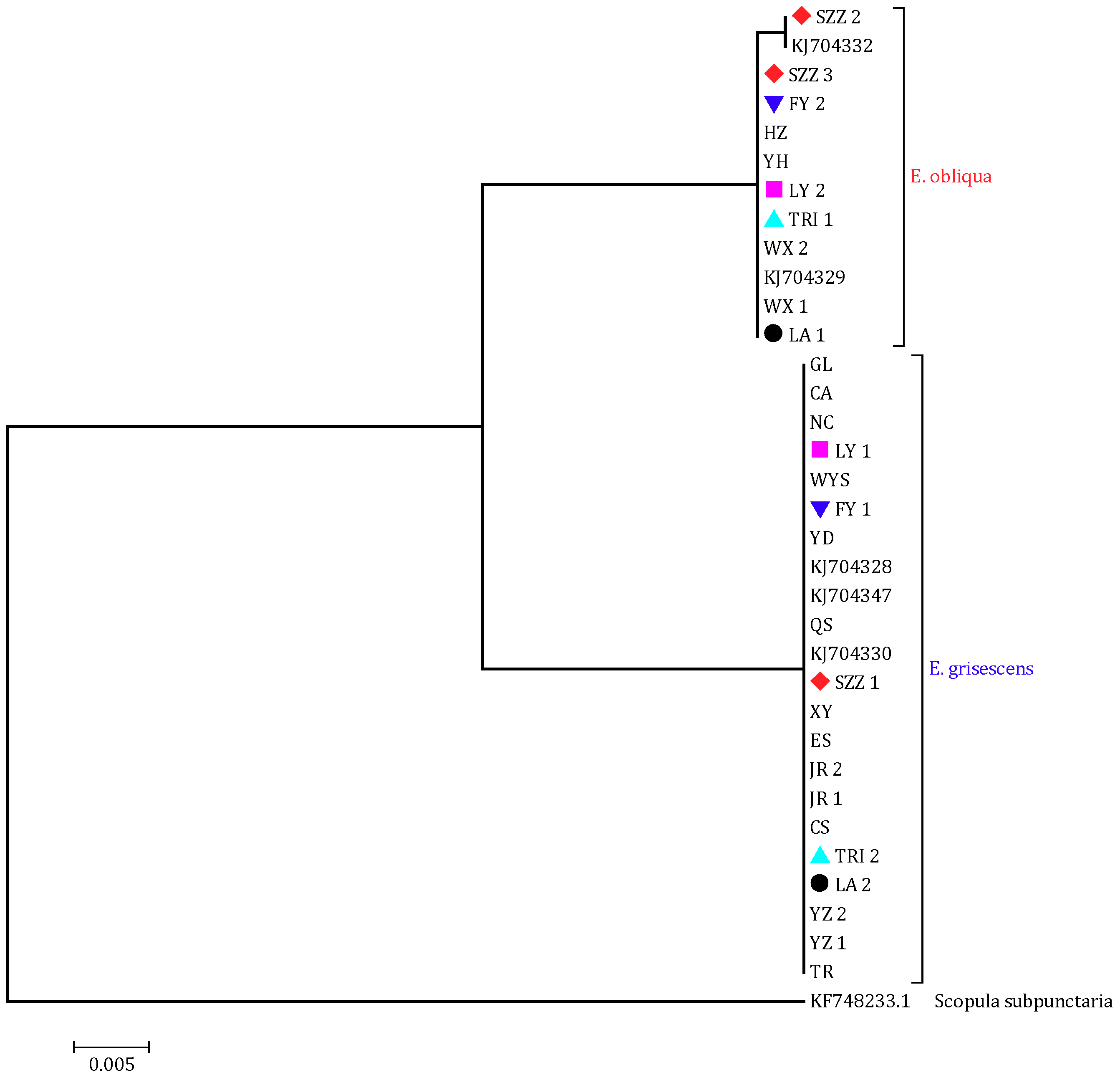
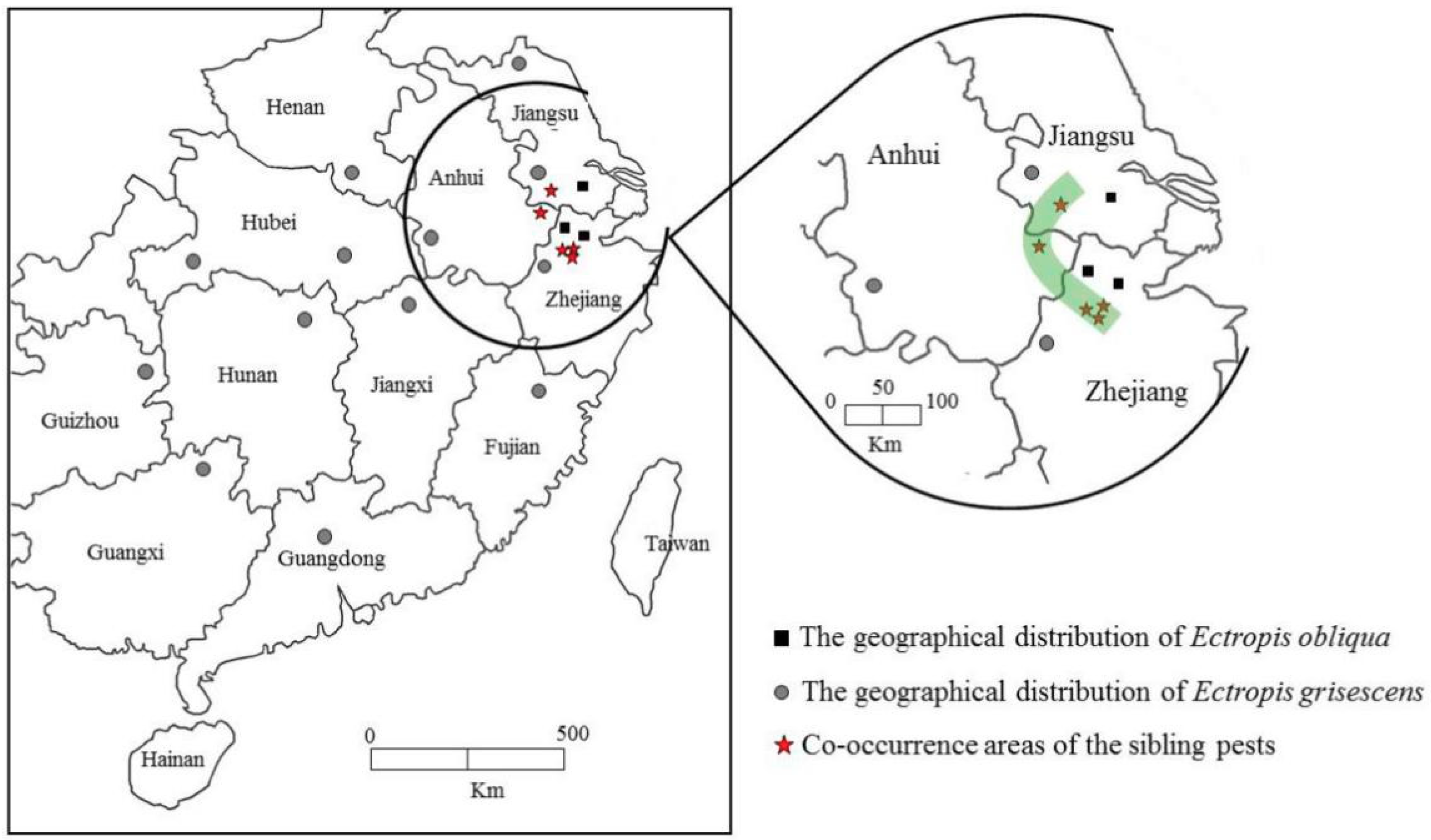
3.1. Distribution of the Two Closely Related Tea Geometrid Species
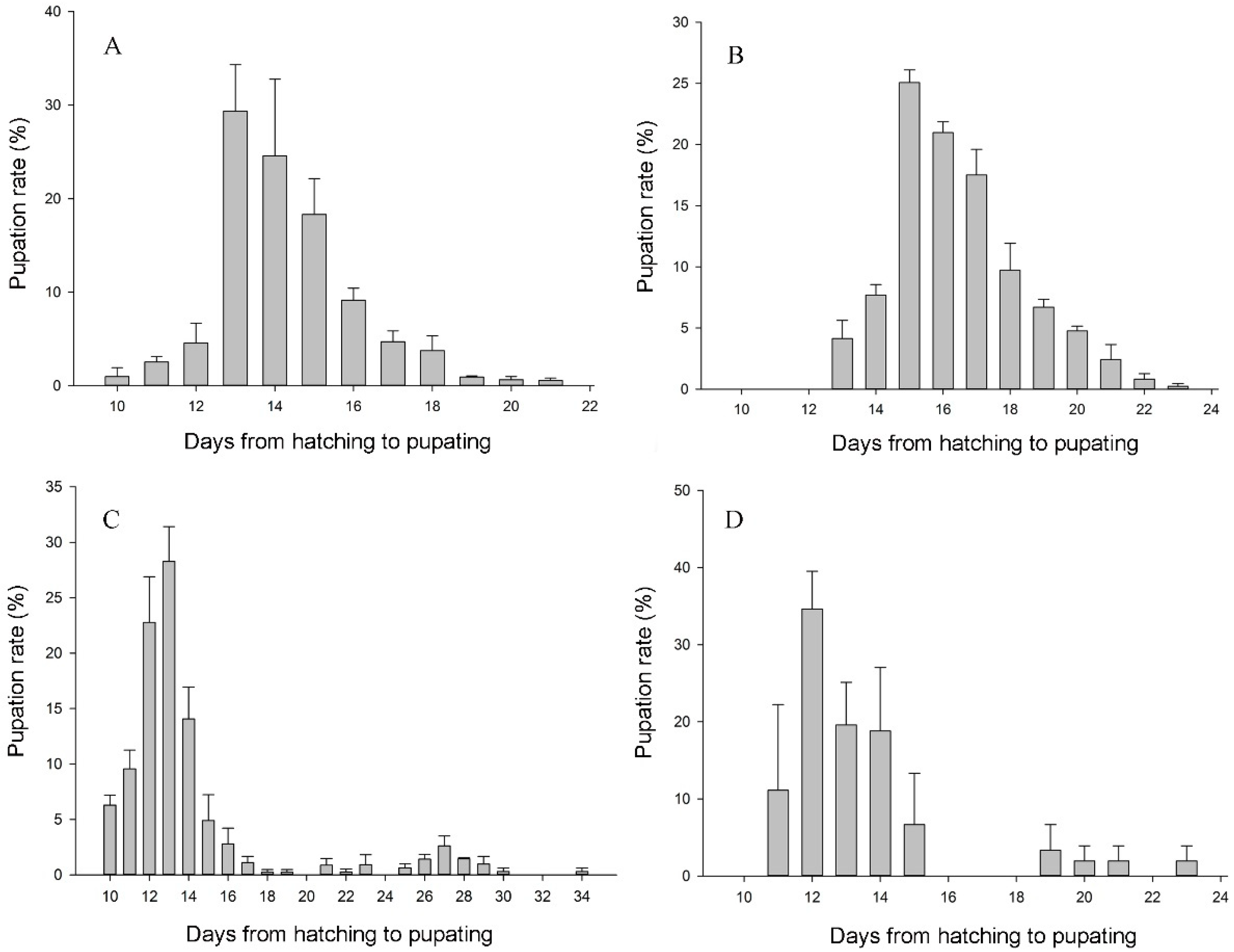
3.2. Development Situation of Filial Generation
3.3. Summary of RNA-Seq
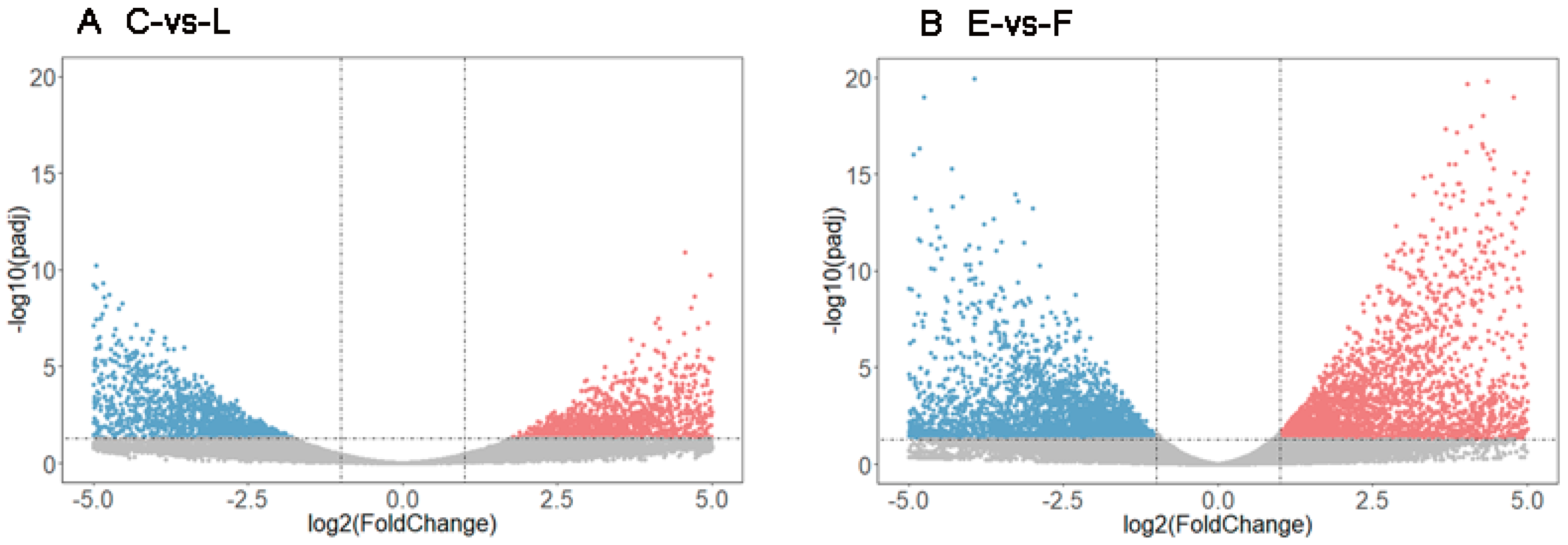
3.4. Analysis of DEGs
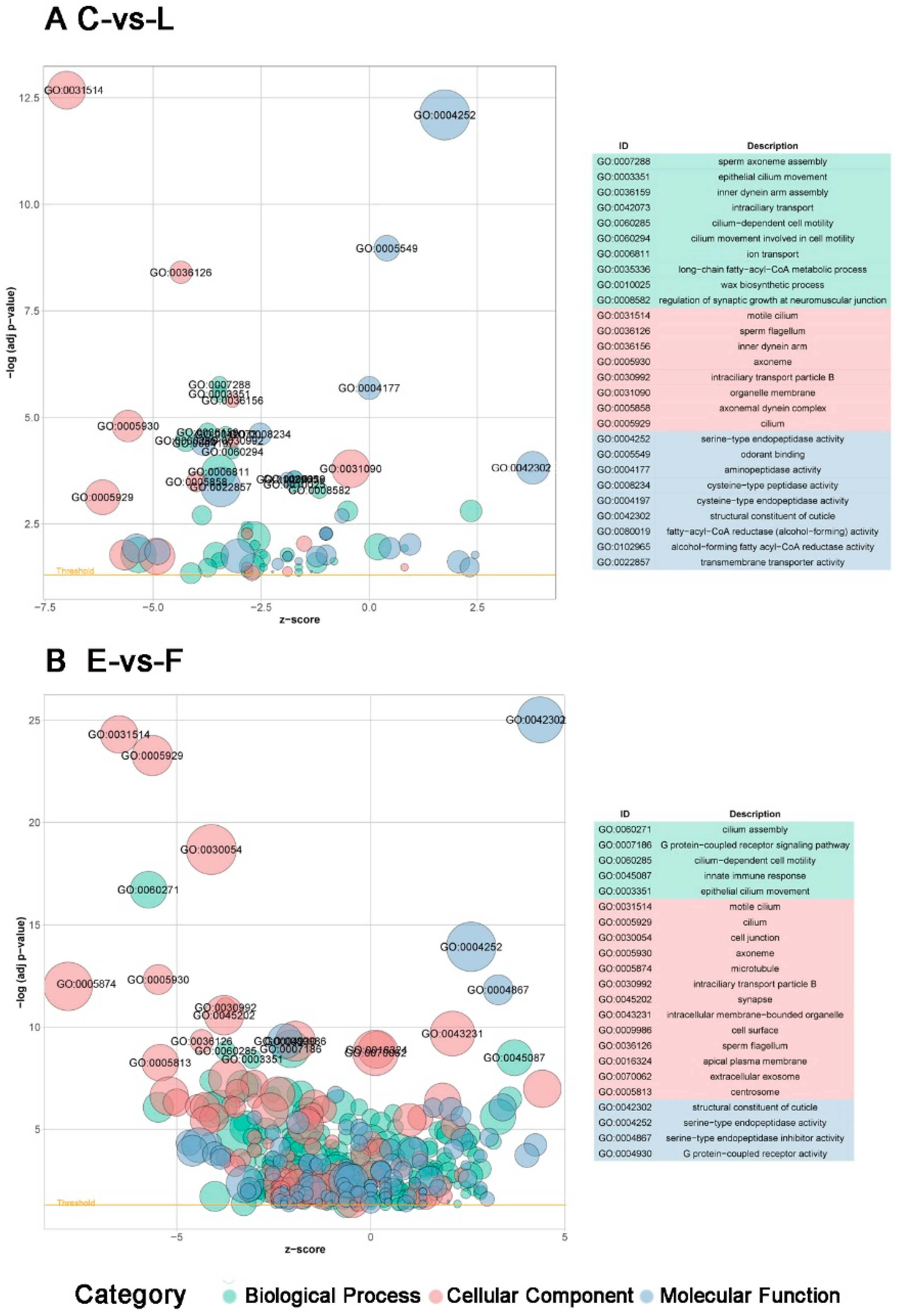
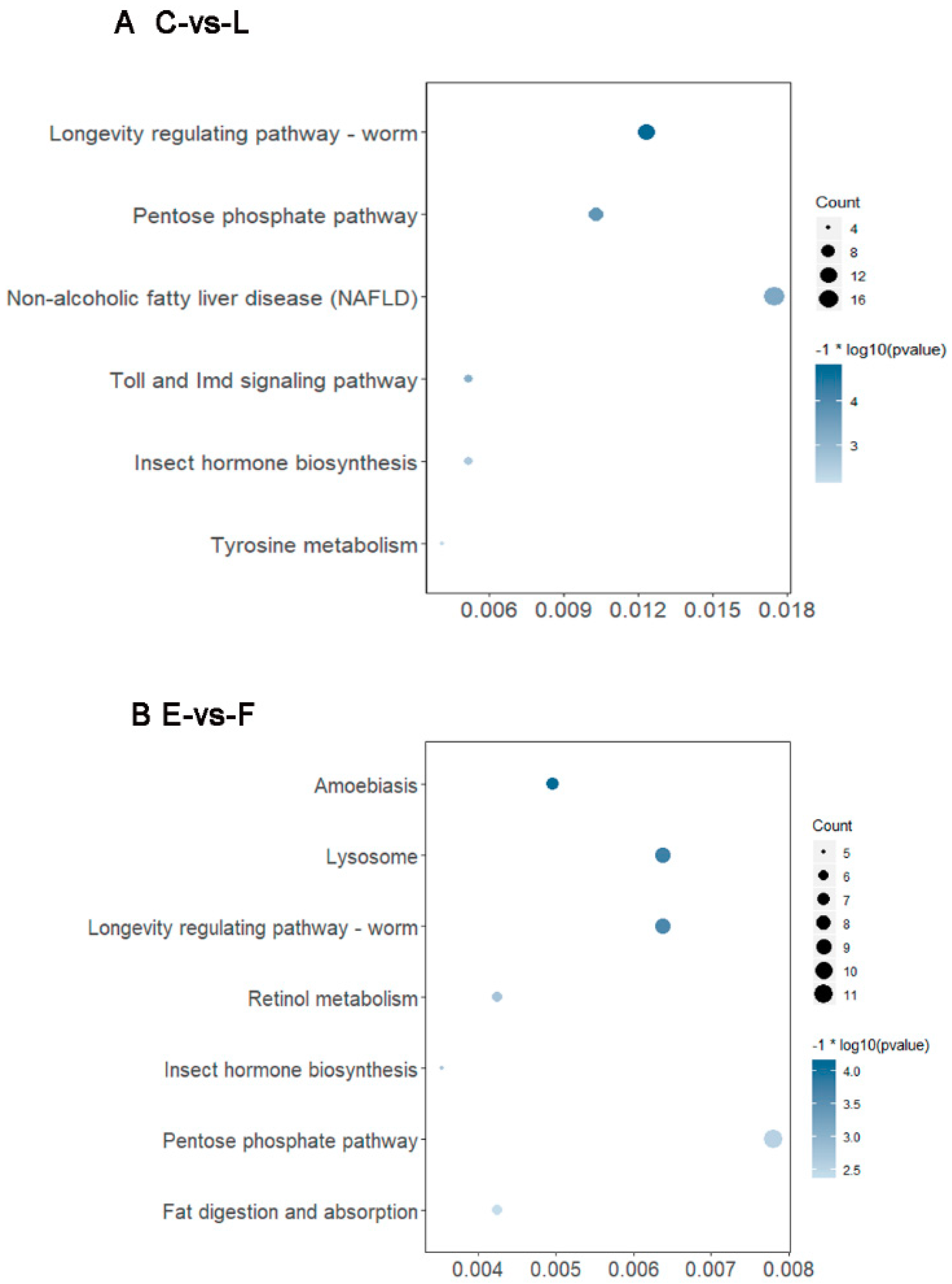
3.5. Functional Classification of DEGs during Development of Filial Generation
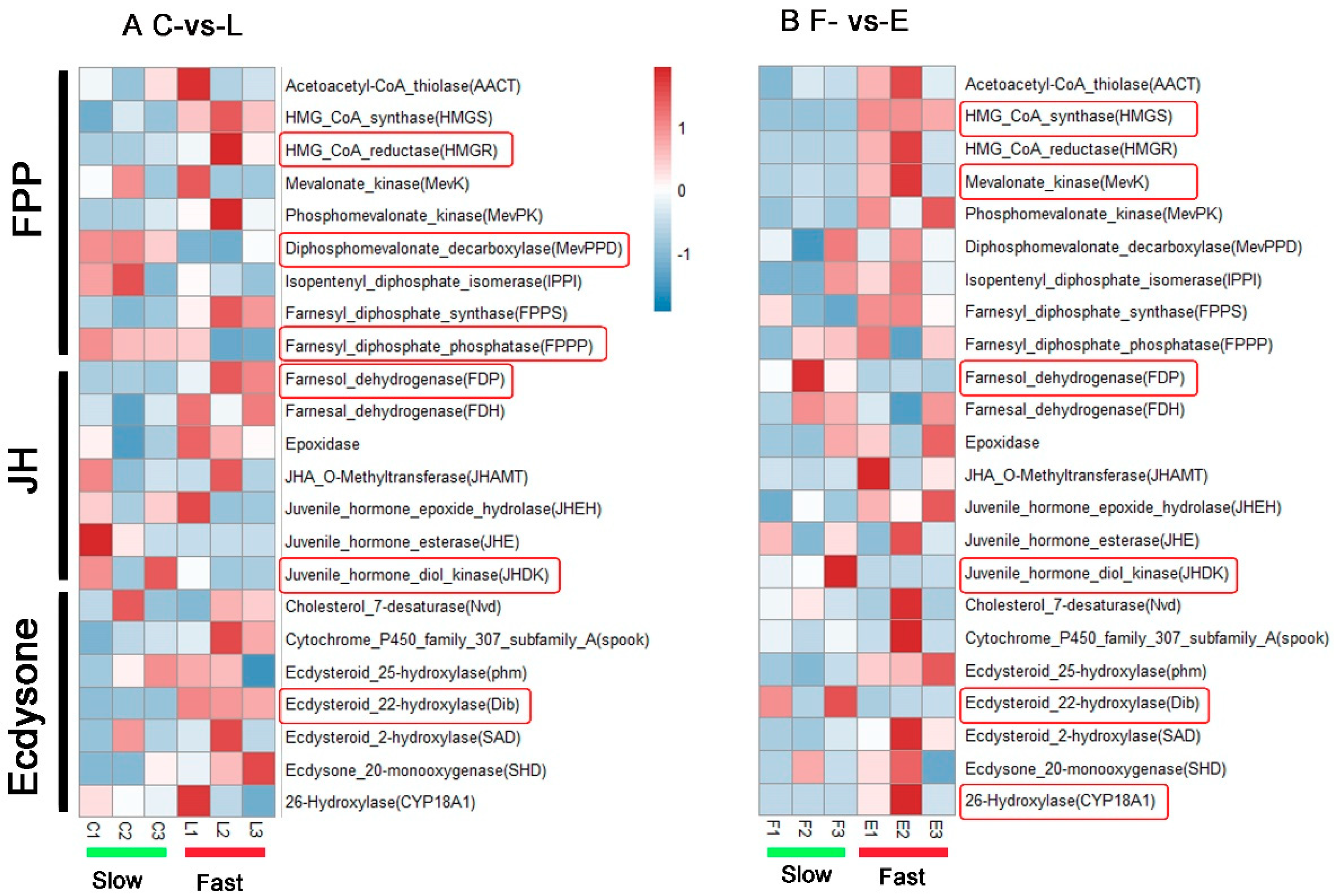
3.6. Identification of Developmental-Related DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.C.; Zhao, Q.Y.; Ma, C.L.; Zhang, Z.H.; Cao, H.L.; Kong, Y.M.; Yue, C.; Hao, X.Y.; Chen, L.; Ma, J.Q. Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genom. 2013, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Qian, W.J.; Li, N.N.; Hao, X.Y.; Wang, L.; Xiao, B.; Wang, X.C.; Yang, Y.J. Metabolic changes of caffeine in tea plant (Camellia sinensis (L.) O. Kuntze) as defense response to Colletotrichum fructicola. J. Agric. Food Chem. 2016, 64, 6685–6693. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Q. Domestic tea production and marketing situation report in 2017. China Tea Process 2018, 151, 67. [Google Scholar]
