Sulfoxaflor Applied via Drip Irrigation Effectively Controls Cotton Aphid (Aphis gossypii Glover)
Abstract
1. Introduction
2. Materials and Methods
2.1. Cotton Seeds and Insecticides
2.2. Field Experimental Site
2.3. Sulfoxaflor Application
2.3.1. Investigations of Cotton Aphid and Natural Enemies
2.3.2. Sample Collection of Cotton Plants, Seeds, and Aphids
2.4. Pretreatment of Cotton Plants, Seeds and Aphid Samples
2.5. Sulfoxaflor Analysis
2.6. Cost Assessment on the Two Application Methods
2.7. Data Analyses
3. Results
3.1. Control Efficacy of Sulfoxaflor with the Two Application Methods
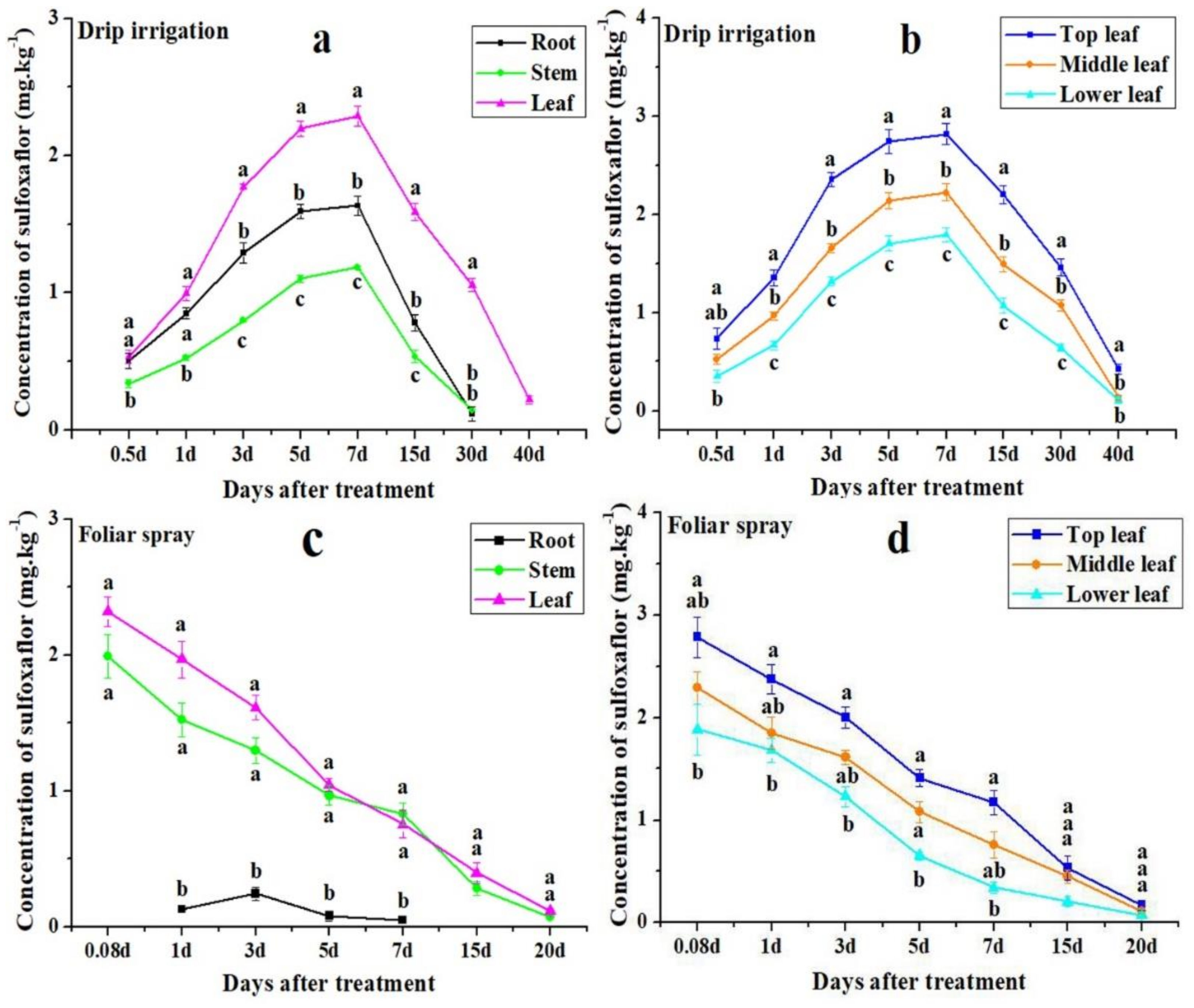
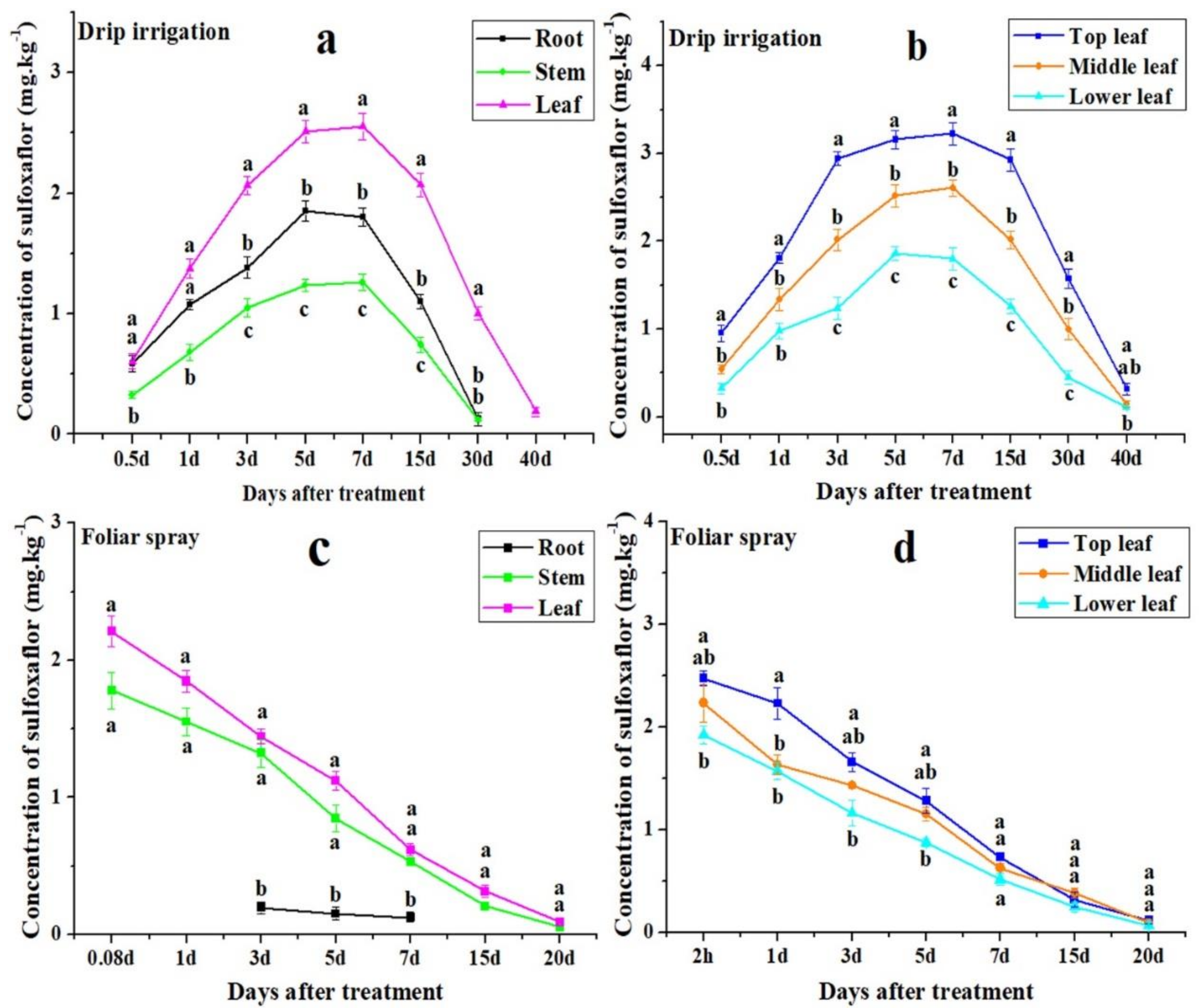
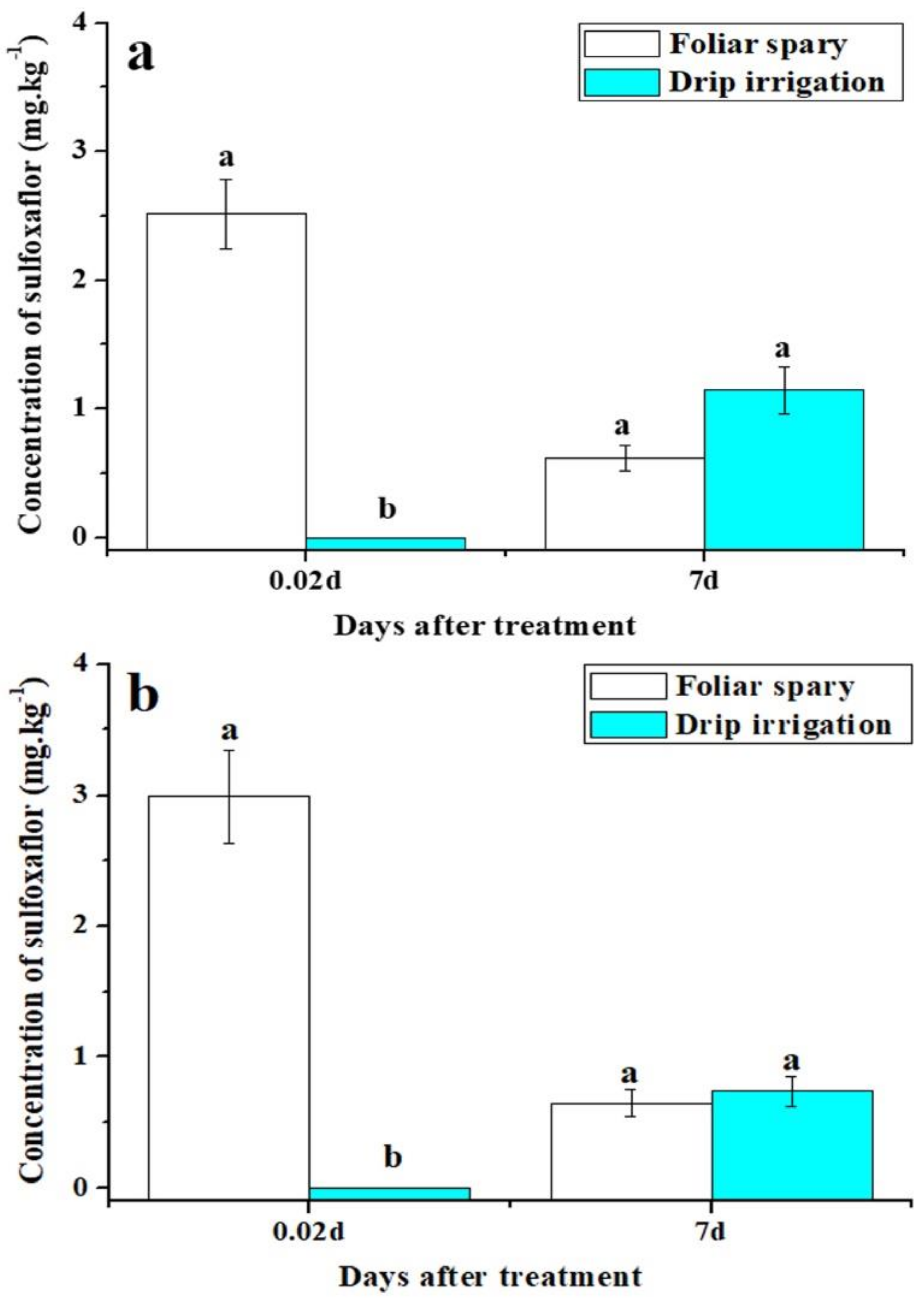
3.2. Distribution of Sulfoxaflor in Cotton Roots, Stems, and Leaves
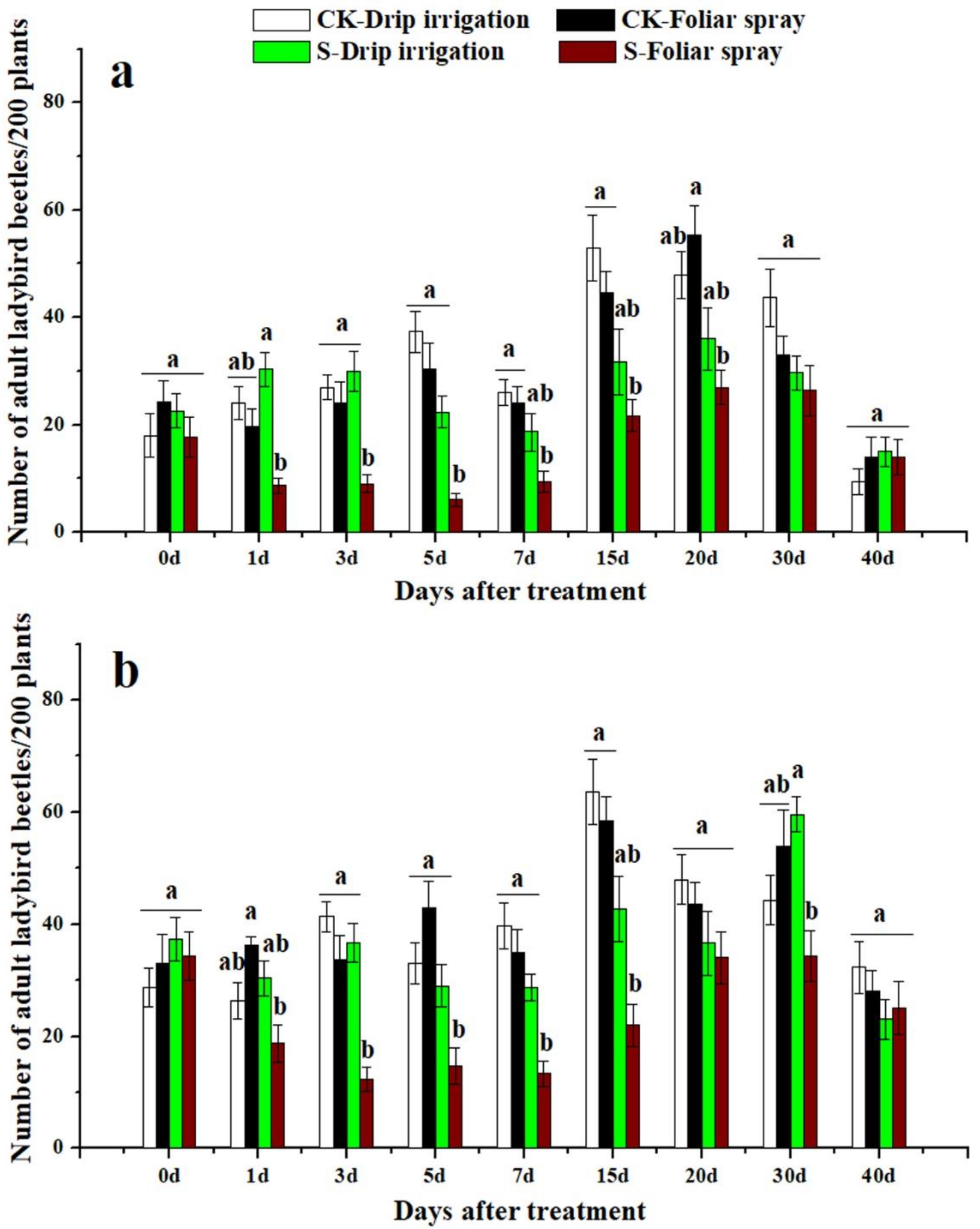
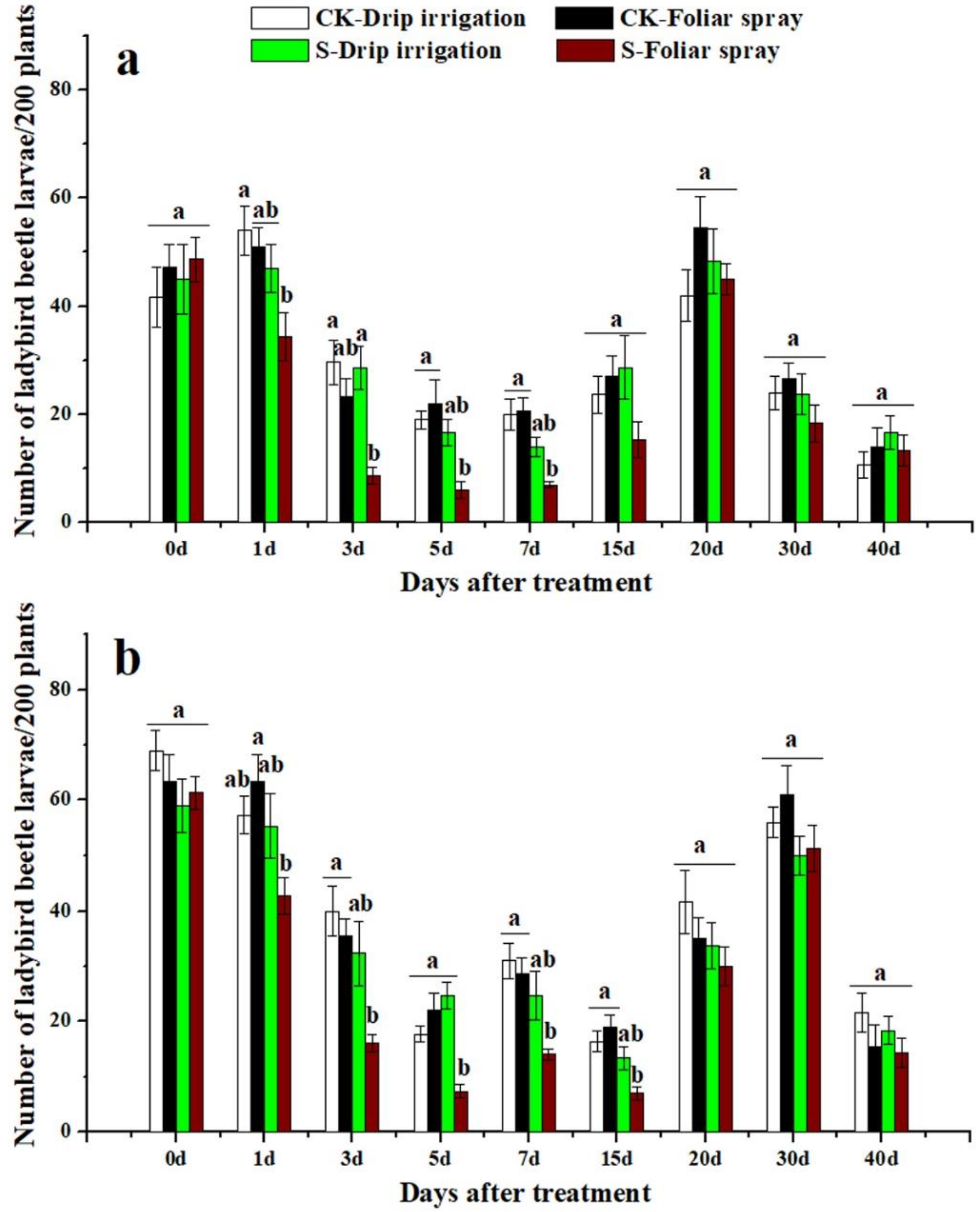
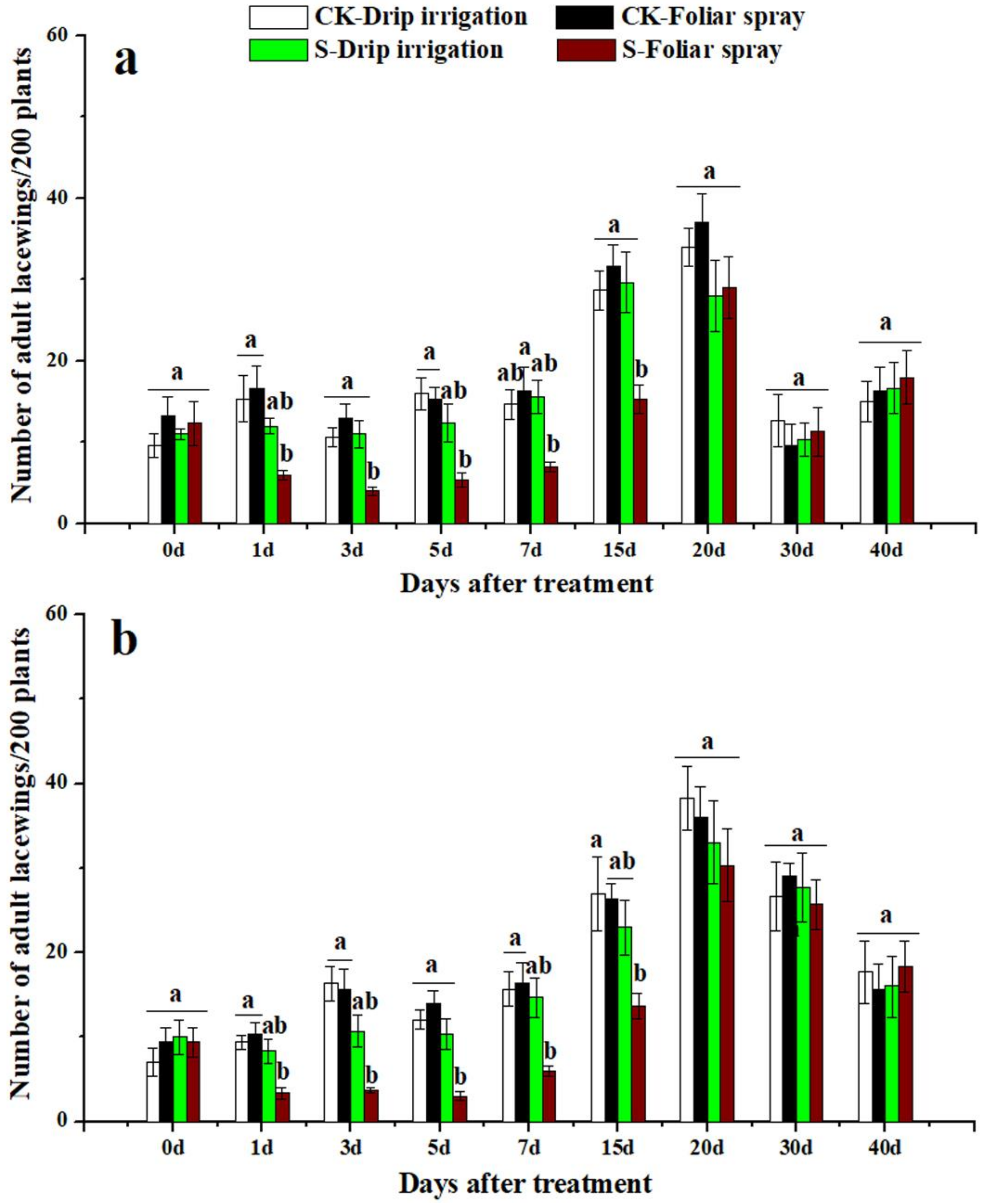
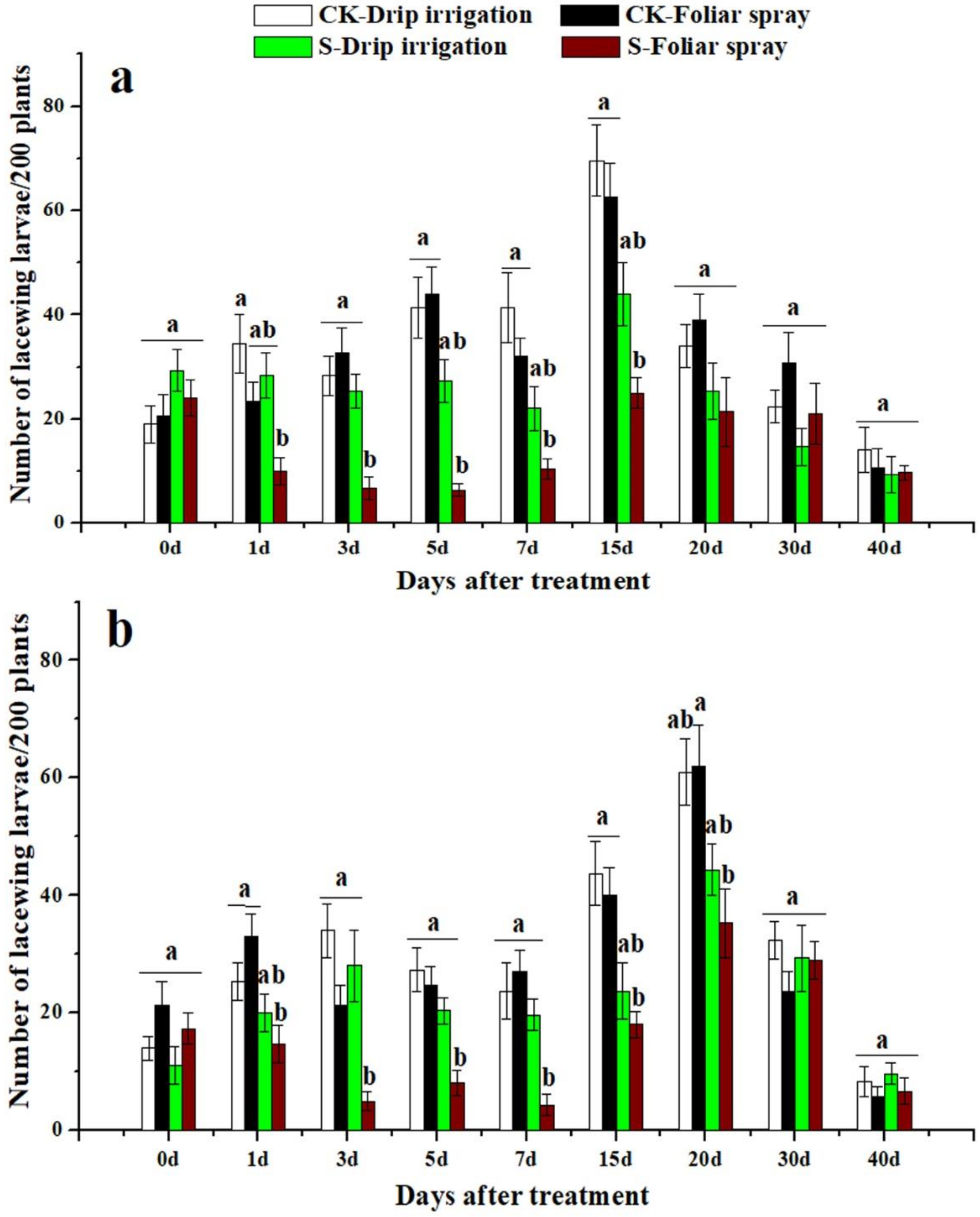
3.3. Effects of Sulfoxaflor on Ladybird Beetles and Lacewings
3.4. Sulfoxaflor Concentration in Cotton Aphids
3.5. Sulfoxaflor Residue in Cotton Plants at Harvest
3.6. Cost of the Two Application Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sarwar, K.; Azam, I.; Iqbal, W.; Rashda, A. Cotton aphid Aphis gossypii L. (Homoptera; Aphididae); a challenging pest; biology and control strategies: A review. Int. J. Appl. Biol. Pharm. Technol. 2014, 5, 290–294. [Google Scholar]
- Moury, B.; Fabre, F.; Senoussi, R. Estimation of the number of virus particles transmitted by an insect vector. Proc. Natl. Acad. Sci. USA 2007, 104, 17891–17896. [Google Scholar] [CrossRef]
- Reddall, A.; Ali, A.; Able, J.A.; Stonor, J.; Tesoriero, L.; Wright, P.R.; Rezaian, M.A.; Wilson, L.J. Cotton bunchy top: An aphid and graft transmitted cotton disease. Aust. Plant Pathol. 2004, 33, 197–202. [Google Scholar] [CrossRef]
- Slosser, J.E.; Parajulee, M.; Hendrix, D.R.; Henneberry, T.J.; Rummel, D.R. Relationship between Aphis gossypii (Homoptera: Aphididae) and sticky lint in cotton. J. Econ. Entomol. 2002, 95, 299–306. [Google Scholar] [CrossRef]
