Initial Exposure of Wax Foundation to Agrochemicals Causes Negligible Effects on the Growth and Winter Survival of Incipient Honey Bee (Apis mellifera) Colonies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Colony Establishment
2.2. Pesticide Treatment of Wax Foundation
2.3. Colony Growth Measurements
2.4. Statistical Analyses
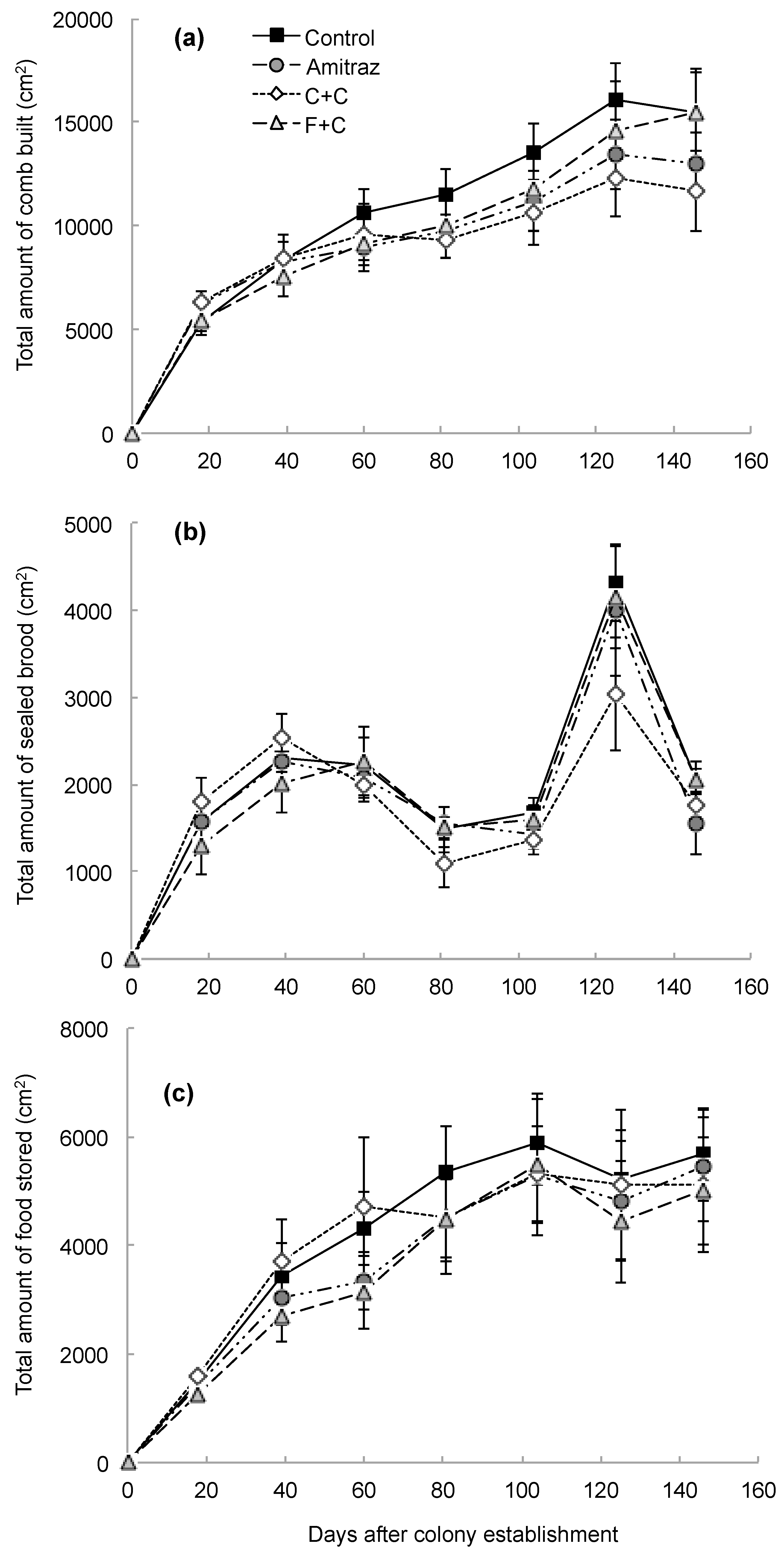
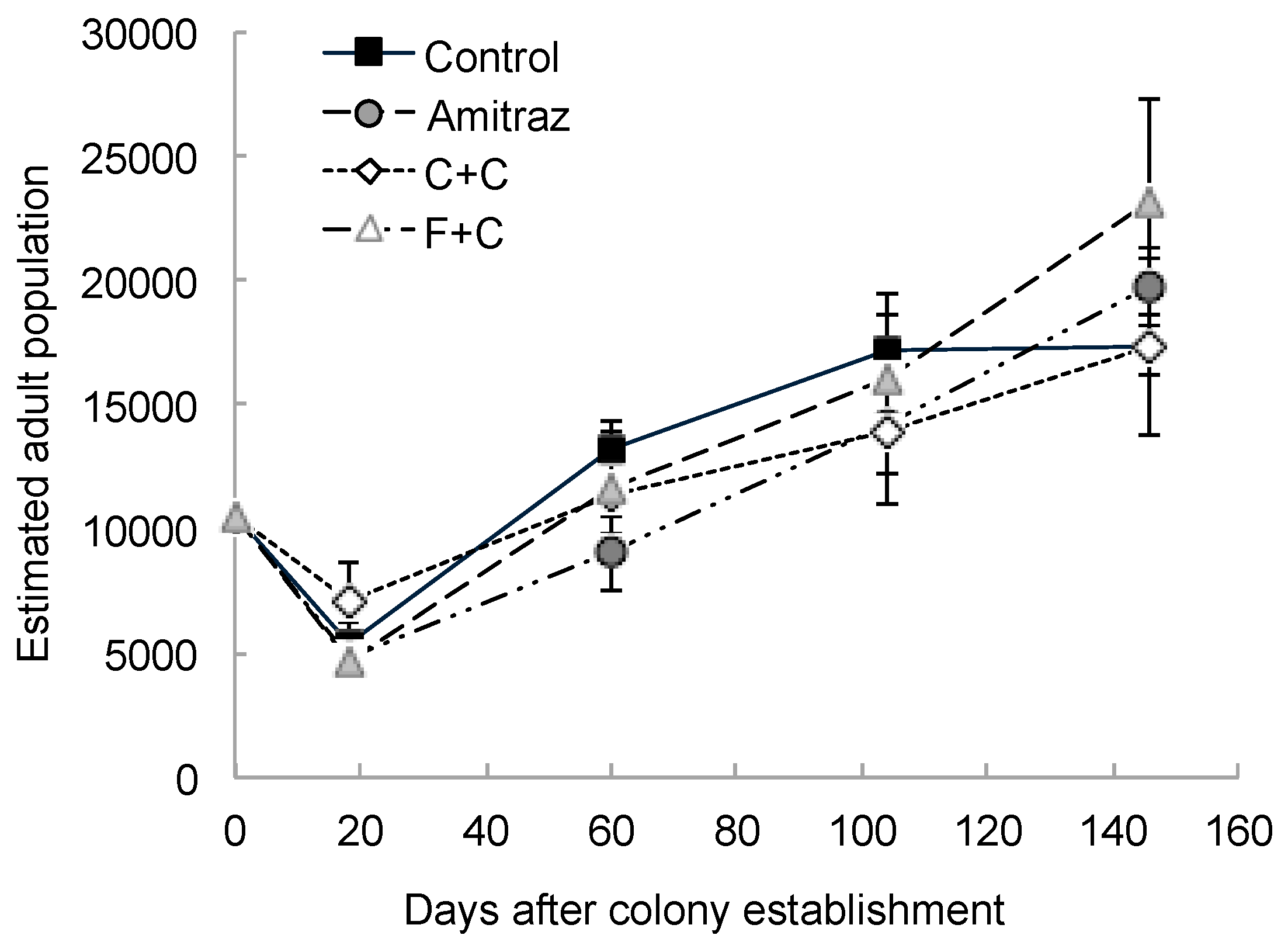