- Zhang, G.H.; Yuan, Z.J.; Zhang, C.X.; Yin, K.S.; Tang, M.J.; Guo, H.W.; Fu, J.Y.; Xiao, Q. Detecting deep divergence in seventeen populations of tea geometrid (Ectropis obliqua Prout) in China by COI mtDNA and cross-breeding. PLoS ONE 2014, 9, e99373. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Liu, S.X.; Xue, D.Y.; Tang, M.J.; Xiao, Q.; Han, H.X. External morphology and molecular identification of two tea Geometrid moth from southern China. Chin. J. Appl. Entomol. 2014, 51, 987–1002. [Google Scholar]
- Sun, L.; Mao, T.F.; Zhang, Y.X.; Wu, J.J.; Bai, J.H.; Zhang, Y.N.; Jiang, X.C.; Yin, K.S.; Guo, Y.Y.; Zhang, Y.J. Characterization of candidate odorant-binding proteins and chemosensory proteins in the tea geometrid Ectropis obliqua Prout (Lepidoptera: Geometridae). Arch. Insect Biochem. Physiol. 2017, 94, e21383. [Google Scholar] [CrossRef] [PubMed]
- Warren, W. New genera and species of Geometridae. Novit. Zool. 1894, 1, 366–466. [Google Scholar] [CrossRef]
- Prout, L.B. The Macrolepidoptera of the World; Alfred Kernen: Stuttgart, Germany, 1912; pp. 1–479. [Google Scholar]
- Wang, Z.; Ma, T.; Mao, T.; Guo, H.; Zhou, X.; Wen, X.; Xiao, Q. Application technology of the sex pheromone of the tea geometrid Ectropis grisescens (Lepidoptera: Geometridae). Int. J. Pest Manag. 2018, 64, 372–378. [Google Scholar] [CrossRef]
