A Clip Domain Serine Protease Involved in Egg Production in Nilaparvata lugens: Expression Patterns and RNA Interference
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
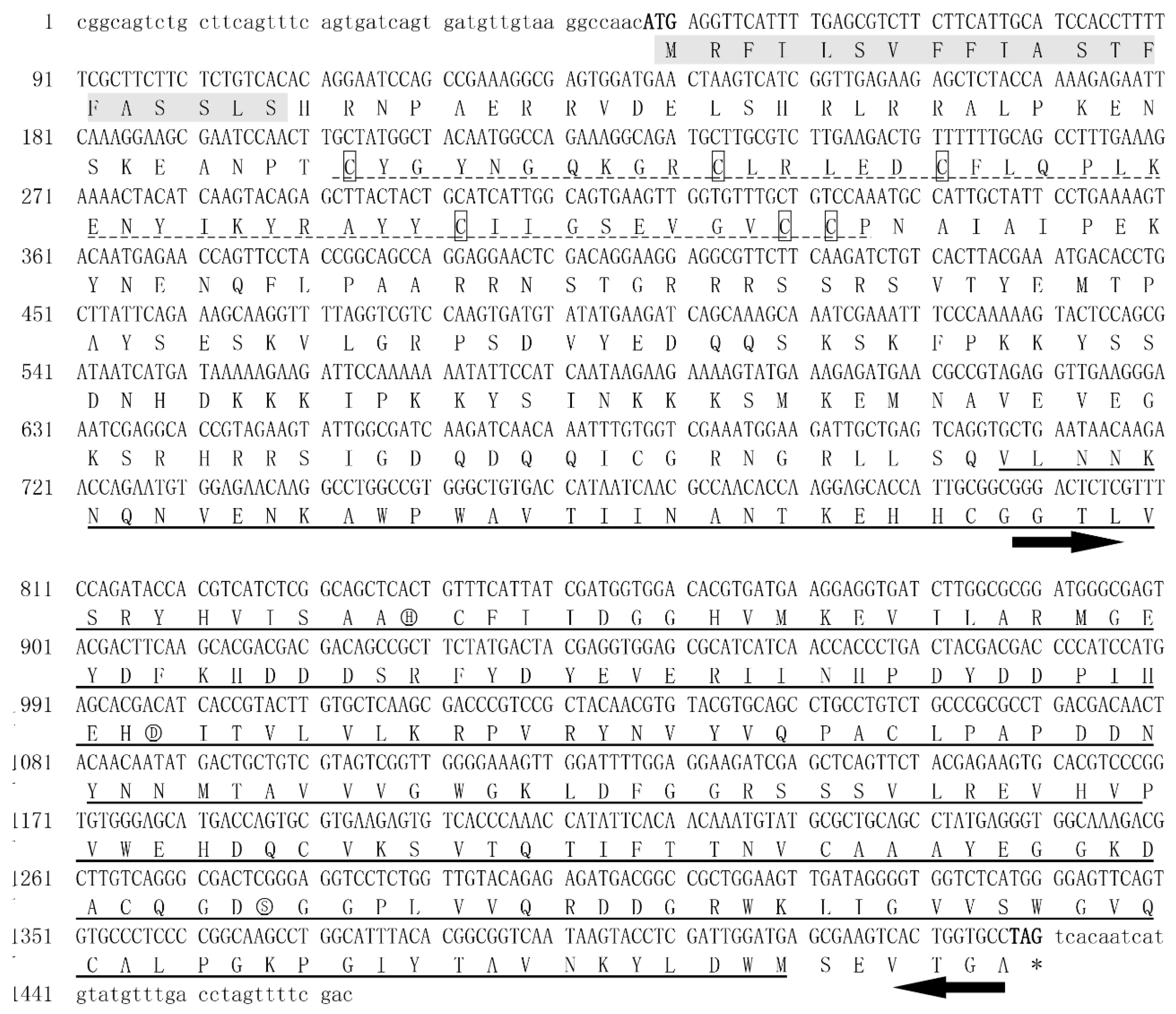
2.2. Cloning the cDNA of NlPCE3
2.3. Sequence Comparison and Phylogenetic Analysis
2.4. Real-time Quantitative PCR Analysis
2.5. RNA Interference
2.6. Dissection Observation and Fertility Analysis
2.7. Immunofluorescence
3. Results
3.1. Identification and Phylogenetic Analysis of NlPCE3
3.2. Developmental and Tissue-specific Expression of NlPCE3
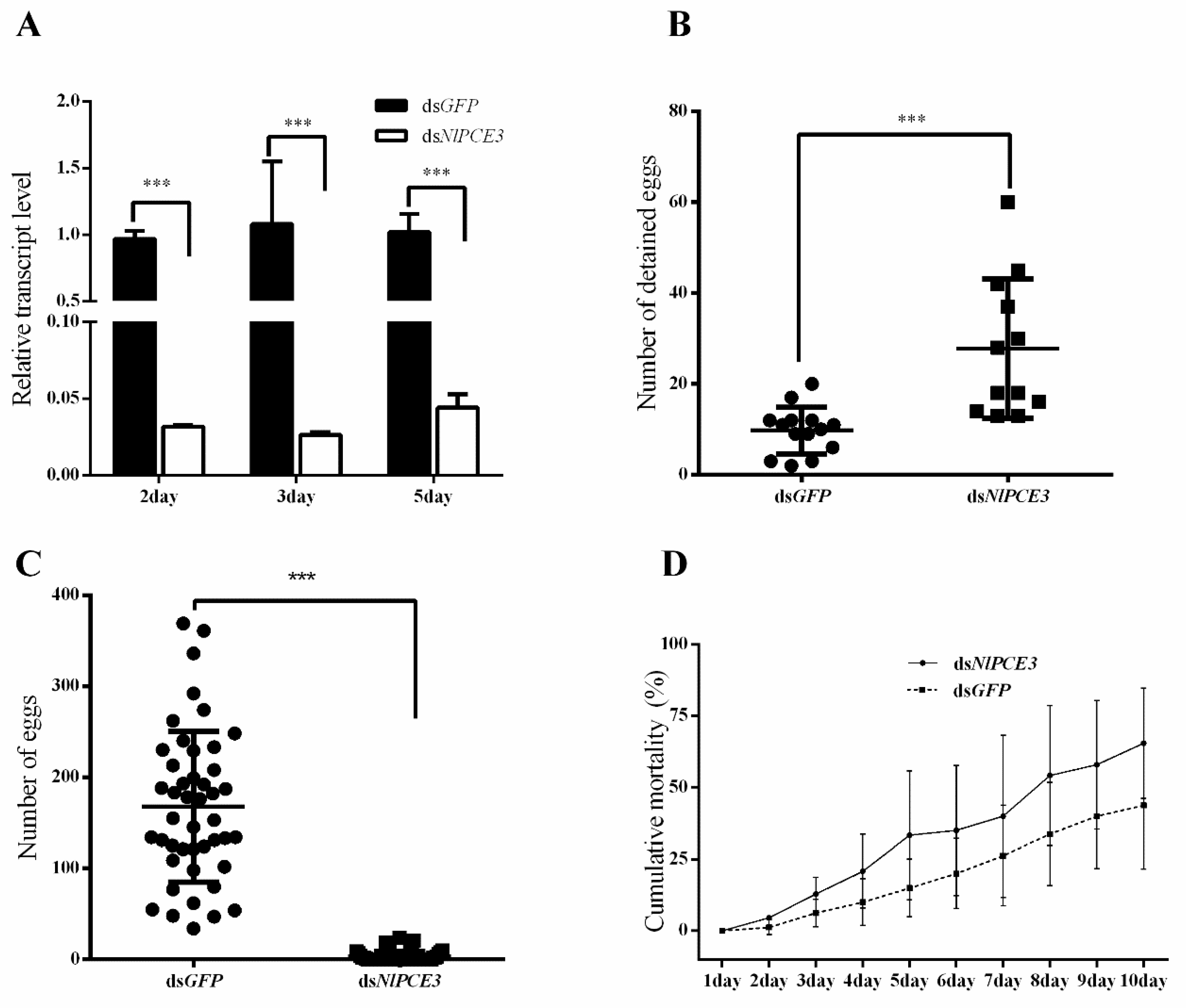
3.3. Effects of RNA Interference
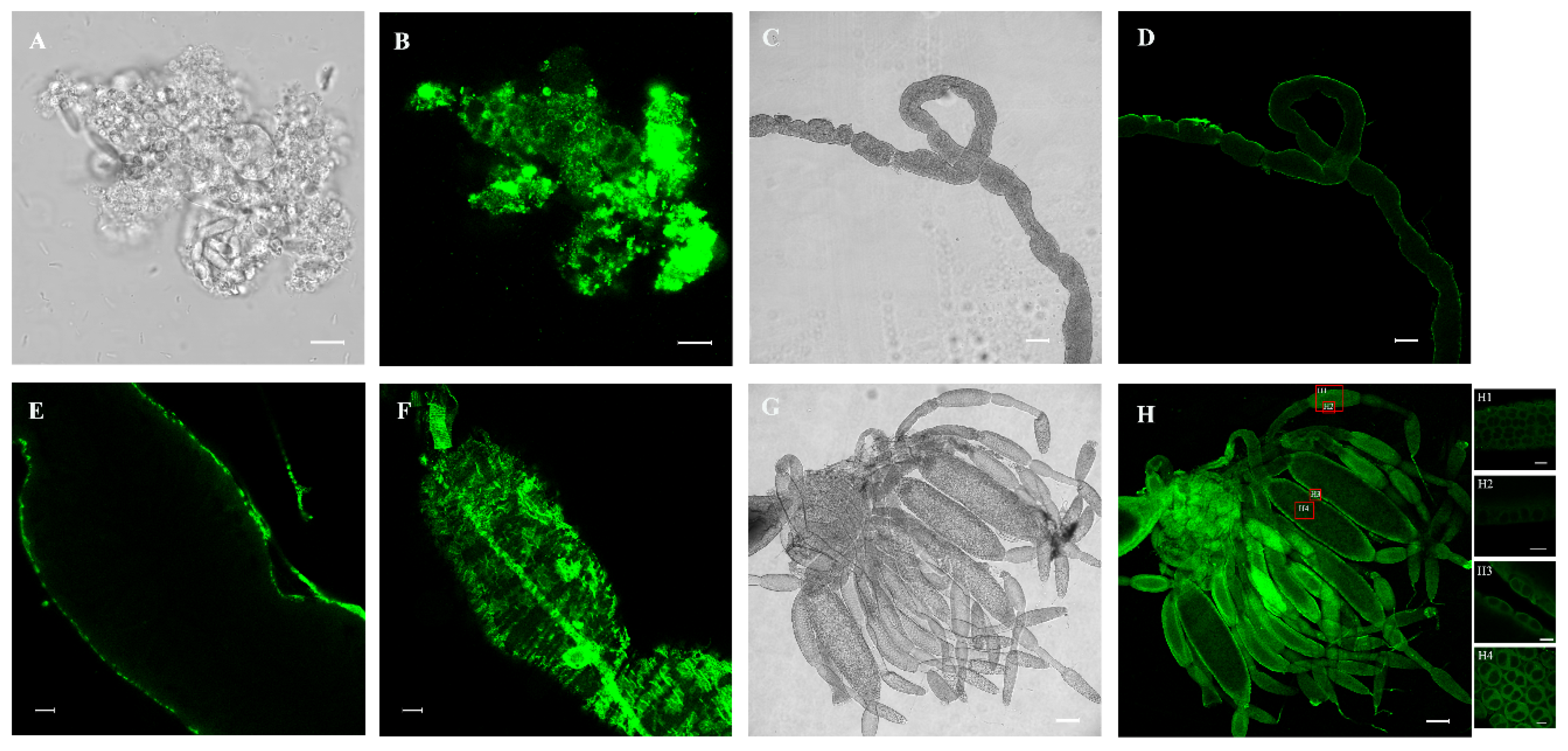
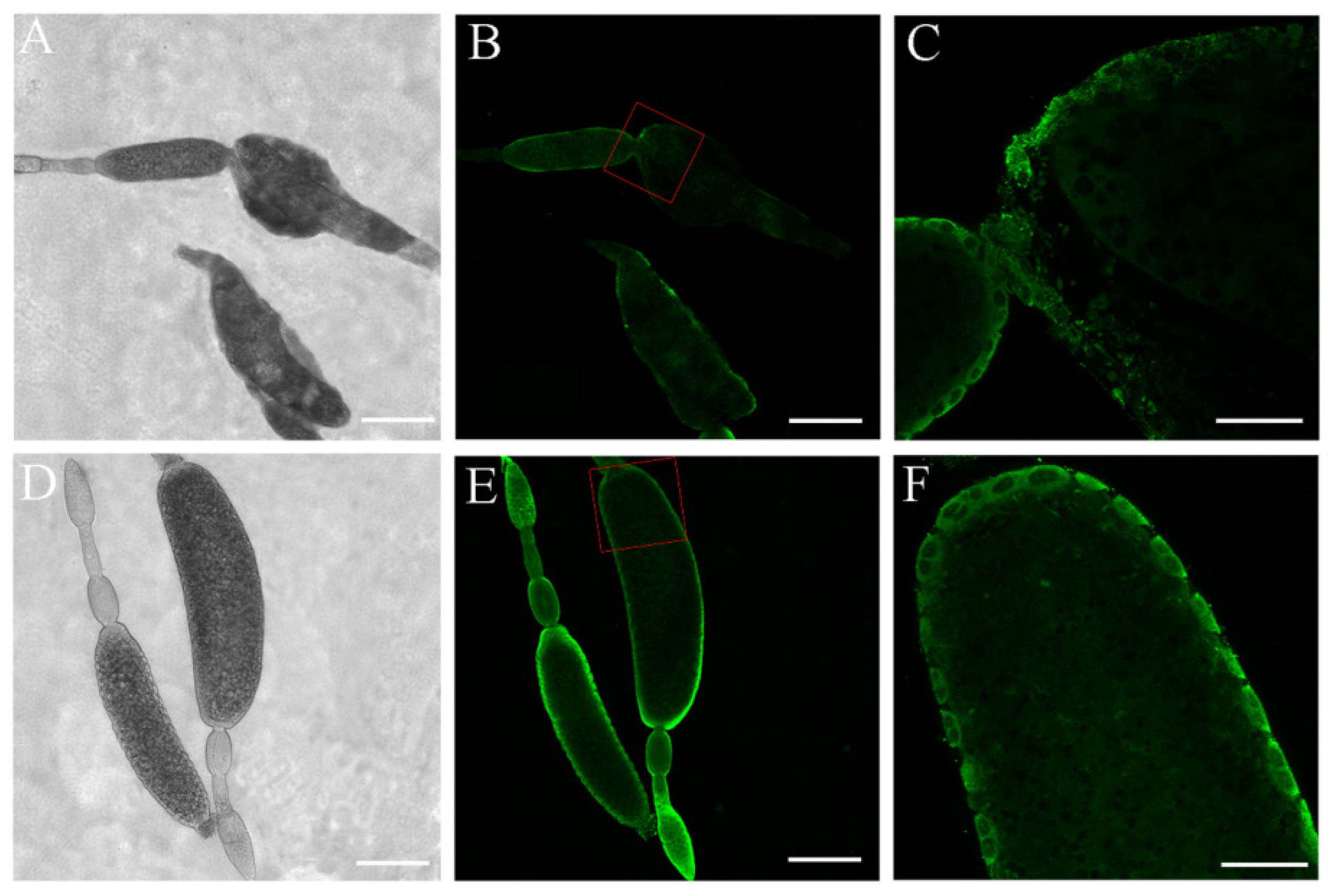
3.4. Immunofluorescence Analysis of NlPCE3 Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jena, K.K.; Kim, S.M. Current status of brown planthopper (BPH) resistance and genetics. Rice 2010, 3, 161–171. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, X.; Ali, E.; Liao, X.; Jin, R.; Ren, Z.; Wan, H.; Li, J. Characterization of nitenpyram resistance in Nilaparvata lugens (Stål). Pestic. Biochem. Physiol. 2019, 157, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Shu, Y.; Zhou, J.; Zhang, X.; Chen, M.; Yao, Q.; Zhou, Q.; Zhang, W. Molecular characterization and RNA interference analysis of vitellogenin receptor from Nilaparvata lugens (Stål). J. Insect Physiol. 