- Kim, J.J. Influence of Lecanicillium attenuatum on the development and reproduction of the cotton aphid, Aphis Gossypii. BioControl 2007, 52, 789–799. [Google Scholar] [CrossRef]
- Zhao, X.F.; Clem, T. The Sustainability of Cotton Production in China and in Australia: Comparative Economic and Environmental Issues. In Sustainable Agriculture: Technology, Planning and Management; Nova Science Publishers: New York, NY, USA, 2009; pp. 265–289. [Google Scholar]
- Dai, J.L.; Dong, H.Z. Intensive cotton farming technologies in China: Achievements, challenges and countermeasures. Field Crops Res. 2014, 155, 99–110. [Google Scholar] [CrossRef]
- Tian, L.W.; Cui, J.P.; Xu, H.J.; Guo, R.S.; Lin, T. Xinjiang cotton production: Status and its problems. J. Anhui Agric. Sci. 2013, 41, 13164–13167. [Google Scholar]
- Razmjou, J.; Moharramipour, S.; Fathipour, Y.; Mirhoseini, S.Z. Demographic parameters of cotton aphid, Aphis gossypii Glover (Homoptera: Aphididae) on five cotton cultivars. Insect Sci. 2006, 13, 205–210. [Google Scholar] [CrossRef]
- Boquel, S.; Zhang, J.; Goyer, C.; Giguère, M.A.; Clark, C.; Pelletier, Y. Effect of insecticide-treated potato plants on aphid behavior and potato virus Y acquisition. Pest Manag. Sci. 2015, 71, 1106–1112. [Google Scholar] [CrossRef]
- Ghidiu, G.; Kuhar, T.P.; Palumbo, J.C.; Schuster, D. Drip chemigation of insecticides as a pest management tool in vegetable production. J. Integr. Pest Manag. 2012, 3, E1–E5. [Google Scholar] [CrossRef]
- Wang, R.S.; Kang, Y.H.; Wan, S.Q. Effects of different drip irrigation regimes on saline-sodic soil nutrients and cotton yield in an arid region of Northwest China. Agric. Water Manag. 2015, 153, 1–8. [Google Scholar] [CrossRef]
- Li, Z.G.; Tian, C.Y.; Zhang, R.H.; Ali, I.M.E.; Liu, Y.; Zhang, G.S.; Pan, J.F.; Chen, F. Plastic mulching with drip irrigation increases soil carbon stocks of natrargid soils in arid areas of northwestern China. Catena 2015, 133, 179–185. [Google Scholar] [CrossRef]
