Lethal and Sub-Lethal Effects of Insecticides on the Pink Hibiscus Mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Experimental Arenas
2.3. Insecticides Evaluated and Treatment Methodology
2.4. Bioassays
2.4.1. Eggs
2.4.2. Nymphs
2.4.3. Adult Females
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, E.E. Remarks on Indian Scale Insects (Coccidae), Part III. With a Catalogue of All Species Hitherto Recorded from the Indian Continent; CABI: Wallingford, UK, 1908; Volume 2, pp. 15–46. [Google Scholar]
- Williams, D. A brief account of the hibiscus mealybug Maconellicoccus hirsutus (Hemiptera: Pseudococcidae), a pest of agriculture and horticulture, with descriptions of two related species from southern Asia. Bull. Entomol. Res. 1996, 86, 617–628. [Google Scholar] [CrossRef]
- Hall, W.J. The Hibiscus Mealy Bug (Phenacoccus Hirsutus, Green) in Egypt in 1925 with Notes on the Introduction of Cryptolœmus Montrouzieri, Muls; Government Press: Dublin, Ireland, 1926.
- Williams, D. The identity and distribution of the genus Maconellicoccus Ezzat (Hemiptera: Pseudococcidae) in Africa. Bull. Entomol. Res. 1986, 76, 351–357. [Google Scholar] [CrossRef]
- Matile-Ferrero, D.; Etienne, J.; Tiego, G. Introduction of two important pests to French Guiana: Maconellicoccus hirsutus and Paracoccus marginatus (Hem., Coccoidea, Pseudococcidae). Bull. Soc. Entomol. Fr. 2000, 105, 485–486. [Google Scholar]
- Germain, J. Four species of invasive scale insects new for Reunion Island (Hemiptera, Coccoidea). Bull. Soc. Entomol. Fr. 2013, 118, 509–511. [Google Scholar]
- Halima-Kamel, M.B.; Germain, J.; Mdellel, L. First records of two mealybugs, Maconellicoccus hirsutus (Green) and Phenacoccus peruvianus Granara de Willink, in Tunisia and the North of Africa. EPPO Bull. 2015, 45, 139–143. [Google Scholar] [CrossRef]
- Marotta, S.; Harten, A.V.; Mahyoub, M. Mealybugs found on agricultural crops in Yemen. Boll. Zool. Agrar. Bachic. 2001, 33, 233–238. [Google Scholar]
- Spodek, M.; Watson, G.; Mendel, Z. The pink hibiscus mealybug, Maconellicoccus hirsutus (Green) (Hemiptera: Coccomorpha: Pseudococcidae), a new threat to Israel’s agriculture and horticulture. EPPO Bull. 2016, 46, 311–312. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Kirk, A.A.; Meyerdirk, D.E. Seasonal phenology and natural enemies of Maconellicoccus hirsutus (Hemiptera: Pseudococcidae) in Australia. Fla. Entomol. 2002, 85, 494–498. [Google Scholar] [CrossRef]
- Chang, L.W.; Miller, C.E. Pathway Risk Assessment: Pink Mealybug from the Caribbean; Planning and Risk Analysis Systems, Policy and Program Development, Animal and Plant Health Inspection Service, US Department of Agriculture: Washington, DC, USA, 1996.
- Sagarra, L.; Peterkin, D. Invasion of the Carribean by the hibiscus mealybug, Maconellicoccus hirsutus Green [Homoptera: Pseudococcidae]. Phytoprotection 1999, 80, 103–113. [Google Scholar] [CrossRef]
- Kairo, M.T.; Pollard, G.V.; Peterkin, D.D.; Lopez, V.F. Biological control of the hibiscus mealybug, Maconellicoccus hirsutus Green (Hemiptera: Pseudococcidae) in the Caribbean. Integr. Pest Manag. Rev. 2000, 5, 241–254. [Google Scholar] [CrossRef]
- Cermeli, M.; Morales Valles, P.; Godoy, F.; Romero, R.; Cárdenas, O.; de Sanidad Agropecuaria, S.A. Precence of the hibiscus pink mealybug Maconellicoccus hirsutus (Green) (Hemiptera: Pseudococcidae) in Venezuela. Entomotrópica 2002. Available online: http://agris.fao.org/agris-search/search.do?recordID=VE2007400217 (accessed on 16 January 2019).