2015, 73, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.X.; Huang, H.J.; Yu, B.; Lou, Y.H.; Fan, H.W.; Zhang, C.X. Bicaudal-C plays a vital role in oogenesis in Nilaparvata lugens (Hemiptera: Delphacidae). J. Insect Physiol. 2015, 79, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.Y.; Qu, L.Y.; Zhao, D.; Chen, L.B.; Jin, H.Y.; Xu, L.M.; Cheng, J.A.; Zhang, C.X. The genome- and transcriptome-wide analysis of innate immunity in the brown planthopper, Nilaparvata lugens. BMC Genomics 2013, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wan, W.; Kong, T.; Zhang, M.; Aweya, J.J.; Gong, Y.; Li, S. A clip domain serine protease regulates the expression of proPO and hemolymph clotting in mud crab, Scylla paramamosain. Fish Shellfish Immunol. 2018, 79, 52–64. [Google Scholar] [CrossRef]
- Jiang, H.; Kanost, M.R. The clip-domain family of serine proteinases in arthropods. Insect Biochem. Mol. Biol. 2000, 30, 95–105. [Google Scholar] [CrossRef]
- Muta, T.; Hashimoto, R.; Miyata, T.; Nishimura, H.; Toh, Y.; Iwanaga, S. Proclotting enzyme from horseshoe crab hemocytes: cDNA cloning, disulfide locations, and subcellular localization. J. Biol. Chem. 1990, 265, 22426–22433. [Google Scholar]
- Iwanaka, S. Biochemical principle of Limulus test for detecting bacterial endotoxins. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 83, 110–119. [Google Scholar] [CrossRef]
- Zhu, L.; Song, L.; Zhao, J.; Xu, W.; Chang, Y. Molecular cloning, characterization and expression of a serine protease with clip-domain homologue from scallop Chlamys farreri. Fish Shellfish Immunol. 2007, 22, 556–566. [Google Scholar] [CrossRef]