- Arrington, A.E.; Kennedy, G.G.; Abney, M.R. Applying insecticides through drip irrigation to reduce wireworm (Coleptera: Elateridae) feeding damage in sweet potato. Pest Manag. Sci. 2016, 72, 1133–1140. [Google Scholar] [CrossRef]
- Boina, D.R.; Bloomquist, J.R. Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag. Sci. 2015, 71, 808–823. [Google Scholar] [CrossRef]
- Van, T.S.; Wise, J.C.; Isaacs, R. Soil application of neonicotinoid insecticides for control of insect pests in wine grape vineyards. Pest Manag. Sci. 2012, 68, 537–542. [Google Scholar]
- Wang, N.X.; Watson, G.; Loso, M.R.; Sparks, T.C. Molecular modeling of sulfoxaflor and neonicotinoid binding in insect nicotinic acetylcholine receptors: Impact of the Myzusβ1 R81T mutation. Pest Manag. Sci. 2016, 72, 1467–1474. [Google Scholar] [CrossRef]
- Cutler, P.; Slater, R.; Edmunds, A.J.F.; Maienfisch, P.; Hall, R.G.; Earley, F.G.; Pitterna, T.; Pal, S.; Paul, V.L.; Goodchild, J.; et al. Investigating the mode of action of sulfoxaflor: A fourth-generation neonicotinoid. Pest Manag. Sci. 2013, 69, 607–619. [Google Scholar] [CrossRef]
- Babcock, J.M.; Gerwick, C.B.; Huang, J.X.; Loso, M.R.; Nakamura, G.; Nolting, S.P.; Rogers, R.B.; Sparks, T.C.; Thomas, J.; Watson, G.B.; et al. Biological characterization of sulfoxaflor, a novel insecticide. Pest Manag. Sci. 2011, 67, 328–334. [Google Scholar] [CrossRef]
- Watson, G.B.; Loso, M.R.; Babcock, J.M.; Hasler, J.M.; Letherer, T.J.; Young, C.D.; Zhu, Y.M.; Casida, J.; Sparks, T.C. Novel nicotinic action of the sulfoximine insecticide sulfoxaflor. Insect Biochem. Mol. Biol. 2011, 41, 432–439. [Google Scholar] [CrossRef]
- Chen, X.W.; Ma, K.S.; Li, F.; Liang, P.Z.; Ying, L.; Guo, T.F.; Song, D.L.; Desneux, N.; Gao, X.W. Sublethal and transgenerational effects of sulfoxaflor on the biological traits of the cotton aphid; Aphis gossypii Glover (Hemiptera: Aphididae). Ecotoxicology 2016, 25, 1841–1848. [Google Scholar] [CrossRef]