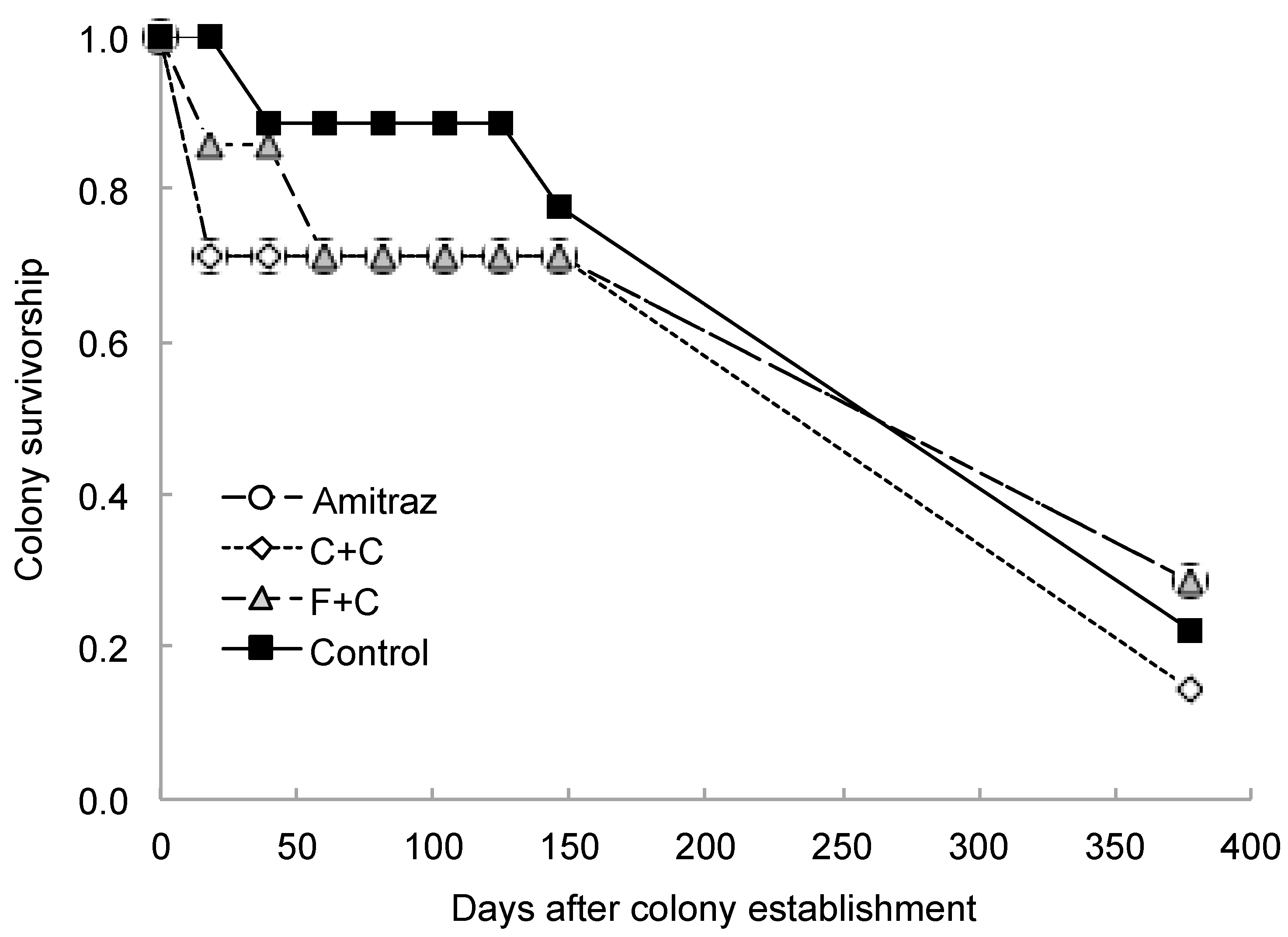
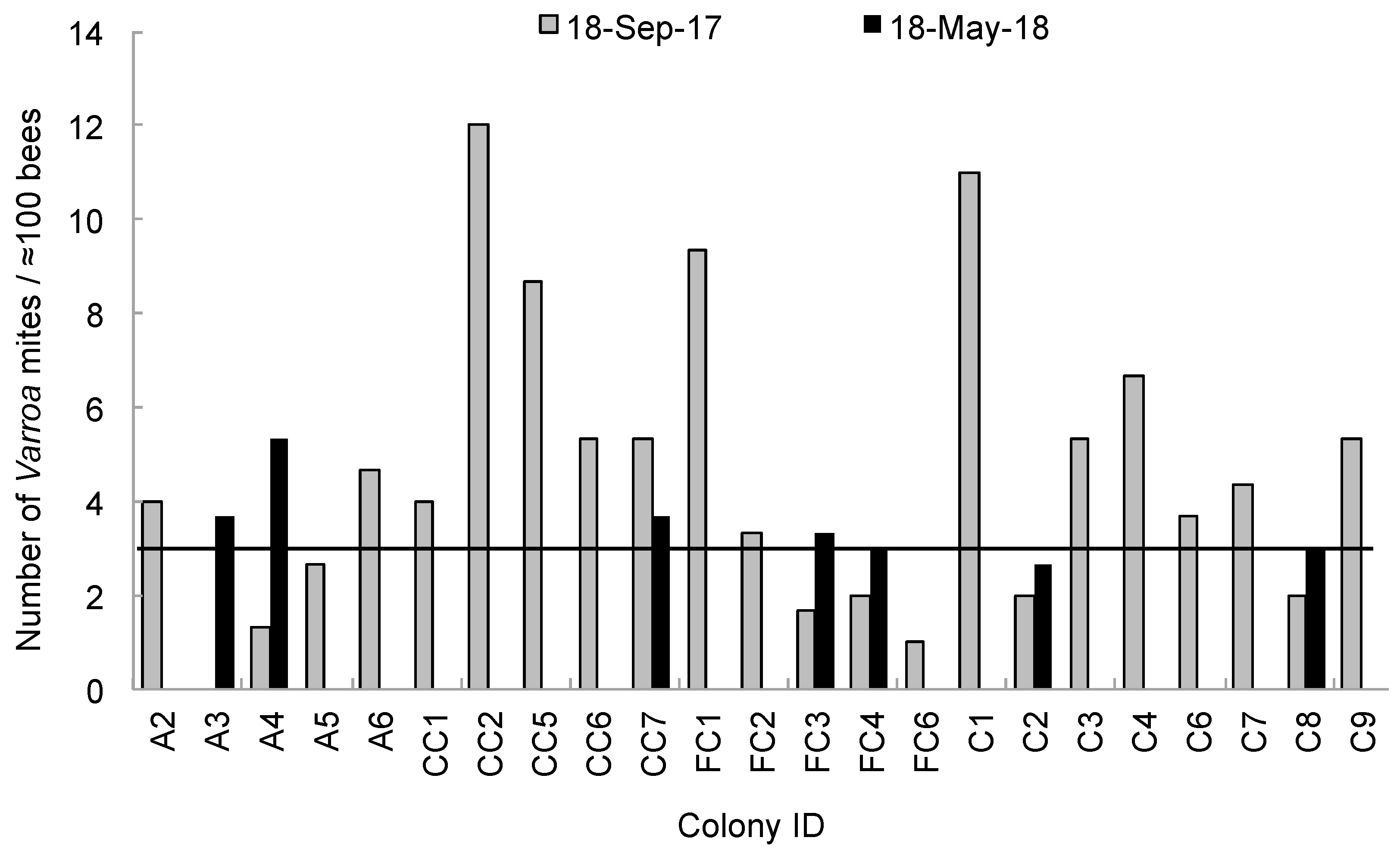
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calderone, N.W. Insect pollinated crops, insect pollinators and us agriculture: Trend analysis of aggregate data for the period 1992–2009. PLoS ONE 2012, 7, e37235. [Google Scholar]
- Watanabe, M.E. Pollination worries rise as honey bees decline. Science 1994, 265, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Delaplane, K.S.; Mayer, D.R.; Mayer, D.F. Crop Pollination by Bees; CABI Publishing: New York, NY, USA, 2000. [Google Scholar]
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Kulhanek, K.; Steinhauer, N.; Rennich, K.; Caron, D.M.; Sagili, R.R.; Pettis, J.S.; Ellis, J.D.; Wilson, M.E.; Wilkes, J.T.; Tarpy, D.R.; et al. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J. Apicult. Res. 2017, 56, 328–340. [Google Scholar]
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.-P. Drivers of colony losses. Curr. Opin. Insect Sci. 2018, 26, 142–148. [Google Scholar] [CrossRef] [PubMed]
- USDA-NASS Census of Agriculture: United States Summary and State Data, Volume 1, Part 51. Available online: www.agcensus.usda.gov/publications/2012 (accessed on 11 July 2018).