- Amparyup, P.; Wiriyaukaradecha, K.; Charoensapsri, W.; Tassanakajon, A. A clip domain serine proteinase plays a role in antibacterial defense but is not required for prophenoloxidase activation in shrimp. Dev. Comp. Immunol. 2010, 34, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Y.; Kanost, M.R. Pro-phenol oxidase activating proteinase from an insect, Manduca sexta: A bacteria-inducible protein similar to Drosophila easter. Proc. Natl. Acad. Sci. USA 1998, 95, 12220–12225. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Y.; Yu, X.Q.; Zhu, Y.; Kanost, M. Prophenoloxidase-activating proteinase-3 (PAP-3) from Manduca sexta hemolymph: A clip-domain serine proteinase regulated by serpin-1J and serine proteinase homologs. Insect Biochem. Mol. Biol. 2003, 33, 1049–1060. [Google Scholar] [CrossRef]
- Gupta, S.; Wang, Y.; Jiang, H. Manduca sexta prophenoloxidase (proPO) activation requires proPO-activating proteinase (PAP) and serine proteinase homologs (SPHs) simultaneously. Insect Biochem. Mol. Biol. 2005, 35, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Shin, S.W.; Alvarez, K.S.; Kokoza, V.; Raikhel, A.S. Distinct melanization pathways in the mosquito Aedes aegypti. Immunity 2010, 32, 41–53. [Google Scholar] [CrossRef]
- Barillas-Mury, C. CLIP proteases and plasmodium melanization in Anopheles gambiae. Trends Parasitol. 2007, 23, 297–299. [Google Scholar] [CrossRef]