- Xi, Y.; Yin, K.S.; Xiao, Q. The susceptibility difference against EoNPV in different geographic populations of tea Geometrid (Ectropis obliqua Prout). J. Tea Sci. 2011, 31, 100–104. [Google Scholar]
- Xi, Y.; Yin, K.S.; Tang, M.J.; Xiao, Q. Geographic populations of the tea geometrid, Ectropis obliqua (Lepidoptera: Geometridae) in Zhejiang, eastern China have differentiated into different species. Acta Entomol. Sin. 2014, 57, 1117–1122. [Google Scholar]
- Ma, T.; Xiao, Q.; Yu, Y.G.; Wang, C.; Zhu, C.Q.; Sun, Z.H.; Chen, X.Y.; Wen, X.J. Analysis of tea Geometrid (Ectropis grisescens) pheromone gland extracts using GC-EAD and GC×GC/TOFMS. J. Agric. Food Chem. 2016, 64, 3161–3166. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.X.; Li, Z.Q.; Cai, X.M.; Bian, L.; Chen, Z.M. Evidence of premating isolation between two sibling moths: Ectropis grisescens and Ectropis obliqua (Lepidoptera: Geometridae). J. Econ. Entomol. 2017, 110, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Yuan, Z.J.; Yin, K.S.; Fu, J.Y.; Tang, M.J.; Xiao, Q. Asymmetrical reproductive interference between two sibling species of tea looper: Ectropis grisescens and Ectropis obliqua. Bull. Entomol. Res. 