- Kondo, T.; Gullan, P.; Portilla, A.A.R. 0265. Report of new invasive scale insects (Hemiptera: Coccoidea), Crypticerya multicicatrices Kondo and Unruh (Monophlebidae) and Maconellicoccus hirsutus (Green)(Pseudococcidae), on the islands of San Andres and Providencia, Colombia, with an updated taxonomic key to iceryine scale insects of South America. Insecta Mundi 2012, 1–17. Available online: https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1775&context=insectamundi (accessed on 16 January 2019).
- Marsaro Júnior, A.; Peronti, A.; Penteado-Dias, A.; Morais, E.; Pereira, P.d.S. First report of Maconellicoccus hirsutus (Green, 1908) (Hemiptera: Coccoidea: Pseudococcidae) and the associated parasitoid Anagyrus kamali Moursi, 1948 (Hymenoptera: Encyrtidae), in Brazil. Braz. J. Biol. 2013, 73, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Broglio, S.; Cordero, E.; dos Santos, J.; Micheletti, L. Record of pink hibiscus mealybug infesting fruit species in Maceió, Alagoas, Brazil. Rev. Caatinga 2015, 28, 242–248. [Google Scholar]
- Villatoro-Moreno, H.; Cisneros, J.; Gómez, J.; Infante, F.; Castillo, A. Mealybugs (Hemiptera: Pseudococcidae) Associated with Rambutan (Nephelium lappaceum L.) in Chiapas, Mexico. J. Kans. Entomol. Soc. 2016, 89, 289–296. [Google Scholar] [CrossRef]
- Beardsley, J. Notes and exhibitions. Proc. Hawaii. Entomol. Soc. 1985, 25, 27–28. [Google Scholar]
- Reddy, G.; Muniappan, R.; Cruz, Z.; Naz, F.; Bamba, J.; Tenorio, J. Present status of Maconellicoccus hirsutus (Hemiptera: Pseudococcidae) in the Mariana Islands and its control by two fortuitously introduced natural enemies. J. Econ. Entomol. 2009, 102, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Michaud, J.; Evans, G. Current status of pink hibiscus mealybug in Puerto Rico including a key to parasitoid species. Fla. Entomol. 2000, 83, 97–101. [Google Scholar] [CrossRef]
- Carter-Lane, S.; Redding, J. Exotic Parasitic Wasps to Attack Invasive Mealybug in California; USDA, APHIS: Washington, DC, USA, 1999.
- Hoy, M.; Hamon, A.; Nguyen, R. Pink Hibiscus Mealybug, Maconellicoccus hirsutus (Green); EENY-029; University of Florida University IFAS Extensión: Gainesville, FL, USA, 2002. [Google Scholar]
- Meyerdirk, D. Status Report on the Pink Hibiscus Biological Control Program; USDA, APHIS, PPQ: Riverdale, MD, USA, 1997.
- [NAPIS] National Agricultural Pest Information System. Pest Tracker. 2017. Available online: https://napis.ceris.purdue.edu/home (accessed on 16 January 2019).
- Mani, M. A review of the pink mealybug—Maconellicoccus hirsutus (Green). Int. J. Trop. Insect Sci. 1989, 10, 157–167. [Google Scholar] [CrossRef]
- Garland, J. Pest Risk Assessment of the Pink Mealybug Maconellicoccus hirsutus (Green), with Particular Reference to Canadian Greenhouses; PRA 96e21; Canadian Food Inspection Agency: Ottawa, ON, Canada, 1998. [Google Scholar]
- Pasiecznik, N.; Smith, I.; Watson, G.; Brunt, A.; Ritchie, B.; Charles, L. CABI/EPPO distribution maps of plant pests and plant diseases and their important role in plant quarantine. EPPO Bull. 2005, 35, 1–7. [Google Scholar] [CrossRef]
- Hall, W.J. The hibiscus mealy bug (Phenacoccus hirsutus, Green). In Bulletin Ministry of Agriculture Egypt Technical and Scientific Service Entomological Section; Government Press: Dublin, Ireland, 1921; Volume 17, pp. 1–28. [Google Scholar]
- Ghose, S. Morpho-histological changes in some economic plants due to the infestation of mealy-bug, Maconellicoccus hirsutus (Green)(Hemiptera: Pseudococcidae). Indian J. Agric. Sci. 1972, 42, 329–334. [Google Scholar]
- Stibick, J. New Pest Response Guidelines-Pink Hibiscus Mealybug, Maconellicoccus hirsutus; US Department of Agriculture, Animal and Plant Health Inspection Service: Riverdale, MD, USA, 1997.
