Eco-Friendly Water-Based Nanolubricants for Industrial-Scale Hot Steel Rolling
Abstract
1. Introduction
2. Materials and Experimental Procedure
2.1. Materials
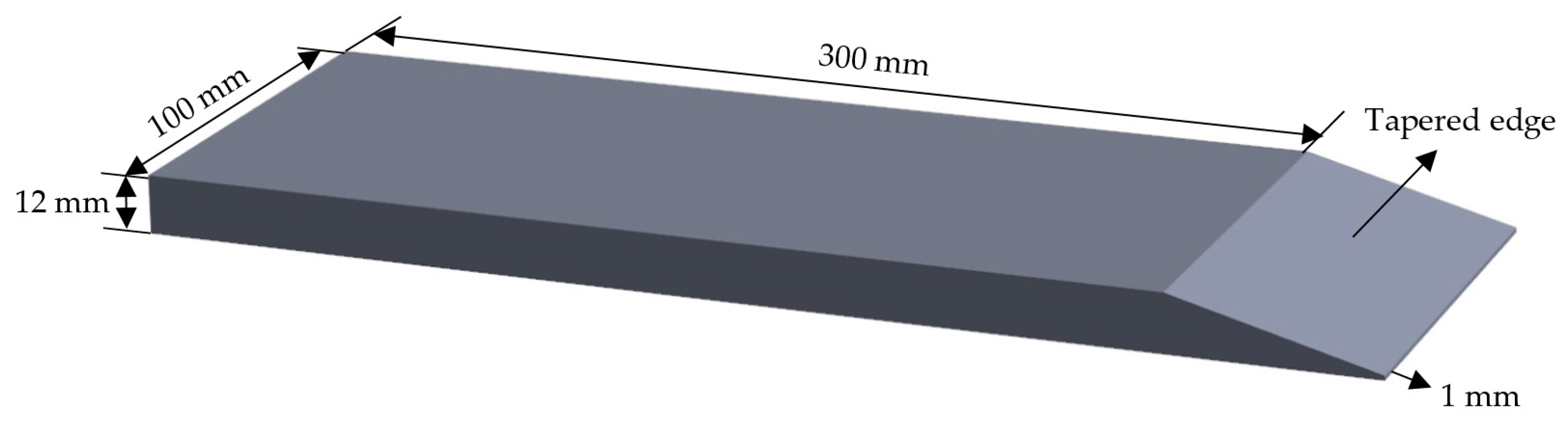