2016, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.; Arnqvist, G. Sexually antagonistic coevolution in a mating system: Combining experimental and comparative approaches to address evolutionary processes. Evolution 2002, 56, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Chapman, T.; Arnqvist, G.; Bangham, J.; Rowe, L. Sexual conflict. Trends Ecol. Evol. 2003, 18, 41–47. [Google Scholar] [CrossRef]
- Xie, P. The Aufhebung and Breakthrough of the Theories on the Origin and Evolution of Life—Life in Philosophy and Philosophy in Life Sciences, 1st ed.; Science Press: Beijing, China, 2014; pp. 1–383. [Google Scholar]
- Darwin, C. On the Origin of Species by Means of Natural Selection or the Preservation of Favoured Races in the Struggle for Life; John Murry: London, UK, 1859. [Google Scholar]
- Reitz, S.R.; Trumble, J.T. Competitive displacement among insects and arachnids. Annu. Rev. Entomol. 2001, 47, 435–465. [Google Scholar] [CrossRef]
- Gao, Y.; Reitz, S.R. Emerging themes in our understanding of species displacements. Annu. Rev. Entomol. 2017, 62, 165–183. [Google Scholar] [CrossRef]
- Chu, D.; Liu, G.X.; Tao, Y.L.; Zhang, Y.J. Diversity of endosymbionts in Bemisia tabaci (Gennadius) complex and their biological implications. Acta Entomol. Sin. 2006, 49, 687–694. [Google Scholar]