- Zhang, A.; Amalin, D.; Shirali, S.; Serrano, M.S.; Franqui, R.A.; Oliver, J.E.; Klun, J.A.; Aldrich, J.R.; Meyerdirk, D.E.; Lapointe, S.L. Sex pheromone of the pink hibiscus mealybug, Maconellicoccus hirsutus, contains an unusual cyclobutanoid monoterpene. Proc. Natl. Acad. Sci. USA 2004, 101, 9601–9606. [Google Scholar] [CrossRef] [PubMed]
- Mani, M.; Shivaraju, C. Mealybugs and Their Management in Agricultural and Horticultural Crops; Springer: Berlin, Germany, 2016. [Google Scholar]
- Moffitt, L. Economic Risk to United States Agriculture of Pink Hibiscus Mealybug Invasion; A Report to the United States Department of Agriculture, Animal and Plant Health Inspection Service; USDA: Washington, DC, USA, 1999.
- Ranjan, R. Economic impacts of pink hibiscus mealybug in Florida and the United States. Stoch. Environ. Res. Risk Assess. 2006, 20, 353. [Google Scholar] [CrossRef]
- Roltsch, W.J.; Meyerdirk, D.E.; Warkentin, R.; Andress, E.R.; Carrera, K. Classical biological control of the pink hibiscus mealybug, Maconellicoccus hirsutus (Green), in southern California. Biol. Control 2006, 37, 155–166. [Google Scholar] [CrossRef]
- Andreason, S.A.; Triapitsyn, S.V.; Perring, T.M. Untangling the Anagyrus pseudococci species complex (Hymenoptera: Encyrtidae), parasitoids of worldwide importance for biological control of mealybugs (Hemiptera: Pseudococcidae): Genetic data corroborates separation of two new, previously misidentified species. Biol. Control 2019, 129, 65–82. [Google Scholar]
- Bliss, D.E.; Lindgren, D.L. The Use of Thiomate ’19’on Dates and Its Effect on Fruit Spoilage; CABI: Wallingford, UK, 1947; pp. 5–9. [Google Scholar]
- Perring, T.M.; Nay, J.E. Evaluation of bunch protectors for preventing insect infestation and preserving yield and fruit quality of dates, Phoenix dactylifera L. J. Econ. Entomol. 2015, 108, 654–661. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Fatima, S.; Hussain, M.; Malik, M.F.; Noureen, N.; ul Ane, N.; Abbas, Z. Field efficacy of some insecticides against hibiscus mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae). Population 2016, 10, 100. [Google Scholar]
- Fatima, S.; Hussain, M.; Shafqat, S.; Faheem Malik, M.; Abbas, Z.; Noureen, N.; Ane, N. Laboratory Evaluation of Different Insecticides against Hibiscus Mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae). Scientifica 2016, 2016, 9312013. [Google Scholar] [CrossRef]
- Anand, P.; Ayub, K. The effect of five insecticides on Maconellicoccus hirsutus (Green)(Homoptera: Pseudococcidae) and its natural enemies Anagyrus kamali Moursi (Hymenoptera: Encyrtidae), and Cryptolaemus montrouzieri Mulsant and Scymnus coccivora Aiyar (Coleoptera: Coccinellidae). Int. Pest Control 2000, 42, 170–173. [Google Scholar]
- Sunitha, N.; Jagginavar, S.; Biradar, A. Bioefficacy botanicals and newer insecticides against grape vine mealy bug, Maconellicoccus hirsutus (Green). Karnataka J. Agric. Sci. 2009, 22, 710–711. [Google Scholar]
- Aida, H.M.; Saber, F.; Ahmed, H.; Sayed, A. Efficiency of certain insecticides on the population (s) of the pink hibiscus mealybug Maconellicoccus hirsutus(Green) and their natural enemies under the field condition in Ismailia governorate. Egypt. Acad. J. Biol. Sci. 2010, 2, 11–17. [Google Scholar]
- Castle, S.; Prabhaker, N. Field evaluation of two systemic neonicotinoid insecticides against pink hibiscus mealybug (Maconellicoccus hirsutus (Green)) on mulberry trees. J. Pest Sci. 2011, 84, 363–371. [Google Scholar] [CrossRef]
- Jacobsen, C.M.; Hara, A.H. Field and postharvest treatments against the pink hibiscus mealybug, Maconellicoccus hirsutus (Green)(Homoptera: Pseudococcidae). Plant Environ. Prot. Sci. 2002. Available online: https://esa.confex.com/esa/2002/techprogram/paper_6895.htm (accessed on 16 January 2019).