- Munger, P.; Bleiholder, H.; Hack, H.; Hess, M.; Stauss, R.; van den Boom, T.; Weber, E. Phenological Growth Stages of the Cotton Plant (Gossypium hirsutum L.): Codification and Description according to the BBCH Scale. J. Agron. Crop Sci. 1998, 180, 143–149. [Google Scholar] [CrossRef]
- Gennari, M.; Gessa, C. Evaluation of Soil Adsorption-Desorption Capacity for the Assessment of Pesticide Bioavailability. In Bioavailability of Organic Xenobiotics in the Environment; Baveye, P., Block, J.-C., Goncharuk, V.V., Eds.; Kluwer: Dordrecht, The Netherlands, 1999; pp. 207–225. [Google Scholar]
- Bailey, G.W.; White, J.L. Factors influencing the adsorption, desorption, and movement of pesticides in soil. Residue Rev. 1970, 32, 29–92. [Google Scholar]
- Adams, R.S. Factors influencing soil adsorption and bioactivity of pesticides. Residue Rev. 1973, 47, 1–54. [Google Scholar]
- Lalah, J.O.; Kaigwara, P.N.; Getenga, Z.; Mghenyi, J.M.; Wandiga, S.O. The major environmental factors that influence rapid disappearance of pesticides from tropical soils in Kenya. Toxicol. Environ. Chem. 2001, 81, 161–197. [Google Scholar] [CrossRef]
- Timmeren, S.V.; Wise, J.C.; VanderVoort, C.; Isaacs, R. Comparison of foliar and soil formulations of neonicotinoid insecticides for control of potato leafhopper, Empoasca fabae (Homoptera: Cicadellidae), in wine grapes. Pest Manag. Sci. 2011, 67, 560–567. [Google Scholar] [CrossRef]
- Wang, X.J.; He, H.J.; Wu, K.M.; Pan, Y.M.; Ma, C.J.; Feng, J.G. Pesticide—Guidelines for the Field Efficacy Trials (II)—Part 75: Insecticides Against Cotton Aphid; GB/T 1798075; Standards Press of China: Beijiang, China, 2004. [Google Scholar]
- SANCO (Directorate General for Health and Consumer Affairs). Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed; Document No. SANCO/10684/2011; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- DOA (Department of Agriculture). National Food Safety Standard—Maximum Residue Limits for Pesticides in Food; GB2763-2014; China Agricultural Press: Beijiang, China, 2014. [Google Scholar]
