Effects of Insecticide Stress on Expression of NlABCG Transporter Gene in the Brown Planthopper, Nilaparvata lugens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects and Insecticides
2.2. Bioassay to Determine Insecticide Concentrations for Treatment
2.3. Insecticide Stress at Known Concentrations
2.4. Total RNA Extraction and cDNA Synthesis
2.5. Cloning of NlABCG
2.6. Sequence Analysis of NlABCG
2.7. Expression Analysis of NlABCG under Insecticide Stress
2.8. Data Analysis
3. Results
3.1. Toxicity of Insecticides against N. lugens
3.2. Identification and Characterization of NlABCG Transporter Genes
3.3. Effect of Thiamethoxam on NlABCG Expression
3.4. Effect of Abamectin on NlABCG Expression
3.5. Effect of Cyantraniliprole on NlABCG Expression
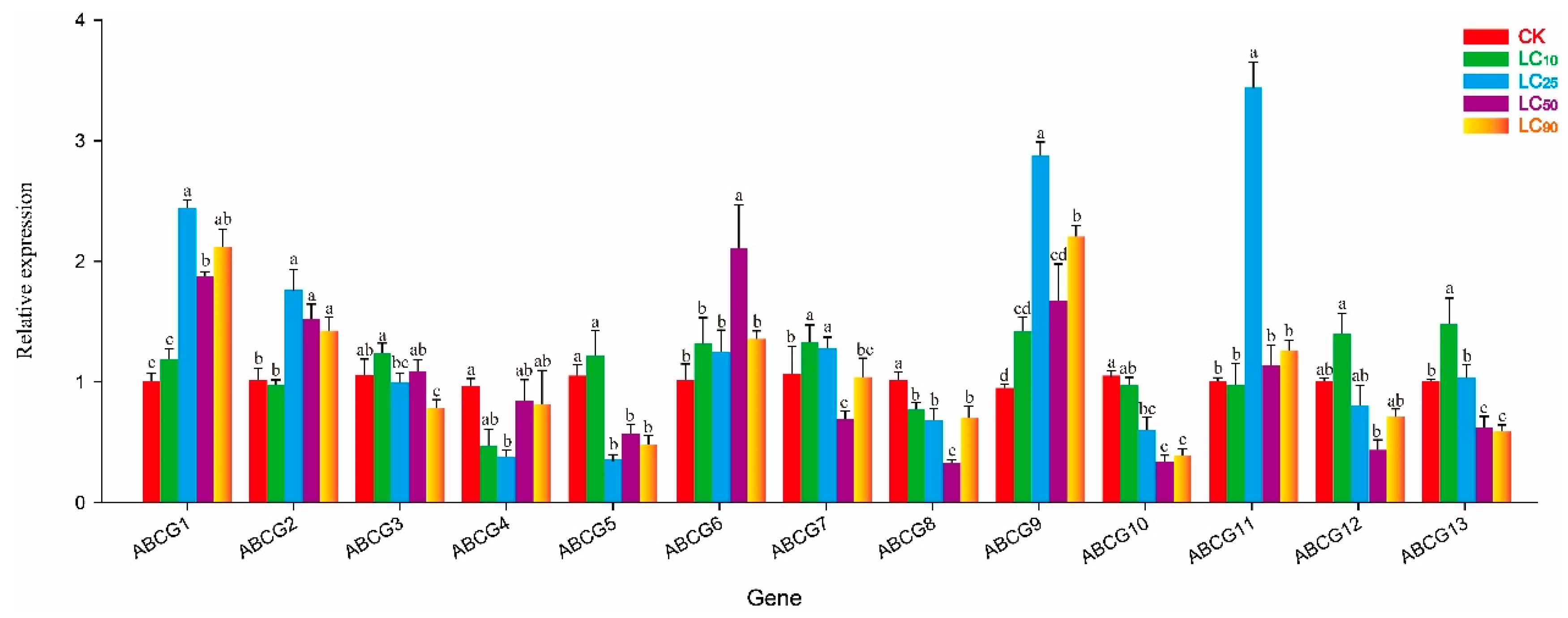
3.6. Co-Induced Expression of NlABCG Genes by Three Insecticide Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ji, Z.; Yang, S.; Zeng, Y.; Liang, Y.; Yang, C.D.; Qian, Q. Pyramiding blast, bacterial blight and brown planthopper resistance genes in rice restorer lines. J. Integr. Agric. 2016, 15, 1432–1440. [Google Scholar] [CrossRef] [Green Version]
- Sarao, P.S.; Sahi, G.K.; Neelam, K.; Mangata, G.S.; Patrac, B.C.; Singh, K. Donors for resistance to brown planthopper Nilaparvata lugens (Stål) from wild rice species. Rice Sci. 2016, 23, 219–224. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.; Mao, K.; Zhang, K.; Wan, H.; Li, J. Insecticide resistance monitoring and correlation analysis of insecticides in field populations of the brown planthopper Nilaparvata lugens (stål) in China 2012–2014. Pestic. Biochem. Physiol. 2016, 132, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Tsurumach, I.M. Insecticide susceptibility of the brown planthopper and the white-backed planthopper collected from Southeast Asia. J. Pestic. Sci. 2001, 26, 82–86. [Google Scholar] [CrossRef]
- Nizamani, I.A.; Talpur, M.A.; Qureshi, K.H. Effectiveness of Different Insecticides Against White-Backed Plant Hopper, Sogatella furcifera (Horv.) on Rice Crop. Asian J. Plant Sci. 2002, 1, 199–200. [Google Scholar]
- Nagata, T. Monitoring on insecticide resistance of the brown planthopper and the white backed planthopper in Asia. J. Asia Pac. Entomol. 2002, 5, 103–111. [Google Scholar] [CrossRef]
- Min, S.; Lee, S.W.; Choi, B.R.; Lee, S.H.; Kwon, D.H. Insecticide resistance monitoring and correlation analysis to select appropriate insecticides against Nilaparvata lugens (Stål), a migratory pest in Korea. J. Asia Pac. Entomol. 2014, 17, 711–716. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Zhu, Y.C.; Ma, C.; Huang, Y.; Shen, J. Susceptibility to neonicotinoids and risk of resistance development in the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Pest Manag. Sci. 2008, 64, 1278–1284. [Google Scholar] [CrossRef]
- Georghiou, G.P.; Mellon, R.B. Pesticide resistance in time and space. In Pest Resistance to Pesticides; Springer: Boston, MA, USA, 1983; pp. 1–46. [Google Scholar]
- Ames, G.F.; Mimura, C.S.; Holbrook, S.R.; Shyamala, V. Traffic ATPases: A superfamily of transport proteins operating from Escherichia coli to humans. Adv. Enzymol. Relat. Areas Mol. Biol. 1992, 65, 1–47. [Google Scholar]