- Liu, S.S.; Barro, P.J.D.; Xu, J.; Luan, J.B.; Zang, L.S.; Ruan, Y.M.; Wan, F.H. Asymmetric mating interactions drive wdespread invasion and displacement in a whitefly. Science 2007, 318, 1769–1772. [Google Scholar] [CrossRef]
- Mayr, E. Principles of Systematic Zoology; McGraw-Hill: New York, NY, USA, 1969. [Google Scholar]
- Wang, C.Z. Interpretation of the biological species concept from interspecific hybridization of two Helicoverpa, species. Sci. Rep. 2007, 52, 284–286. [Google Scholar] [CrossRef]
- Xu, G.; Guo, Y.Y. Research progresses on interspecific hybridization of Heliothinae. Entomol. Knowl. 2001, 38, 8–11. [Google Scholar]
- Dobzhansky, T. Genetics of the evolutionary process. J. Med. Genet. 1970, 8, 545. [Google Scholar]
- Houtkooper, R.H.; Mouchiroud, L.; Ryu, D.; Moullan, N.; Katsyuba, E.; Knott, G.; Williams, R.W.; Auwerx, J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 2013, 497, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Antebi, A. Ageing: When less is more. Nature 2007, 447, 536–537. [Google Scholar] [CrossRef] [PubMed]
- Peiró, C.; Romacho, T.; Azcutia, V.; Villalobos, L.; Fernández, E.; Bolaños, J.P.; Moncada, S.; Sánchez-Ferrer, C.F. Inflammation, glucose, and vascular cell damage: The role of the pentose phosphate pathway. Cardiovasc. Diabetol. 2016, 15, 82. [Google Scholar] [CrossRef] [PubMed]