- Stanley, J.; Preetha, G. Pesticide Toxicity to Parasitoids: Exposure, Toxicity and Risk Assessment Methodologies. In Pesticide Toxicity to Non-Target Organisms; Springer: Berlin, Germany, 2016; pp. 99–152. [Google Scholar]
- Ganjisaffar, F.; Perring, T.M. Prey stage preference and functional response of the predatory mite Galendromus flumenis to Oligonychus pratensis. Biol. Control 2015, 82, 40–45. [Google Scholar] [CrossRef]
- Ganjisaffar, F.; Perring, T.M. A life table analysis to evaluate biological control of banks grass mite using the predatory mite, Galendromus flumenis (Acari: Phytoseiidae). Syst. Appl. Acarol. 2017, 22, 7–13. [Google Scholar] [CrossRef]
- Hamby, K.A.; Alifano, J.A.; Zalom, F.G. Total effects of contact and residual exposure of bifenthrin and λ-cyhalothrin on the predatory mite Galendromus occidentalis (Acari: Phytoseiidae). Exp. Appl. Acarol. 2013, 61, 183–193. [Google Scholar] [CrossRef]
- de Morais, M.R.; Zanardi, O.Z.; Rugno, G.R.; Yamamoto, P.T. Impact of five insecticides used to control citrus pests on the parasitoid Ageniaspis citricola Longvinovskaya (Hymenoptera: Encyrtidae). Ecotoxicology 2016, 25, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.; Sharma, J. Bio-efficacy of various acaricides against the phytophagous mites on apple and their sensitivity to the phytoseiid mites. Pest Manag. Econ. Zool. 2010, 18, 116–121. [Google Scholar]
- Alzoubi, S.; Cobanoglu, S. Toxicity of some pesticides against Tetranychus urticae and its predatory mites under laboratory conditions. Am. Eurasian J. Agric. Environ. Sci. 2008, 3, 30–37. [Google Scholar]
- Kavya, M.; Srinivasa, N.; Vidyashree, A.; Ravi, G. Bioefficacy of newer acaricides against two spotted spider mite, Tetranychus urticae and phytoseiid predator, Neoseiulus longispinosus on brinjal under field condition. Plant Arch. 2015, 15, 493–497. [Google Scholar]