- Johnson, R.M.; Ellis, M.D.; Mullin, C.A.; Frazier, M. Pesticides and honey bee toxicity–USA. Apidologie 2010, 41, 312–331. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Pesticide residues and bees—A risk assessment. PLoS ONE 2014, 9, e94482. [Google Scholar]
- Traynor, K.S.; Pettis, J.S.; Tarpy, D.R.; Mullin, C.A.; Frazier, J.L.; Frazier, M. In-hive pesticide exposome: Assessing risks to migratory honey bees from in-hive pesticide contamination in the eastern united states. Sci. Rep. 2016, 6, 33207. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; Pettis, J.S. High levels of miticides and agrochemicals in north american apiaries: Implications for honey bee health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [PubMed]
- Chensheng, L.; Warchol, K.M.; Callahan, R.A. In situ replication of honey bee colony collapse disorder. Bull. Insect. 2012, 65, 99–106. [Google Scholar]
- Cresswell, J.E.; Page, C.J.; Uygun, M.B.; Holmbergh, M.; Li, Y.; Wheeler, J.G.; Laycock, I.; Pook, C.J.; de Ibarra, N.H.; Smirnoff, N.; et al. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 2012, 115, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Pilling, E.; Campbell, P.; Coulson, M.; Ruddle, N.; Tornier, I. A four-year field program investigating long-term effects of repeated exposure of honey bee colonies to flowering crops treated with thiamethoxam. PLoS ONE 2013, 8, e77193. [Google Scholar] [CrossRef] [PubMed]
- Rangel, J.; Keller, J.; Tarpy, D. The effects of honey bee (Apis mellifera L.) queen reproductive potential on colony growth. Insect. Soc. 2013, 60, 65–73. [Google Scholar] [CrossRef]
- Rangel, J.; Böröczky, K.; Schal, C.; Tarpy, D.R. Honey bee (Apis mellifera) queen reproductive potential affects queen mandibular gland pheromone composition and worker retinue response. PLoS ONE 2016, 11, e0156027. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C. Weights of workers and drones. Am. Bee J. 1970, 110, 468–469. [Google Scholar]
- Seeley, T.D.; Visscher, P.K. Choosing a home: How the scouts in a honey bee swarm perceive the completion of their group decision making. Behav. Ecol. Sociobiol. 2003, 54, 511–520. [Google Scholar] [CrossRef]
- Seeley, T. The Wisdom of the Hive; Harvard University Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Rangel, J.; Seeley, T. Colony fissioning in honey bees: Size and significance of the swarm fraction. Insect. Soc. 2012, 59, 453–462. [Google Scholar] [CrossRef]
- Macedo, P.A.; Wu, J.; Ellis, M.D. Using inert dusts to detect and assess varroa infestations in honey bee colonies. J. Apicult. Res. 2002, 41, 3–7. [Google Scholar] [CrossRef]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard methods for varroa research. J. Apicult. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Littell, R.; Henry, P.; Ammerman, C. Statistical analysis of repeated measures data using sas procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-meier estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar] [PubMed]
- Ostiguy, N.; Drumond, F.A.; Aronstein, K.; Eitzer, B.; Ellis, J.D.; Spivak, M.; Shepherd, W.S. Pesticide exposure to honey bees in a four-year nationwide study. Insects 2018. submitted for publication. [Google Scholar]
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.D.; Pettis, J. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insest Physiol. 2012, 58, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A., II; Coleman, C.; Hoffmann, C.; Fritz, B.; Rangel, J. The effects of the insect growth regulators methoxyfenozide and pyriproxyfen and the acaricide bifenazate on honey bee (Hymenoptera: Apidae) forager survival. J. Econ. Entomol. 2018, 111, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A., II; Coleman, J.C.; Hoffmann, C.; Fritz, B.; Rangel, J. The synergistic effects of almond protection fungicides on honey bee (Hymenoptera: Apidae) forager survival. J. Econ. Entomol. 2017, 110, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, F.D.; Margotta, J.W.; Pittman, J.M.; Danka, R.G.; Tarver, M.R.; Ottea, J.A.; Healy, K.B. Genetics, synergists, and age affect insecticide sensitivity of the honey bee, Apis mellifera. PLoS ONE 2015, 10, e0139841. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 2014, 9, e77547. [Google Scholar] [CrossRef] [PubMed]