2.2. Hot Rolling Test
2.3. Characterisation Methods
3. Results
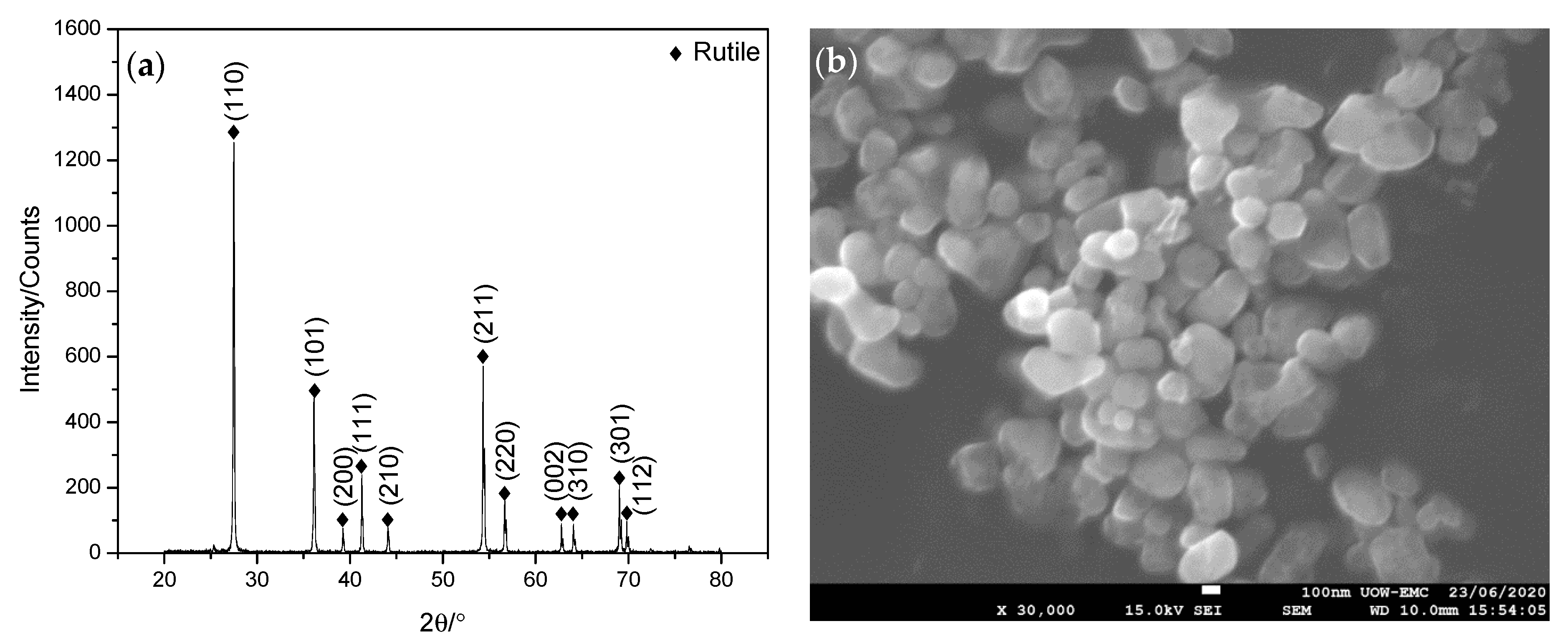