- Palumbo, J.C. Systemic efficacy of Coragen applied through drip irrigation on romaine lettuce, fall 2007. Arthropod Manag. Tests 2008, 33, E24. [Google Scholar] [CrossRef]
- Ghidiu, G.M. Control of insect pests of eggplant with insecticides applied through a drip irrigation system under black plastic. In Vegetable Entomology Research Results, Rutgers University Cooperative Extension Bulletin; 104R; Rutgers University Press: New Brunswick, NJ, USA, 2009; pp. 8–11. [Google Scholar]
- Kuhar, T.P.; Doughty, H.; Hitchner, E.; Cassell, M. Evaluation of insecticide treatments for the control of lepidopteran pests in bell peppers in Virginia; 2007. Arthropod Manag. Tests 2008, 33, E7. [Google Scholar] [CrossRef]
- Kuhar, T.P.; Walgenbach, J.F.; Doughty, H.B. Control of Helicoverpa zea in tomatoes with chlorantraniliprole applied through drip chemigation. Plant Health Prog. 2010, 11, 21. [Google Scholar] [CrossRef]
- Sparks, T.C.; Watson, G.B.; Loso, M.R.; Geng, C.X.; Babcock, J.M.; Thomas, J.D. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic. Biochem. Phys. 2013, 107, 1–7. [Google Scholar] [CrossRef]
- Guerrieri, E.; Digilio, M.C. Aphid-plant interactions: A review. J. Plant Interact. 2008, 3, 223–232. [Google Scholar] [CrossRef]
- He, J.T.; Zhou, L.; Yao, Q.; Liu, B.; Xu, H.; Huang, J. Greenhouse and field-based studies on the distribution of dimethoate in cotton and its effect on Tetranychus urticae by drip irrigation. Pest Manag. Sci. 2018, 74, 225–233. [Google Scholar] [CrossRef]
- Celini, L.; Vaillant, J. A model of temporal distribution of Aphis gossypii (Glover) (Hem, Aphididae) on cotton. J. Appl. Ent. 2004, 128, 133–139. [Google Scholar] [CrossRef]
- Farha, W.; Abd El-Aty, A.M.; Rahman, M.M.; Shin, H.C.; Shim, J.H. An overview on common aspects influencing the dissipation pattern of pesticides: A review. Environ. Monit. Assess. 2016, 188, 693. [Google Scholar] [CrossRef]