- Haarmann, T.; Spivak, M.; Weaver, D.; Weaver, B.; Glenn, T. Effects of fluvalinate and coumaphos on queen honey bees (hymenoptera: Apidae) in two commercial queen rearing operations. J. Econ. Entomol. 2002, 95, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Williams, V.; Evans, J. Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insest Mol. Biol. 2004, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Pettis, J.S.; Collins, A.M.; Wilbanks, R.; Feldlaufer, M.F. Effects of coumaphos on queen rearing in the honey bee, Apis mellifera. Apidologie 2004, 35, 605–610. [Google Scholar] [CrossRef]
- Rangel, J.; Tarpy, D.R. The combined effects of miticides on the mating health of honey bee (Apis mellifera L.) queens. J. Apicult. Res. 2015, 54, 275–283. [Google Scholar] [CrossRef]
- Walsh, E.; Sweet, S.; Rangel, J. Honey bee (Apis mellifera L.) queen rearing environment affects behavior and physiology. In Proceedings of the 2018 American Bee Research Conference, Reno, NV, USA, 11–12 January 2018; Volume 95, pp. 47–72. [Google Scholar]
- De Guzman, L.I.; Rinderer, T.E.; Lancaster, V.A.; Delatte, G.T.; Stelzer, A. Varroa in the mating yard: III. The effects of formic acid gel formulation on drone production. Am. Bee J. 1999, 139, 304–307. [Google Scholar]
- Rinderer, T.E.; De Guzman, L.; Lancaster, V.A.; Delatte, G.T.; Stelzer, J.A. Varroa in the mating yard: I. The effects of Varroa jacobsoni and Apistan on drone honey bees. Am. Bee J. 1999, 139, 134–139. [Google Scholar]
- Fell, R.; Tignor, K. Miticide effects on the reproductive physiology of queens and drones. Am. Bee J. 2001, 141, 888–889. [Google Scholar]
- Burley, L.M. The Effects of Miticides on the Reproductive Physiology of Honey Bee (Apis mellifera L.) Queens and Drones. Master’s Thesis, Virginia Tech, Blacksburg, VA, USA, 2007. [Google Scholar]
- Burley, L.M.; Fell, R.D.; Saacke, R.G. Survival of honey bee (Hymenoptera: Apidae) spermatozoa incubated at room temperature from drones exposed to miticides. J. Econ. Entomol. 2008, 101, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A., II; Rangel, J. Exposure to pesticides during development negatively affects honey bee (Apis mellifera) drone sperm viability. PLoS ONE 2018. under review. [Google Scholar]
- Christensen, K.; Harper, B.; Luukinen, B.; Buhl, K.; Stone, D. Chlorpyrifos Technical Fact Sheet; National Pesticide Information Center, Oregon State University Extension Services: Corvallis, OR, USA, 2009. [Google Scholar]
- Dai, P.; Jack, C.J.; Mortensen, A.N.; Bustamante, T.A.; Bloomquist, J.R.; Ellis, J.D. Chronic toxicity of clothianidin, imidacloprid, chlorpyrifos, and dimethoate to Apis mellifera L. larvae reared in vitro. Pest Manag. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tillman, R.W.; Siegel, M.R.; Long, J.W. Mechanism of action and fate of the fungicide chlorothalonil (2, 4, 5, 6-tetrachloroisophthalonitrile) in biological systems: I. Reactions with cells and subcellular components of Saccharomyces pastorianus. Pest. Biochem. Physiol. 1973, 3, 160–167. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Evans, J.D.; Donovall, L.; Mullin, C.; Frazier, M.; Frazier, J.; Tarpy, D.R.; Hayes, J., Jr.; Pettis, J.S. “Entombed pollen”: A new condition in honey bee colonies associated with increased risk of colony mortality. J. Invert. Pathol. 2009, 101, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Lodesani, M.; Colombo, M.; Spreafico, M. Ineffectiveness of apistan® treatment against the mite Varroa jacobsoni oud. in several districts of lombardy (Italy). Apidologie 1995, 26, 67–72. [Google Scholar] [CrossRef]