- Grafton-Cardwell, E.E.; Gu, P. Conserving vedalia beetle, Rodolia cardinalis (Mulsant) (Coleoptera: Coccinellidae), in citrus: A continuing challenge as new insecticides gain registration. J. Econ. Entomol. 2003, 96, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, R.A.; Dickinson, A. Effect of insecticides on mealybug destroyer (Coleoptera: Coccinellidae) and parasitoid Leptomastix dactylopii (Hymenoptera: Encyrtidae), natural enemies of citrus mealybug (Homoptera: Pseudococcidae). J. Econ. Entomol. 2006, 99, 1596–1604. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Akey, D.H. Conservation of natural enemies in cotton: comparative selectivity of acetamiprid in the management of Bemisia tabaci. Pest Manag. Sci. 2005, 61, 555–566. [Google Scholar] [CrossRef]
- Laurin, M.C.; Bostanian, N.J. Laboratory studies to elucidate the residual toxicity of eight insecticides to Anystis baccarum (Acari: Anystidae). J. Econ. Entomol. 2007, 100, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Lin, R.; Lin, T.; You, Y.; Zeng, Z.; Zhou, X.; Zhou, Y.; Jiang, H.; Wei, H.; Fu, J.; et al. Effects of acetamiprid on life cycle development of predatory mite Amblyseius cucumeris (Acari: Phytoseiidae) after contact exposure. Chemosphere 2018, 210, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Barbar, Z. Evaluation of three pesticides against phytophagous mites and their impact on phytoseiid predators in an eggplant open-field. Acarologia 2017, 57, 529–539. [Google Scholar]
- Poletti, M.; Maia, A.; Omoto, C. Toxicity of neonicotinoid insecticides to Neoseiulus californicus and Phytoseiulus macropilis (Acari: Phytoseiidae) and their impact on functional response to Tetranychus urticae (Acari: Tetranychidae). Biol. Control 2007, 40, 30–36. [Google Scholar] [CrossRef]