- Schuster, D.J.; Shurtleff, A.; Kalb, S. Management of armyworms and leaf miners on fresh market tomatoes, fall 2007. Arthropod Manag. Tests 2009, 34, E79. [Google Scholar]
- Ghidiu, G.M.; Ward, D.L.; Rogers, G.S. Control of European corn borer in bell peppers with chlorantraniliprole applied through a drip irrigation system. Int. J. Veg. Sci. 2009, 15, 193–201. [Google Scholar] [CrossRef]
- Aajoud, A.; Raveton, M.; Aouadi, H.; Michel, T.; Ravanel, P. Uptake and Xylem Transport of Fipronil in Sunflower. J. Agric. Food Chem. 2006, 54, 5055–5060. [Google Scholar] [CrossRef]
- Baker, E.A.; Hunt, G.M. Developmental changes in leaf epicuticular waxes in relation to foliar penetration. New Phytol. 1981, 88, 731–747. [Google Scholar] [CrossRef]
- Wamhoff, H.; Schneider, V. Photodegradation of imidacloprid. J. Agric. Food Chem. 1999, 47, 1730–1734. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Zhao, Y.; Ren, Y.; Mu, W.; Liu, F. Efficacy of granular applications of clothianidin and nitenpyram against Aphis gossypii (Glover) and Apolygus lucorum (Meyer-Dür) in cotton fields in China. Crop Prot. 2015, 78, 27–34. [Google Scholar] [CrossRef]
- Seagraves, M.P. Lady beetle oviposition behavior in response to the trophic environment. Biol. Control 2009, 51, 313–322. [Google Scholar] [CrossRef]
- Evans, E.W. Searching and reproductive behaviour of female aphidophagous ladybirds (Coleoptera: Coccinellidae): A review. Eur. J. Entomol. 2003, 100, 1–10. [Google Scholar] [CrossRef]
- Pan, H.; Jiang, Y.; Wang, P.; Liu, J.; Lu, Y. Research progress in the status evolution and integrated control of cotton pests in Xinjiang. Plant Protect. 2018, 44, 42–50. (In Chinese) [Google Scholar]
- Wu, L.; Zhang, F.; Wang, H.; Zhou, H.; Zhou, J.; Fei, L. Simulation of cotton leaf area index under deficit irrigation in Xinjiang. J. Agric. Eng. 2015, 46, 249–258. [Google Scholar]
| Dates | Experiments |
|---|---|
| 4 May 2016 | Sow seeds |