- Higgins, C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef]
- Wu, C.; Chakrabarty, S.; Jin, M.; Liu, K.; Xiao, Y. Insect ATP-Binding Cassette (ABC) transporters: Roles in xenobiotic detoxification and Bt insecticidal activity. Int. J. Mol. Sci. 2019, 20, 2829. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H. ABC transporters and their role in protecting insects from pesticides and their metabolites. Adv. Insect. Physiol. 2014, 46, 1–72. [Google Scholar]
- Dassa, E.; Bouige, P. The ABC of ABCs: A phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001, 152, 211–229. [Google Scholar] [CrossRef]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef]
- Aurade, R.M.; Jayalakshmi, S.K.; Sreeramulu, K. P-glycoprotein ATPase from the resistant pest, Helicoverpa armigera: Purification, characterization and effect of various insecticides on its transport function. Biochim. Biophys. Acta 2010, 1798, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Denecke, S.; Fusetto, R.; Batterham, P. Describing the role of Drosophila melanogaster ABC transporters in insecticide biology using CRISPR-Cas9 knockouts. Insect Biochem. Mol. Biol. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Yang, B.; Fang, J.C.; Liu, Z.W. Transcriptomic responses to different doses of cycloxaprid involved in detoxification and stress response in the whitebacked planthopper, Sogatella furcifera. Entomol. Exp. Appl. 2016, 158, 248–257. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, H.; Wang, Z.; Long, G.Y.; Jin, D.C. Protective and Detoxifying Enzyme Activity and ABCG Subfamily Gene Expression in Sogatella furcifera Under Insecticide Stress. Front. Physiol. 2019, 9, 1890. [Google Scholar] [CrossRef]
- Sun, H.; Pu, J.; Chen, F.; Wang, J.; Han, Z. Multiple ATP-binding cassette transporters are involved in insecticide resistance in the small brown planthopper, Laodelphax striatellus. Insect Mol. Biol. 2017, 26, 343–355. [Google Scholar] [CrossRef]
- Xiao, L.F.; Zhang, W.; Jing, T.X.; Zhang, M.Y.; Miao, Z.Q.; Wei, D.D.; Yuan, G.R.; Wang, J.J. Genome-wide identification, phylogenetic analysis, and expression profiles of ATP-binding cassette transporter genes in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Roth, C.W.; Holm, I.; Graille, M.; Dehoux, P.; Rzhetsky, A.; Wincker, P.; Weissenbach, J.; Brey, P.T. Identification of the Anopheles gambiae ATP-binding cassette transporter superfamily genes. Mol. Cells 2003, 15, 150–158. [Google Scholar] [PubMed]
- Sturm, A.; Cunningham, P.; Dean, M. The ABC transporter gene family of Daphnia pulex. BMC Genom. 2009, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, S.; Tian, L.; Guo, E.; Luan, Y.; Zhang, J.; Li, S. Genome-wide identification and characterization of ATP-binding cassette transporters in the silkworm, Bombyx mori. BMC Genom. 2011, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Cheng, T.; Wang, G.; Duan, J.; Niu, W.; Xia, Q. Genome-wide analysis of the ATP-binding cassette (ABC) transporter gene family in the silkworm, Bombyx mori. Mol. Biol. Rep. 2012, 39, 7281–7291. [Google Scholar] [CrossRef]