- Argolo, P.S.; Jacas, J.A.; Urbaneja, A. Comparative toxicity of pesticides in three phytoseiid mites with different life-style occurring in citrus: Euseius stipulatus, Neoseiulus californicus and Phytoseiulus persimilis. Exp. Appl. Acarol. 2014, 62, 33–46. [Google Scholar] [CrossRef]
- Villanueva, R.T.; Walgenbach, J.F. Development, oviposition, and mortality of Neoseiulus fallacis (Acari: Phytoseiidae) in response to reduced-risk insecticides. J. Econ. Entomol. 2005, 98, 2114–2120. [Google Scholar] [CrossRef]
- Beers, E.H.; Schmidt, R.A. Impacts of orchard pesticides on Galendromus occidentalis: Lethal and sublethal effects. Crop Prot. 2014, 56, 16–24. [Google Scholar] [CrossRef]
- Campbell, J.W.; Cabrera, A.R.; Stanley-Stahr, C.; Ellis, J.D. An evaluation of the honey bee (Hymenoptera: Apidae) safety profile of a new systemic insecticide, flupyradifurone, under field conditions in Florida. J. Econ. Entomol. 2016, 109, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.K.; Alves, T.M.; Koch, R.L. Potential for sulfoxaflor to improve conservation biological control of Aphis glycines (Hemiptera: Aphididae) in soybean. J. Econ. Entomol. 2016, 109, 2105–2114. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.; Suma, P.; Mazzeo, G.; Lebdi, K.G.; Russo, A. Evaluating side effects of newer insecticides on the vine mealybug parasitoid Anagyrus sp. near pseudococci, with implications for integrated pest management in vineyards. Phytoparasitica 2011, 39, 369–376. [Google Scholar] [CrossRef]
- Venkatesan, T.; Jalali, S.; Ramya, S.; Prathibha, M. Insecticide resistance and its management in mealybugs. In Mealybugs and Their Management in Agricultural and Horticultural Crops; Springer: Berlin, Germany, 2016; pp. 223–229. [Google Scholar]
- Suma, P.; Mazzeo, G. Laboratory evaluation of pesticide secondary effects on Anagyrus sp. nov. near pseudococci, parasitoid of the citrus mealybug Planococcus citri. IOBC/WPRS Bull. 2008, 38, 99–103. [Google Scholar]

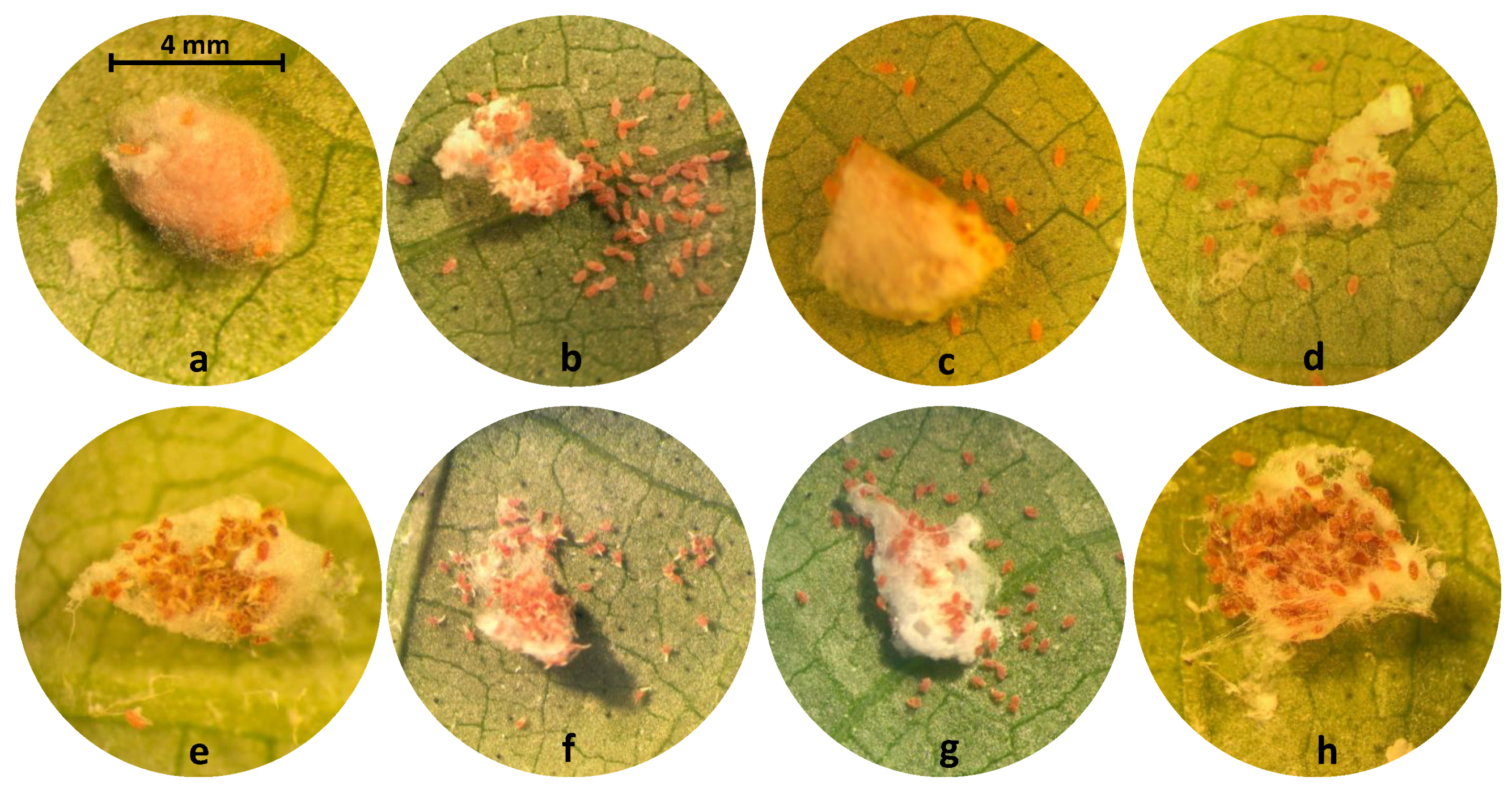