| 3 June 2016 | Counting the number of cotton aphids and two kinds of natural enemies before sulfoxaflor application |
| 3 June 2016 | Applying sulfoxaflor through drip irrigation or foliar spray |
| 3 June 2016–13 July 2016 | Counting the number of cotton aphids and natural enemies, and collecting cotton root, stem and leaves |
| 24 April 2017 | Sow seeds |
| 21 June 2017 | Counting the number of cotton aphids and natural enemies before sulfoxaflor application |
| 21 June 2017 | Applying sulfoxaflor through drip irrigation or foliar spray |
| 21 June 2017–31 July 2017 | Counting the number of cotton aphids and natural enemies, collecting cotton root, stem and leaves |
| Time (Year) | Application Method | Leaf Position | Corrected Mortality (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 day | 3 days | 5 days | 7 days | 15 days | 20 days | 30 days | 40 days | |||
| 2016 | Drip | Top | 50.76 ± 5.68aB | 80.70 ±3.69aB | 96.79 ± 0.35aA | 99.00 ± 0.20aA | 98.36 ± 0.24aA | 92.95 ± 1.27aA | 87.84 ± 2.01aA | 82.19 ± 2.97aA |
| Middle | 31.16 ± 6.43abB | 63.54 ± 2.62bB | 88.53 ± 2.93aA | 95.70 ± 0.61aA | 94.16 ± 1.18aA | 86.22 ± 1.49abA | 77.63 ± 3.08abA | 72.08 ± 2.08aA | ||
| Lower | 12.07 ± 5.39bB | 41.28 ± 4.74cB | 68.27 ± 3.77bA | 78.19 ± 3.37bA | 83.79 ± 3.34bA | 76.38 ± 3.71bA | 68.76 ± 4.67bA | 57.41 ± 3.31bA | ||
| Foliar spray | Top | 76.82 ± 3.46aA | 96.66 ± 0.72aA | 95.10 ± 0.86aA | 89.51 ± 1.46aB | 77.85± 4.72aB | 53.96 ± 4.94aB | 31.83 ± 5.12aB | 2.97 ± 6.67aB | |
| Middle | 70.25 ± 3.02abA | 81.15 ± 2.73bA | 88.04 ± 2.09aA | 78.05 ± 2.78abB | 70.59 ± 4.48aB | 43.40 ± 3.41aB | 21.71 ± 6.23aB | 2.55 ± 5.75aB | ||
| Lower | 57.00 ± 3.38bA | 74.14 ± 2.66bA | 75.56 ± 4.24bA | 66.00 ± 4.57bA | 58.73 ± 5.09aB | 39.95 ± 3.69aB | 17.01 ± 5.44aB | −3.47 ± 5.36aB | ||
| 2017 | Drip | Top | 44.41 ± 3.42aB | 75.37 ± 3.48aB | 92.02 ± 1.30aA | 97.18 ± 1.35aA | 96.52 ± 1.08aA | 94.86 ± 1.41aA | 89.58 ± 3.48aA | 70.27 ± 2.90aA |
| Middle | 28.30 ± 3.81abB | 59.18 ± 3.85bB | 85.24 ± 3.18aA | 92.45 ± 1.12aA | 92.28 ± 1.19abA | 89.21 ± 2.10abA | 80.40 ± 4.14abA | 67.23 ± 2.44aA | ||