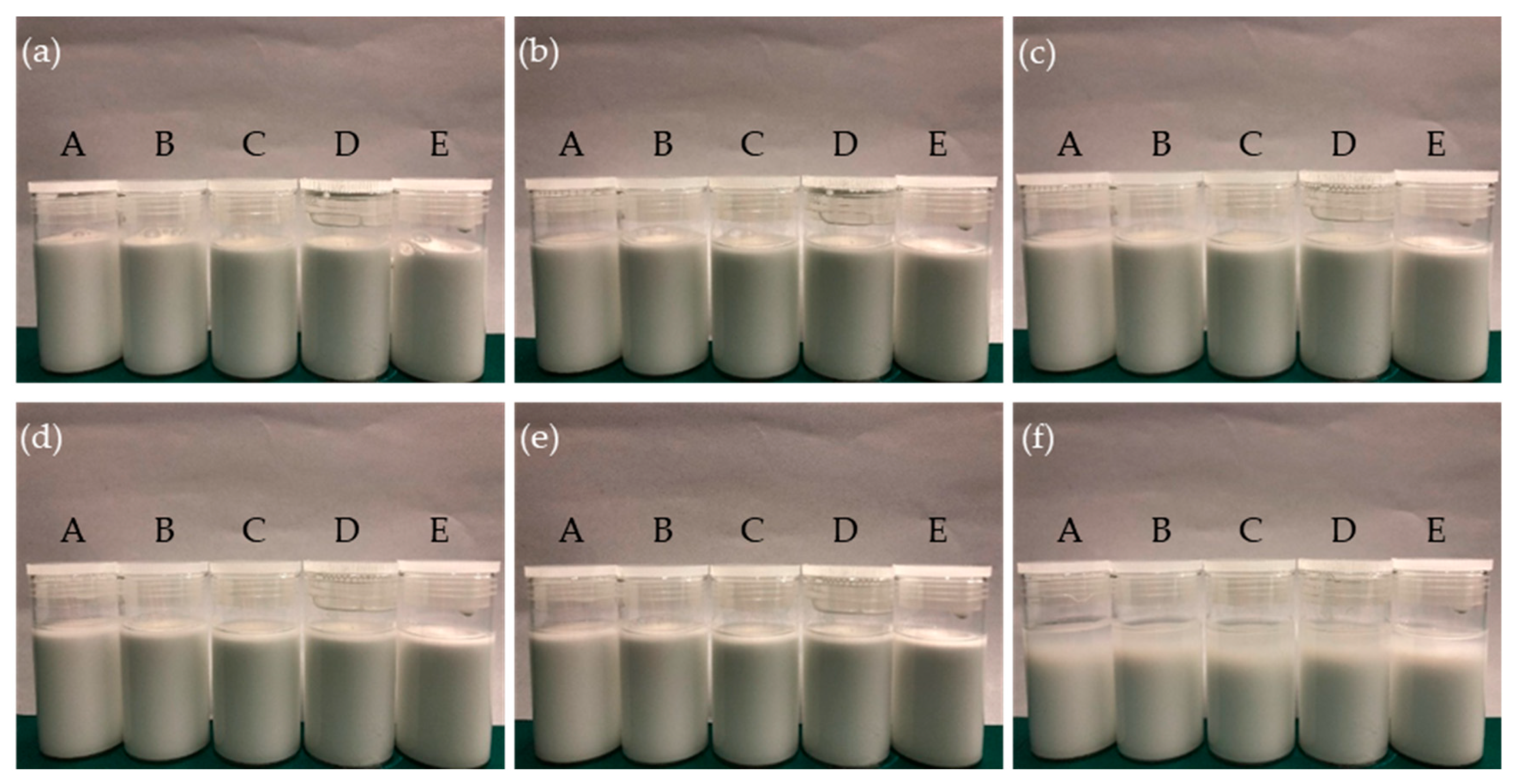
3.1. Characterisation of Nanolubricant
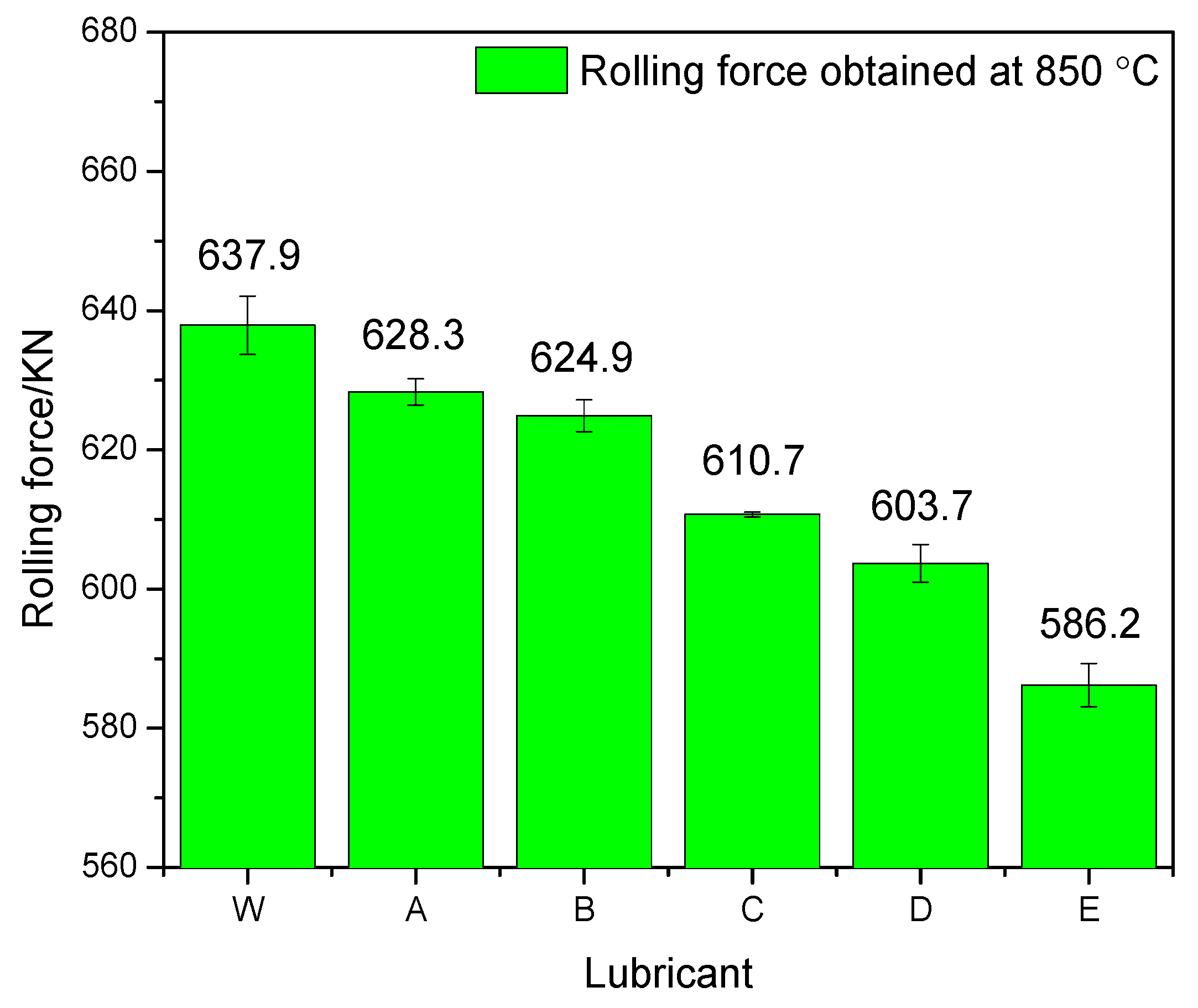