- Horecker, B.L. The pentose phosphate pathway. J. Biol. Chem. 2002, 277, 47965–47971. [Google Scholar] [CrossRef]
- Niwa, Y.S.; Niwa, R. Transcriptional regulation of insect steroid hormone biosynthesis and its role in controlling timing of molting and metamorphosis. Dev. Growth Differ. 2016, 58, 94–105. [Google Scholar] [CrossRef]
- Venkataraman, V.; O’Mahony, P.J.; Manzcak, M.; Jones, G. Regulation of juvenile hormone esterase gene transcription by juvenile hormone. Genesis 2010, 15, 391–400. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Jang, H.J.; Yoon, S.H.; Kim, J.Y.; Lee, S.G.; Choi, E.S.; Kim, S.W. Engineered heterologous FPP synthases-mediated Z, E-FPP synthesis in E. coli. Metab. Eng. 2013, 18, 53–59. [Google Scholar] [CrossRef]
- Maxwell, R.A.; Welch, W.H.; Schooley, D.A. Juvenile hormone diol kinase. I. Purification, characterization, and substrate specificity of juvenile hormone-selective diol kinase from Manduca sexta. J. Biol. Chem. 2002, 277, 21874–21881. [Google Scholar] [CrossRef]
- Warren, J.T.; Petryk, A.; Marqués, G.; Jarcho, M. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2002, 99, 11043–11048. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.T.; Petryk, A.; Guillermo, M. Phantom encodes the 25-hydroxylase of Drosophila melanogaster and Bombyx mori: A P450 enzyme critical in ecdysone biosynthesis. Insect Biochem. Mol. Biol. 2004, 34, 991–1010. [Google Scholar] [CrossRef] [PubMed]
| Sample | Raw Reads | Clean Reads | Valid (%) | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| C1 | 52,103,142 | 50,962,816 | 97.81 | 97.88 | 94.11 | 51.64 |
| C2 | 50,825,206 | 49,705,732 | 97.80 | 97.56 | 93.46 | 52.31 |
| C3 | 44,598,296 | 43,010,684 | 96.44 | 97.48 | 93.45 | 55.64 |
| L1 | 46,276,016 | 45,479,152 | 98.28 | 97.91 | 94.02 | 51.80 |
| L2 | 54,087,972 | 52,918,552 | 97.84 | 97.21 | 92.42 | 51.85 |
| L3 | 55,174,098 | 53,559,112 | 97.07 | 97.60 | 93.32 | 54.47 |
| E1 | 59,386,648 | 58,378,284 | 98.30 | 97.21 | 92.49 | 46.61 |
| E2 | 56,732,666 | 55,961,382 | 98.64 | 97.24 | 92.57 | 47.98 |
| E3 | 74,080,310 | 72,958,276 | 98.49 | 97.35 | 92.77 | 47.70 |
| F1 | 63,019,000 | 61,842,676 | 98.13 | 97.24 | 92.57 | 47.51 |
| F2 | 63,144,758 | 62,126,664 | 98.39 | 97.15 | 92.34 | 46.71 |
| F3 | 61,542,756 | 60,005,800 | 97.50 | 97.34 | 92.81 | 46.