- Broehan, G.; Kroeger, T.; Lorenzen, M.; Merzendorfer, H. Functional analysis of the ATP-binding cassette (ABC) transporter gene family of Tribolium castaneum. BMC Genom. 2013, 14, 6. [Google Scholar] [CrossRef]
- Dermauw, W.; Osborne, E.; Clark, R.; Grbić, M.; Tirry, L.; Leeuwen, T. A burst of ABC genes in the genome of the polyphagous spider mite Tetranychus urticae. BMC Genom. 2013, 14, 317. [Google Scholar] [CrossRef]
- Tian, L.; Song, T.; He, R.; Zeng, Y.; Xie, W.; Wu, Q.; Wang, S.; Zhou, X.; Zhang, Y. Genome-wide analysis of ATP-binding cassette (ABC) transporters in the sweetpotato whitefly, Bemisia tabaci. BMC Genom. 2017, 18, 330. [Google Scholar] [CrossRef]
- He, Q.; Yan, Z.; Si, F.; Zhou, Y.; Fu, W.; Chen, B. ATP-Binding Cassette (ABC) Transporter Genes Involved in Pyrethroid Resistance in the Malaria Vector Anopheles sinensis: Genome-Wide Identification, Characteristics, Phylogenetics, and Expression Profile. Int. J. Mol. Sci. 2019, 20, 1409. [Google Scholar] [CrossRef]
- Jin, M.; Liao, C.; Chakrabarty, S.; Wu, K.; Xiao, Y. Transcriptional response of ATP-binding cassette (ABC) transporters to insecticides in the cotton bollworm, Helicoverpa armigera. Pestic. Biochem. Physiol. 2019, 154, 46–59. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, H.; Yang, H.; Wang, Z.; Long, G.Y.; Jin, D.C. Effects of sublethal concentrations of deltamethrin on fitness of white-backed planthopper, Sogatella furcifera (Horváth). Int. J. Pest Manag. 2019, 65, 165–170. [Google Scholar] [CrossRef]
- Wang, Y.H.; Gao, C.F.; Zhu, Y.C.; Chen, J.; Li, W.H.; Zhuang, Y.L.; Dai, D.J.; Zhou, W.J.; Yong, C.; Shen, J.L. Imidacloprid susceptibility survey and selection risk assessment in field populations of Nilaparvata lugens (Homoptera: Delphacidae). J. Econ. Entomol. 2008, 101, 515–522. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.C.; Xue, J.; Lu, J.B.; Huang, H.J.; Xu, H.J.; Zhang, C.X. Effect of RNAi-mediated knockdown of NlTOR gene on fertility of male Nilaparvata lugens. J. Insect Physiol. 2017, 98, 149–159. [Google Scholar] [CrossRef]
- Deeley, R.G.; Westlake, C.; Cole, S.P.C. Transmembrane transport of endo-and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol. Rev. 2006, 86, 849–899. [Google Scholar] [CrossRef]
- Xu, C.; Li, C.Y.T.; Kong, A.N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005, 28, 249. [Google Scholar] [CrossRef]
- Ferreira, M.; Costa, J.; Reis-Henriques, M.A. ABC transporters in fish species: A review. Front. Physiol. 2014, 5, 266. [Google Scholar] [CrossRef]
- Labbé, R.; Caveney, S.; Donly, C. Genetic analysis of the xenobiotic resistance-associated ABC gene subfamilies of the Lepidoptera. Insect Mol. Biol. 2011, 20, 243–256. [Google Scholar] [CrossRef]
- Mastrantonio, V.; Ferrari, M.; Negri, A.; Sturmo, T.; Favia, G.; Porretta, D.; Epis, S.; Urbanelli, S. Insecticide exposure triggers a modulated expression of ABC transporter genes in larvae of Anopheles gambiae ss. Insects 2019, 10, 66. [Google Scholar] [CrossRef]