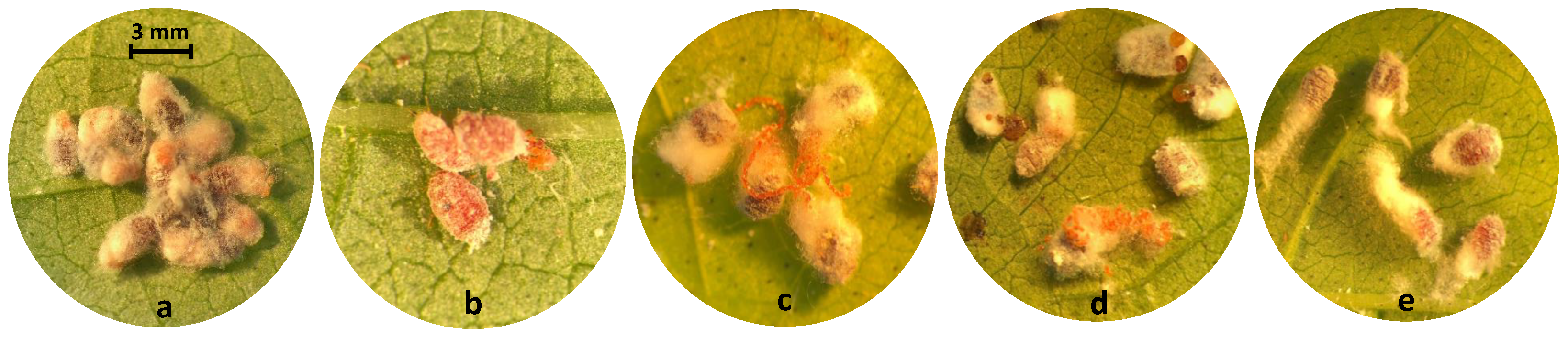
| Common Name | Trade Name | Chemical Class | Mode of Action | Max. Label Rate mL or g AI | Bioassay Rate or g AI mL DI Water |
|---|---|---|---|---|---|
| Spirotetramat | Movento 240 SC | Tetramic acid | 23 | 731 | 0.78 |
| Buprofezin | Applaud DF | Buprofezin | 16 | 1680 | 0.00180 |
| Acetamiprid | Assail 70 WP | Neonicotinoid | 4A | 237 | 0.00025 |
| Sulfoxaflor | Closer SC | Sulfoximine | 4C | 300 | 0.32 |
| Flupyradifurone | Sivanto Prime | Butenolide | 4D | 1023 | 1.09 |
| Bifenthrin | Brigade 2 EC | Pyrethroid | 3 | 937 | 1.00 |
| Fenpropathrin | Danitol 2.4 EC | Pyrethroid | 3 | 1535 | 1.64 |
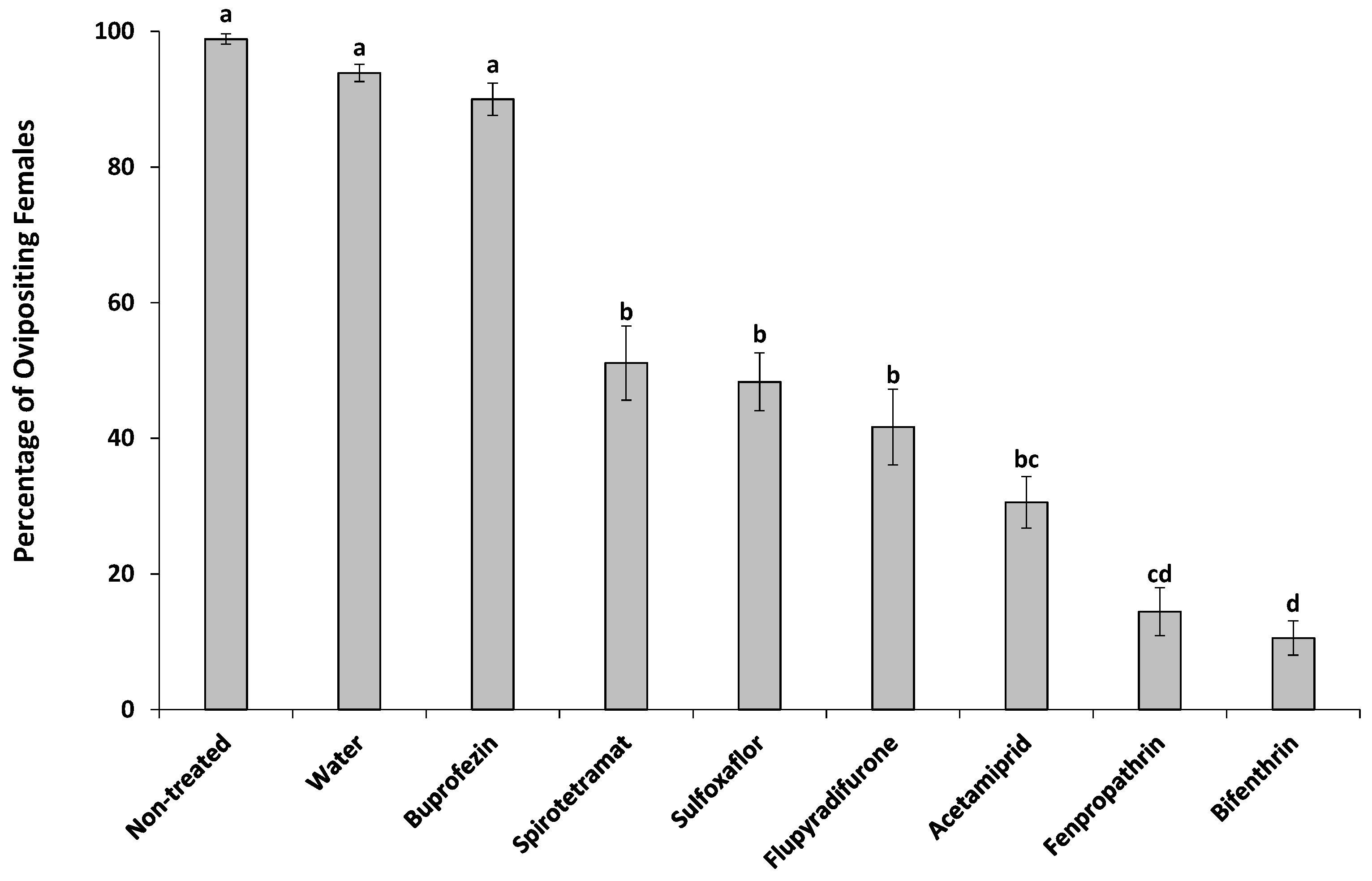
| Treatment | % Hatched Eggs in Each Ovisac | % Survived Crawlers after Emergence |
|---|---|---|
| Non-treated | a | (12) |
| Water | a | (12) |
| Spirotetramat | b | (12) |
| Bifenthrin | b | (12) |
| Flupyradifurone | b | (12) |
| Fenpropathrin | b | 0.0 (12) |
| Buprofezin | b | (12) |
| Sulfoxaflor | c | 0.0 (7) |
| Acetamiprid | c | 0.0 (1) |
| Stage Tested | Treatment | % Mortality | |||||
|---|---|---|---|---|---|---|---|
| 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | ||
| Nymphs | Non-treated | a | a | a | a | a | a |
| Water | a | a | a,b | a | a | a | |
| Spirotetramat | a | a,b | b | b | b | b | |
| Buprofezin | a | a,b | a,b | b | b | b | |
| Sulfoxaflor | a | b | c | c | c | c | |
| Acetamiprid | b | c | d | d | c,d | c,d | |
| Flupyradifurone | c | d | e | e | d | d | |
| Fenpropathrin | b,c | c,d | d,e | d,e | d | d | |
| Bifenthrin | c | d | d,e | d,e | d | d | |
| Adults (♀) | Non-treated | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| Water | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | |
| Spirotetramat | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | a | |
| Buprofezin | 0.0 a | 0.0 a | 0.0 a | a | a | a | |
| Sulfoxaflor | 0.0 a | 0.0 a | 0.0 a | a | a | a | |