- Volz, J.; Osta, M.A.; Kafatos, F.C.; Muller, H.M. The roles of two clip domain serine proteases in innate immune responses of the malaria vector Anopheles gambiae. J. Biol. Chem. 2005, 280, 40161–40168. [Google Scholar] [CrossRef]
- Kan, H.; Kim, C.H.; Kwon, H.M.; Park, J.W.; Roh, K.B.; Lee, H.; Park, B.J.; Zhang, R.; Zhang, J.; Soderhall, K.; et al. Molecular control of phenoloxidase-induced melanin synthesis in an insect. J. Biol. Chem. 2008, 283, 25316–25323. [Google Scholar] [CrossRef]
- Kwon, T.H.; Kim, M.S.; Choi, H.W.; Joo, C.H.; Cho, M.Y.; Lee, B.L. A masquerade-like serine proteinase homologue is necessary for phenoloxidase activity in the coleopteran insect, Holotrichia diomphalia larvae. Eur. J. Biochem. 2000, 267, 6188–6196. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, M.Y.; Hyun, J.H.; Lee, K.M.; Homma, K.I.; Natori, S.; Kawabata, S.I.; Iwanaga, S.; Lee, B.L. Molecular cloning of cDNA for pro-phenol-oxidase-activating factor I, a serine protease is induced by lipopolysaccharide or 1,3-beta-glucan in coleopteran insect, Holotrichia diomphalia larvae. Eur. J. Biochem. 1998, 257, 615–621. [Google Scholar] [CrossRef]
- Satoh, D.; Horii, A.; Ochiai, M.; Ashida, M. Prophenoloxidase-activating enzyme of the silkworm, Bombyx mori. Purification, characterization, and cDNA cloning. J. Biol. Chem. 1999, 274, 7441–7453. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Nam, H.; Lee, W. CLIP-domain serine proteases in Drosophila innate immunity. BMB Rep. 2008, 41, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Kanost, M.R.; Jiang, H. Clip-domain serine proteases as immune factors in insect hemolymph. Curr. Opin. Insect Sci. 2015, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.V. Pinning down positional information: Dorsal-ventral polarity in the Drosophila embryo. Cell 1998, 95, 439–442. [Google Scholar] [CrossRef]
- Kanost, M.R.; Clem, R.J. Insect proteases. In Insect Molecular Biology and Biochemistry; Gilbert, I.L., Ed.; Academic Press: San Diego, CA, USA, 2012; Volume 10, pp. 346–364. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Qin, X.; Yu, B.; Chen, L.B.; Wang, Z.C.; Zhang, C.X. Genomic insights into the serine protease gene family and expression profile analysis in the planthopper, Nilaparvata lugens. BMC Genomics 2014, 15, 507. [Google Scholar] [CrossRef]
- Milligan, J.F.; Groebe, D.R.; Witherell, G.W.; Unlenbeck, O.C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987, 15, 8783–8798. [Google Scholar] [CrossRef]
- Nan, G.H.; Xu, Y.P.; Yu, Y.W.; Zhao, C.X.; Zhang, C.X.; Yu, X.P. Oocyte vitellogenesis triggers the entry of yeast-like symbionts into the oocyte of brown planthopper (Hemiptera: Delphacidae). Ann. Entomol. Soc. Am. 2016, 109, 753–758. [Google Scholar] [CrossRef]
- Horne-Badovinac, S.; Bilder, D. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev. Dyn. 2005, 232, 559–574. [Google Scholar] [CrossRef]