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Bai, J.; Liu, Y.; Li, H.; Zhan, S.; Xiao, Q. Transcriptomic Analysis Reveals Insect Hormone Biosynthesis Pathway Involved in Desynchronized Development Phenomenon in Hybridized Sibling Species of Tea Geometrids (Ectropis grisescens and Ectropis obliqua). Insects 2019, 10, 381. https://doi.org/10.3390/insects10110381
Wang Z, Bai J, Liu Y, Li H, Zhan S, Xiao Q. Transcriptomic Analysis Reveals Insect Hormone Biosynthesis Pathway Involved in Desynchronized Development Phenomenon in Hybridized Sibling Species of Tea Geometrids (Ectropis grisescens and Ectropis obliqua). Insects. 2019; 10(11):381. https://doi.org/10.3390/insects10110381
Chicago/Turabian StyleWang, Zhibo, Jiahe Bai, Yongjian Liu, Hong Li, Shuai Zhan, and Qiang Xiao. 2019. "Transcriptomic Analysis Reveals Insect Hormone Biosynthesis Pathway Involved in Desynchronized Development Phenomenon in Hybridized Sibling Species of Tea Geometrids (Ectropis grisescens and Ectropis obliqua)" Insects 10, no. 11: 381. https://doi.org/10.3390/insects10110381
APA StyleWang, Z., Bai, J., Liu, Y., Li, H., Zhan, S., & Xiao, Q. (2019). Transcriptomic Analysis Reveals Insect Hormone Biosynthesis Pathway Involved in Desynchronized Development Phenomenon in Hybridized Sibling Species of Tea Geometrids (Ectropis grisescens and Ectropis obliqua). Insects, 10(11), 381. https://doi.org/10.3390/insects10110381