| Lower | 13.58 ± 4.25bB | 33.75 ± 3.54cB | 72.39 ± 3.13bA | 81.39 ± 3.67bA | 84.91 ± 2.81bA | 79.65 ± 3.79bA | 70.58 ± 3.74bA | 51.78 ± 3.81bA | ||
| Foliar spray | Top | 82.53 ± 4.30aA | 94.57 ± 1.04aA | 93.36 ± 1.82aA | 92.74 ± 1.97aA | 80.22 ± 2.44aB | 66.91 ± 6.43aB | 29.43 ± 5.72aB | 18.39 ± 3.84aB | |
| Middle | 78.70 ± 2.57abA | 84.34 ± 4.89abA | 82.23 ± 3.29abA | 81.53 ± 4.01abA | 73.18± 5.09aB | 57.60 ± 4.42aB | 24.53 ± 4.20aB | 10.57 ± 4.98aB | ||
| Lower | 63.76 ± 3.48bA | 72.16 ± 2.69bA | 69.42 ± 4.40bA | 67.61 ± 3.38bA | 54.10 ± 5.01bB | 41.35 ± 6.60aB | 11.49 ± 5.22aB | 6.14 ± 5.57aB | ||
| Item | Drip Irrigation 1 | Foliar Spray |
|---|---|---|
| Insecticide cost ($ ha−1) 2 | −202.39 | −43.48 |
| Labor cost ($ ha−1) 3 | −1.49 | −14.88 |
| Machinery and diesel costs ($ ha−1) 4 | 0 | −59.52 |
| Mechanical damage ($ ha−1) 5 | +89.29 | −89.29 |
| Yield difference 6 | +95.00 | −95.00 |
| Accumulative cost ($ ha−1) 7 | −19.59 | −302.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Wu, H.; Chen, J.; Tian, Y.; Zhang, Z.; Xu, H. Sulfoxaflor Applied via Drip Irrigation Effectively Controls Cotton Aphid (Aphis gossypii Glover). Insects 2019, 10, 345. https://doi.org/10.3390/insects10100345
Jiang H, Wu H, Chen J, Tian Y, Zhang Z, Xu H. Sulfoxaflor Applied via Drip Irrigation Effectively Controls Cotton Aphid (Aphis gossypii Glover). Insects. 2019; 10(10):345. https://doi.org/10.3390/insects10100345
Chicago/Turabian StyleJiang, Hui, Hanxiang Wu, Jianjun Chen, Yongqing Tian, Zhixiang Zhang, and Hanhong Xu. 2019. "Sulfoxaflor Applied via Drip Irrigation Effectively Controls Cotton Aphid (Aphis gossypii Glover)" Insects 10, no. 10: 345. https://doi.org/10.3390/insects10100345
APA StyleJiang, H., Wu, H., Chen, J., Tian, Y., Zhang, Z., & Xu, H. (2019). Sulfoxaflor Applied via Drip Irrigation Effectively Controls Cotton Aphid (Aphis gossypii Glover). Insects, 10(10), 345. https://doi.org/10.3390/insects10100345