- Yu, H.Z.; Xu, J.P.; Wang, X.Y.; Ma, Y.; Yu, D.; Fei, D.Q.; Zhang, S.Z.; Wang, W.L. Identification of four ATP-binding cassette transporter genes in Cnaphalocrocis medinalis and their expression in response to insecticide treatment. J. Insect Sci. 2017, 17, 44. [Google Scholar] [CrossRef]
- Sun, H.; Buchon, N.; Scott, J.G. Mdr65 decreases toxicity of multiple insecticides in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2017, 89, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Gellatly, K.J.; Yoon, K.S.; Doherty, J.J.; Sun, W.; Pittendrigh, B.R.; Clark, J.M. RNAi validation of resistance genes and their interactions in the highly DDT-resistant 91-R strain of Drosophila melanogaster. Pestic. Biochem. Physiol. 2015, 121, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Insecticide | Toxic Regression Equation | LC10 (mg/L) (95% CL a) | LC25 (mg/L) (95% CL) | LC50 (mg/L) (95% CL) | LC90 (mg/L) (95% CL) | Chi-Square Value (χ2) |
|---|---|---|---|---|---|---|
| Thiamethoxam | Y = −0.275 + 1.261x | 0.159 (0.073–0.265) | 0.483 (0.296–0.685) | 1.653 (1.232–2.220) | 17.147 (10.256–37.496) | 1.103 |
| Abamectin | Y = 0.639 + 1.769x | 0.082 (0.056–0.109) | 0.181 (0.141–0.221) | 0.435 (0.368–0.513) | 2.308 (1.759–3.302) | 1.358 |
| Cyantraniliprole | Y = −2.397 + 2.561x | 2.727 (0.248–5.158) | 4.704 (0.959–7.492) | 8.633 (3.988–12.235) | 27.329 (18.047–12.974) | 5.800 |
| Gene Name | Accession Number | Product Size (bp) | Size of ORF (aa) | Molecular Weight | Theoretical pI |
|---|---|---|---|---|---|
| NlABCG1 | MN326305 | 1917 | 631 | 71,025.02 | 8.66 |
| NlABCG2 | MN326306 | 2246 | 680 | 75,896.09 | 9.20 |
| NlABCG3 | MN326307 | 2046 | 665 | 74,512.47 | 7.50 |
| NlABCG4 | MN326308 | 2004 | 618 | 70,498.96 | 8.91 |
| NlABCG5 | MN326309 | 3184 | 970 | 106,439.26 | 9.35 |
| NlABCG6 | MN326310 | 2451 | 615 | 69,011.27 | 8.83 |
| NlABCG7 | MN326311 | 2263 | 711 | 79,592.84 | 7.14 |
| NlABCG8 | MN326312 | 2184 | 630 | 71,071.50 | 8.52 |
| NlABCG9 | MN326313 | 1896 | 607 | 68,417.12 | 9.10 |
| NlABCG10 | MN326314 | 2343 | 642 | 71,090.70 | 9.12 |
| NlABCG11 | MN326315 | 1964 | 603 | 68,158.16 | 8.79 |
| NlABCG12 | MN326316 | 1925 | 621 | 70,006.10 | 8.70 |
| NlABCG13 | MN326317 | 2298 | 722 | 82,264.20 | 7.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhou, C.; Yang, X.-B.; Long, G.-Y.; Jin, D.-C. Effects of Insecticide Stress on Expression of NlABCG Transporter Gene in the Brown Planthopper, Nilaparvata lugens. Insects 2019, 10, 334. https://doi.org/10.3390/insects10100334
Yang H, Zhou C, Yang X-B, Long G-Y, Jin D-C. Effects of Insecticide Stress on Expression of NlABCG Transporter Gene in the Brown Planthopper, Nilaparvata lugens. Insects. 2019; 10(10):334. https://doi.org/10.3390/insects10100334
Chicago/Turabian StyleYang, Hong, Cao Zhou, Xi-Bin Yang, Gui-Yun Long, and Dao-Chao Jin. 2019. "Effects of Insecticide Stress on Expression of NlABCG Transporter Gene in the Brown Planthopper, Nilaparvata lugens" Insects 10, no. 10: 334. https://doi.org/10.3390/insects10100334
APA StyleYang, H., Zhou, C., Yang, X.-B., Long, G.-Y., & Jin, D.-C. (2019). Effects of Insecticide Stress on Expression of NlABCG Transporter Gene in the Brown Planthopper, Nilaparvata lugens. Insects, 10(10), 334. https://doi.org/10.3390/insects10100334