| Acetamiprid | 0.0 a | a | a | a | a | a | |
| Flupyradifurone | 0.0 a | 0.0 a | a | a | a | a | |
| Fenpropathrin | a | a | a | a | a | a | |
| Bifenthrin | a | a | a | a | a | a | |
| Treatment | % Nymphs That Stopped Feeding | |||||
|---|---|---|---|---|---|---|
| 1 d | 2 d | 3 d | 4 d | 5 d | 6 d | |
| Non-treated | a | a | a | a | a | a |
| Water | a | a | a | a | a | a |
| Spirotetramat | a | a | a | a | ab | b |
| Buprofezin | a | a | a | a | ab | b |
| Sulfoxaflor | b | 100.0 b | 100.0 b | 100.0 b | 100.0 b | 100.0 b |
| Acetamiprid | b | 100.0 b | 100.0 b | 100.0 b | 100.0 b | 100.0 b |
| Flupyradifurone | 100.0 b | 100.0 b | 100.0 b | 100.0 b | 100.0 b | 100.0 b |
| Fenpropathrin | 100.0 b | 100.0 b | 100.0 b | 100.0 b | 100.0 b | 100.0 b |
| Bifenthrin | 100.0 b | 100.0 b | 100.0 b | 100.0 b | 100.0 b | 100.0 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganjisaffar, F.; Andreason, S.A.; Perring, T.M. Lethal and Sub-Lethal Effects of Insecticides on the Pink Hibiscus Mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae). Insects 2019, 10, 31. https://doi.org/10.3390/insects10010031
Ganjisaffar F, Andreason SA, Perring TM. Lethal and Sub-Lethal Effects of Insecticides on the Pink Hibiscus Mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae). Insects. 2019; 10(1):31. https://doi.org/10.3390/insects10010031
Chicago/Turabian StyleGanjisaffar, Fatemeh, Sharon A. Andreason, and Thomas M. Perring. 2019. "Lethal and Sub-Lethal Effects of Insecticides on the Pink Hibiscus Mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae)" Insects 10, no. 1: 31. https://doi.org/10.3390/insects10010031
APA StyleGanjisaffar, F., Andreason, S. A., & Perring, T. M. (2019). Lethal and Sub-Lethal Effects of Insecticides on the Pink Hibiscus Mealybug, Maconellicoccus hirsutus (Hemiptera: Pseudococcidae). Insects, 10(1), 31. https://doi.org/10.3390/insects10010031