3.2. Rolling Force
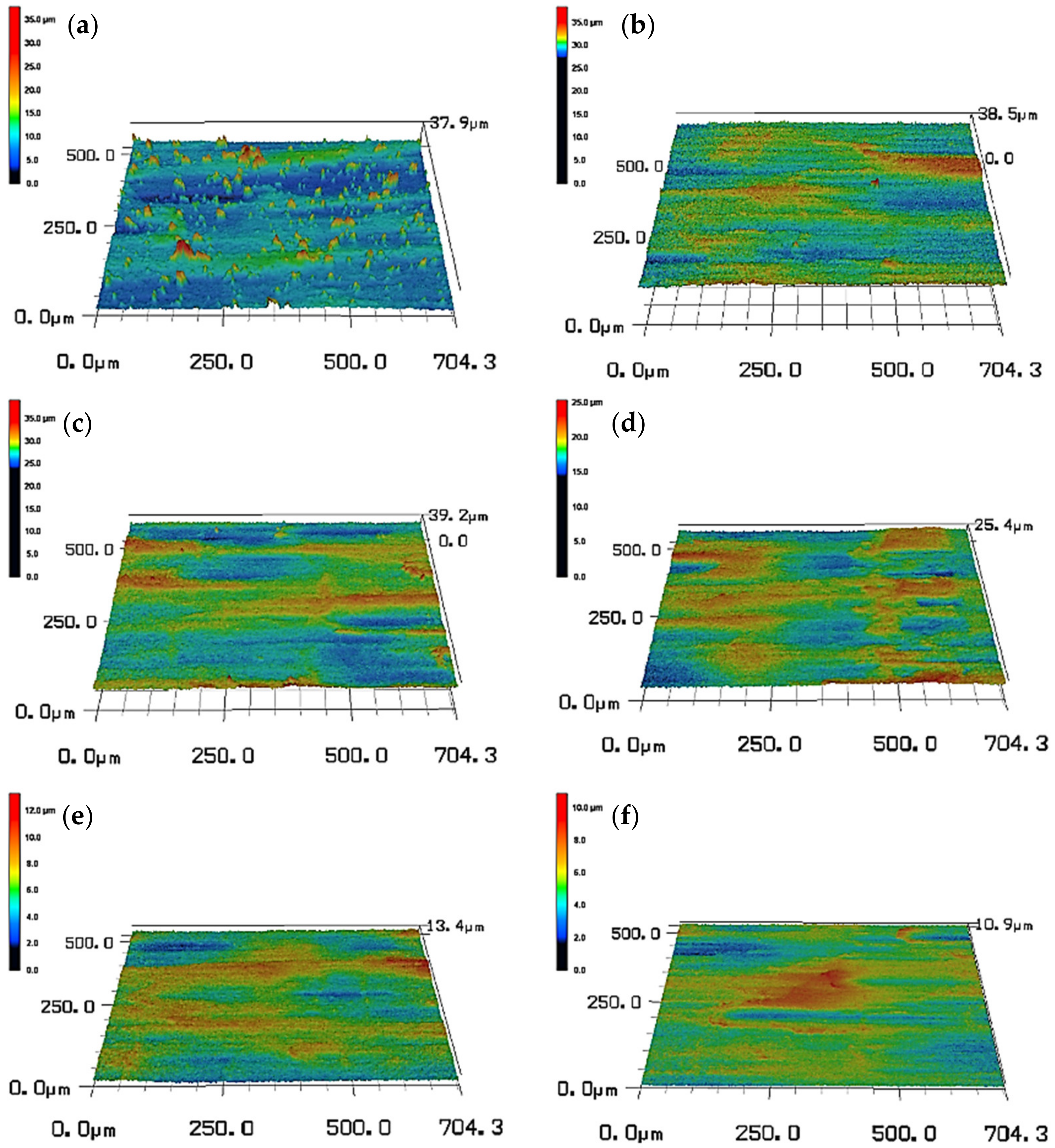
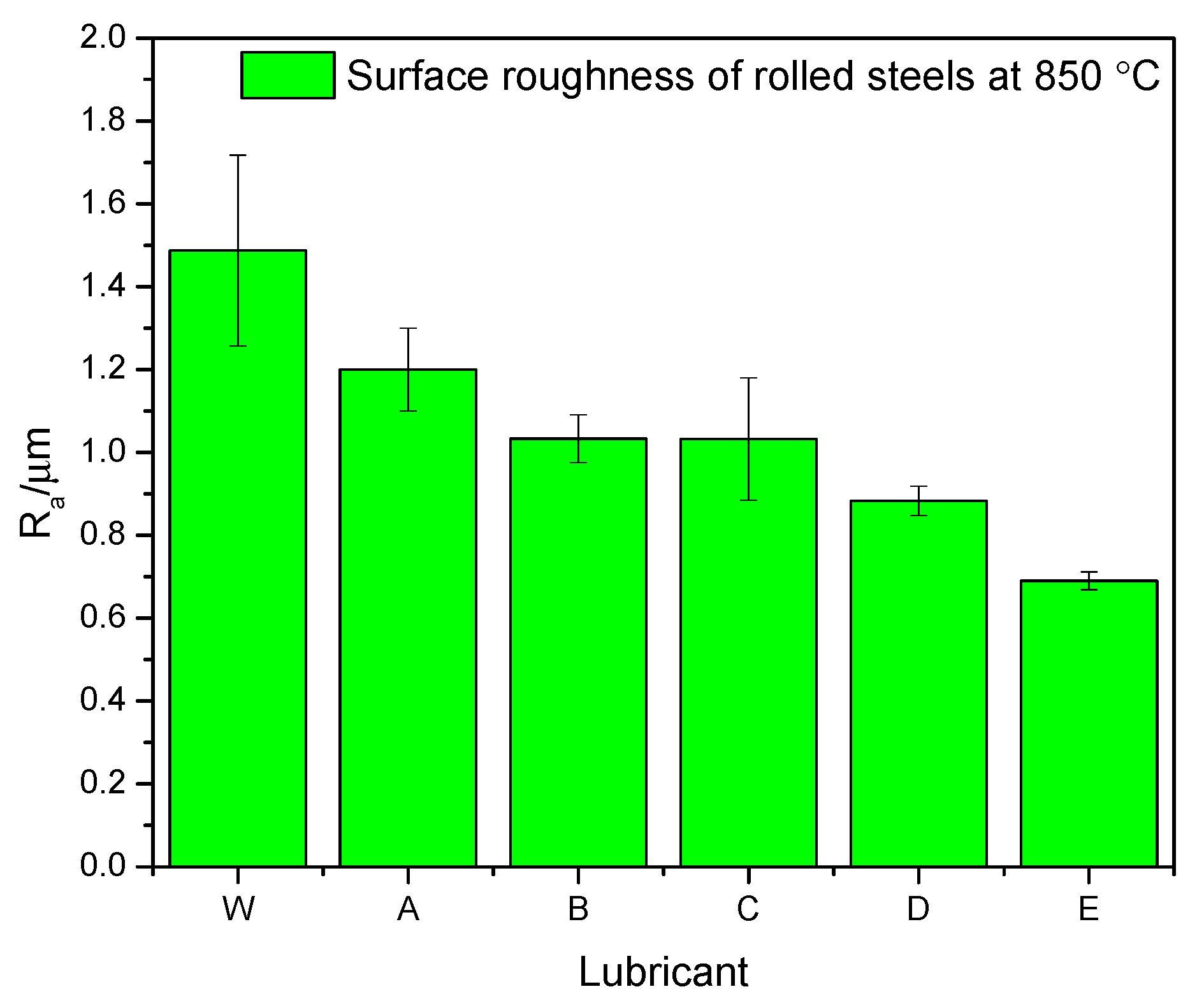