- Baxter, J.; Eischen, F.; Pettis, J.; Wilson, W.; Shimanuki, H. Detection of fluvalinate-resistant varroa mites in us honey bees. Am. Bee J. 1998, 138, 291. [Google Scholar]
- Elzen, P.J.; Eischen, F.A.; Baxter, J.R.; Elzen, G.W.; Wilson, W.T. Detection of resistance in U.S. Varroa jacobsoni oud. (mesostigmata: Varroidae) to the acaricide fluvalinate. Apidologie 1999, 30, 13–17. [Google Scholar] [CrossRef]
- Elzen, P.J.; Baxter, J.R.; Spivak, M.; Wilson, W.T. Control of Varroa jacobsoni oud. Resistant to fluvalinate and amitraz using coumaphos. Apidologie 2000, 31, 437–441. [Google Scholar] [CrossRef]
- Elzen, P.; Westervelt, D. Detection of coumaphos resistance in Varroa destructor in Florida. Am. Bee J. 2002, 142, 291–292. [Google Scholar]
- Boecking, O.; Genersch, E. Varroosis-the ongoing crisis in bee keeping. J. Verbr. Lebensm. 2008, 3, 221–228. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Eccles, L.; Calvete, Y.; Mcgowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Borba, R.; Wilson, M.; Spivak, M. Propolis counteracts some threats to honey bee health. Insects 2017, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Borba, R.S.; Klyczek, K.K.; Mogen, K.L.; Spivak, M. Seasonal benefits of a natural propolis envelope to honey bee immunity and colony health. J. Exp. Biol. 2015, 218, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.V.; Goblirsch, M.; McDermott, E.; Tarpy, D.R.; Spivak, M. Is the brood pattern within a honey bee colony a reliable indicator of queen quality? Insects 2018, in press. [Google Scholar]
- O’Neal, S.T.; Anderson, T.D.; Wu-Smart, J.Y. Interactions between pesticides and pathogen susceptibility in honey bees. Curr. Opin. Insect Sci. 2018, 26, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS ONE 2011, 6, e14720. [Google Scholar] [CrossRef] [PubMed]
| Parameter Measured | Effect of Pesticide Treatment | Effect of Sampling Day | Interaction Effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFnu | DFden. | F-Value | p-Value | DFnu | DFden. | F-Value | p-Value | DFnu | DFden. | F-Value | p-Value | |
| Amount of comb built | 3 | 21 | 0.49 | 0.69 | 7 | 136 | 131.18 | <0.0001 | 21 | 136 | 1.36 | 0.15 |
| Amount of brood produced | 3 | 20 | 0.34 | 0.80 | 7 | 136 | 68.00 | <0.0001 | 21 | 136 | 0.88 | 0.61 |
| Amount of food stored | 3 | 21 | 0.28 | 0.84 | 7 | 136 | 55.70 | <0.0001 | 21 | 136 | 0.34 | 1.00 |
| Estimated adult population | 3 | 21 | 0.34 | 0.79 | 4 | 79 | 48.77 | <0.0001 | 12 | 79 | 1.29 | 0.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payne, A.N.; Walsh, E.M.; Rangel, J. Initial Exposure of Wax Foundation to Agrochemicals Causes Negligible Effects on the Growth and Winter Survival of Incipient Honey Bee (Apis mellifera) Colonies. Insects 2019, 10, 19. https://doi.org/10.3390/insects10010019
Payne AN, Walsh EM, Rangel J. Initial Exposure of Wax Foundation to Agrochemicals Causes Negligible Effects on the Growth and Winter Survival of Incipient Honey Bee (Apis mellifera) Colonies. Insects. 2019; 10(1):19. https://doi.org/10.3390/insects10010019
Chicago/Turabian StylePayne, Alexandria N., Elizabeth M. Walsh, and Juliana Rangel. 2019. "Initial Exposure of Wax Foundation to Agrochemicals Causes Negligible Effects on the Growth and Winter Survival of Incipient Honey Bee (Apis mellifera) Colonies" Insects 10, no. 1: 19. https://doi.org/10.3390/insects10010019
APA StylePayne, A. N., Walsh, E. M., & Rangel, J. (2019). Initial Exposure of Wax Foundation to Agrochemicals Causes Negligible Effects on the Growth and Winter Survival of Incipient Honey Bee (Apis mellifera) Colonies. Insects, 10(1), 19. https://doi.org/10.3390/insects10010019