- Went, D.F.; Junquera, P. Embryonic development of insect eggs formed without follicular epithelium. Dev. Biol. 1981, 86, 100–110. [Google Scholar] [CrossRef]
- Gutzeit, H.O.; Haas-Assenbaum, A. The somatic envelopes around the germ-line cells of polytrophic insect follicles: Structural and functional aspects. Tissue Cell 1991, 23, 853–865. [Google Scholar] [CrossRef]
- Heifetz, Y.; Tram, U. Oogenesis, final oocyte maturation and ovulation, Insects. In Encyclopedia of Reproduction, 2nd ed.; Skinner, K.M., Ed.; Academic Press: San Diego, CA, USA, 2018; Volume 6, pp. 239–245. [Google Scholar]
- Irles, P.; Ramos, S.; Piulachs, M.D. SPARC preserves follicular epithelium integrity in insect ovaries. Dev. Biol. 2017, 422, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, K.; Zhou, Q. Dicer1 is crucial for the oocyte maturation of telotrophic ovary in Nilaparvata lugens (Stål) (Hemiptera: Geometroidea). Arch. Insect Biochem. Physiol. 2013, 84, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.X.; Pan, P.L.; Xu, J.Y.; Shen, Z.F.; Kang, D.; Lu, J.B.; Hu, Q.L.; Huang, H.J.; Lou, Y.H.; Zhou, N.M.; et al. Forkhead box transcription factor L2 activates Fcp3C to regulate insect chorion formation. Open Biol. 2017, 7, 170061. [Google Scholar] [CrossRef] [PubMed]


| Primers | Primer Sequence (5′–3′) |
|---|---|
| for cloning cDNA | |
| NlPCE3-F | CGGCAGTCTGCTTCAGTTT |
| NlPCE3-R | GTCGAAAACTAGGTCAAACAT |
| for qRT-PCR | |
| NlPCE3-qF | CGAAATGGAAGATTGCTGAGTC |
| NlPCE3-qR | TGGTGTTGGCGTTGATTATGG |