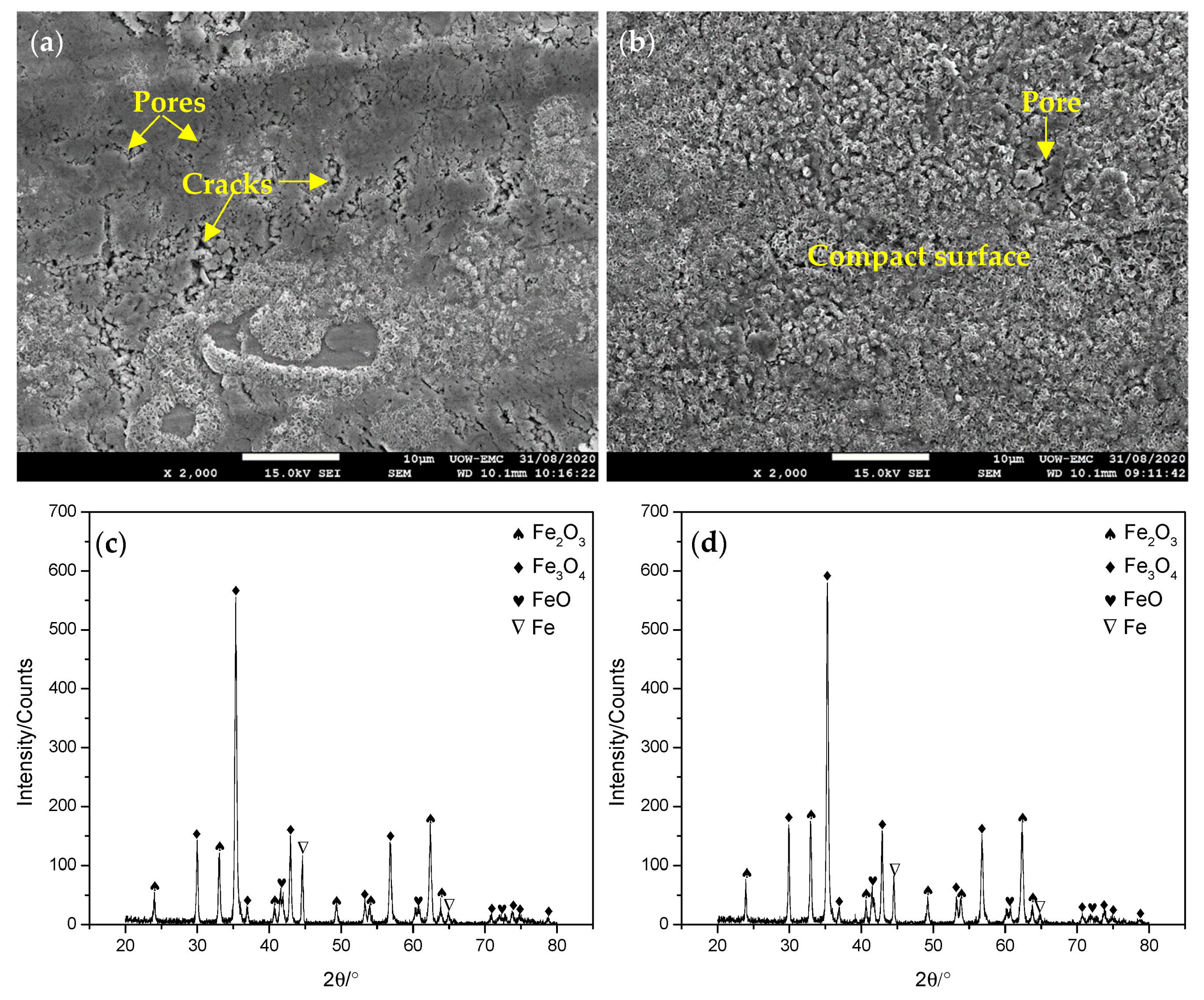
3.3. Surface Morphologies
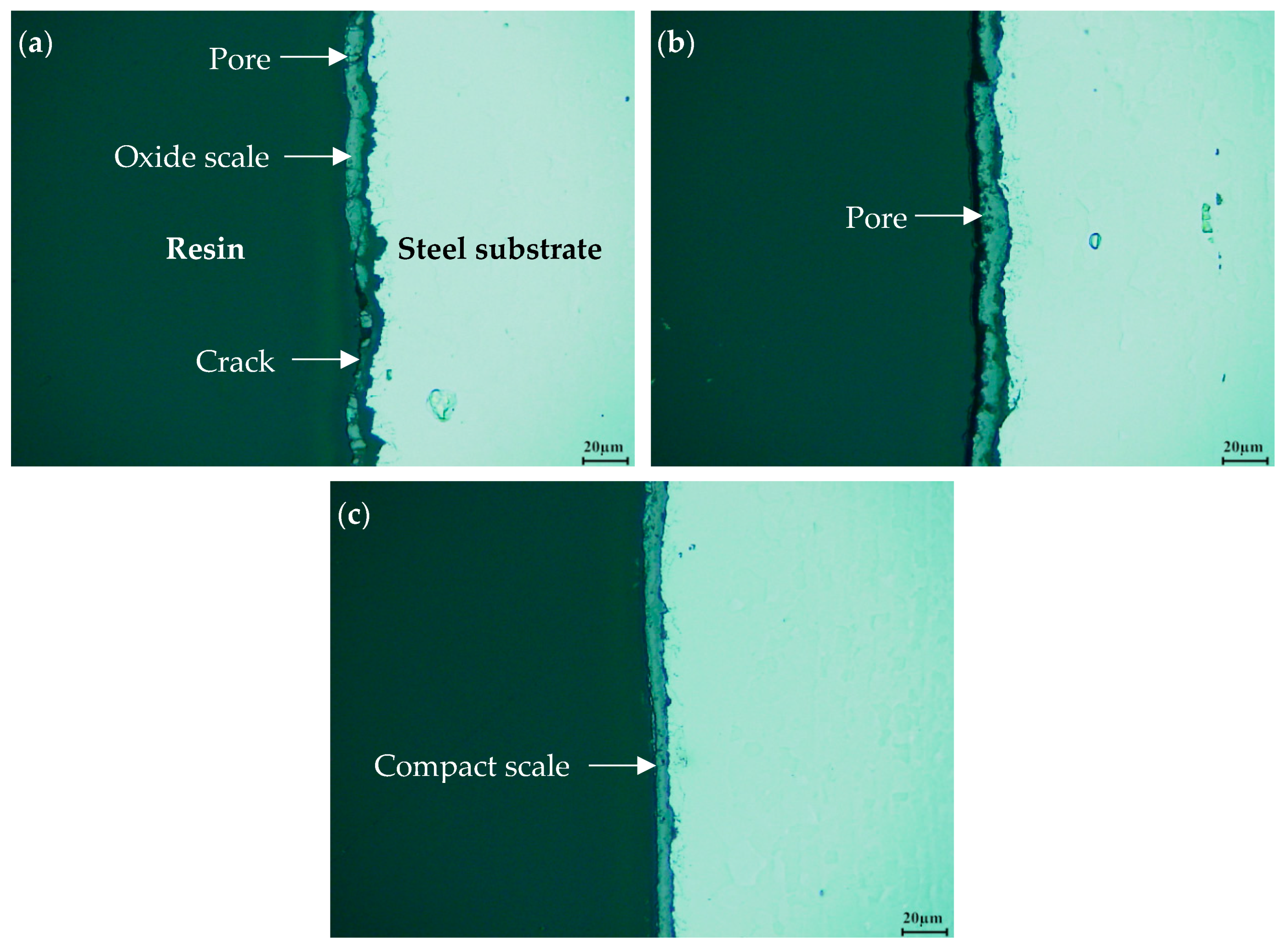
3.4. Thickness of Oxide Scale
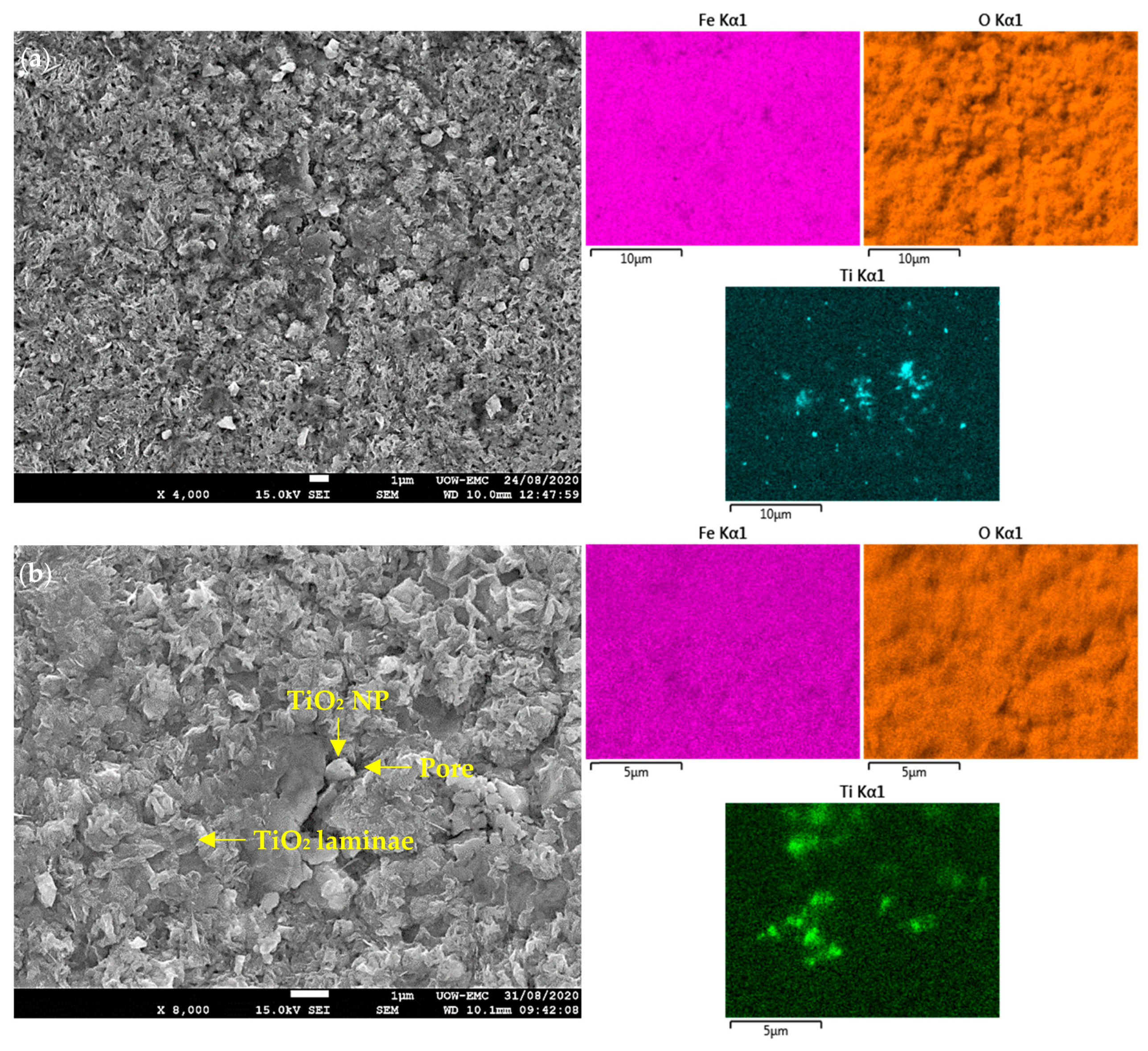
3.5. Surface Microstructure and Hardness