| Nl18S-qF | GTAACCCGCTGAACCTCC |
| Nl18S-qR | GTCCGAAGACCTCACTAAATCA |
| for synthesizing dsRNA | |
| T7-NlPCE3-dsF | GGATCCTAATACGACTCACTATAGGGACTCTCGTTTCCAGATACC |
| T7-NlPCE3-dsR | GGATCCTAATACGACTCACTATAGGCACCAGTGACTTCGCTC |
| T7-GFP-dsF | GGATCCTAATACGACTCACTATAGGGATACGTGCAGGAGAGGAC |
| T7-GFP-dsR | GGATCCTAATACGACTCACTATAGGGCAGATTGTGTGGACAGG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-m.; Zheng, R.-e.; Zhang, R.-j.; Ji, J.-l.; Yu, X.-p.; Xu, Y.-p. A Clip Domain Serine Protease Involved in Egg Production in Nilaparvata lugens: Expression Patterns and RNA Interference. Insects 2019, 10, 378. https://doi.org/10.3390/insects10110378
Wu J-m, Zheng R-e, Zhang R-j, Ji J-l, Yu X-p, Xu Y-p. A Clip Domain Serine Protease Involved in Egg Production in Nilaparvata lugens: Expression Patterns and RNA Interference. Insects. 2019; 10(11):378. https://doi.org/10.3390/insects10110378
Chicago/Turabian StyleWu, Jia-min, Rong-er Zheng, Rui-juan Zhang, Jin-liang Ji, Xiao-ping Yu, and Yi-peng Xu. 2019. "A Clip Domain Serine Protease Involved in Egg Production in Nilaparvata lugens: Expression Patterns and RNA Interference" Insects 10, no. 11: 378. https://doi.org/10.3390/insects10110378
APA StyleWu, J.-m., Zheng, R.-e., Zhang, R.-j., Ji, J.-l., Yu, X.-p., & Xu, Y.-p. (2019). A Clip Domain Serine Protease Involved in Egg Production in Nilaparvata lugens: Expression Patterns and RNA Interference. Insects, 10(11), 378. https://doi.org/10.3390/insects10110378