4. Discussion
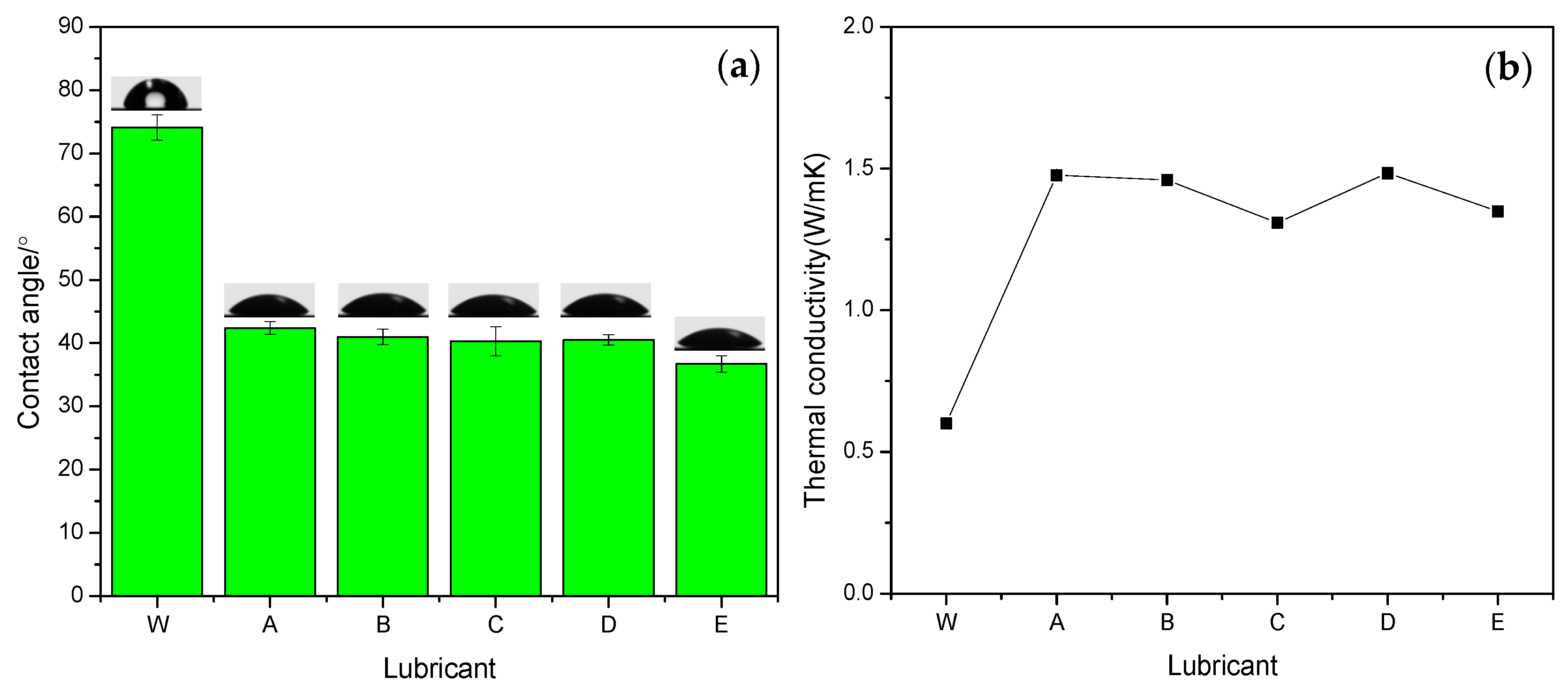
4.1. Wettability and Thermal Conductivity
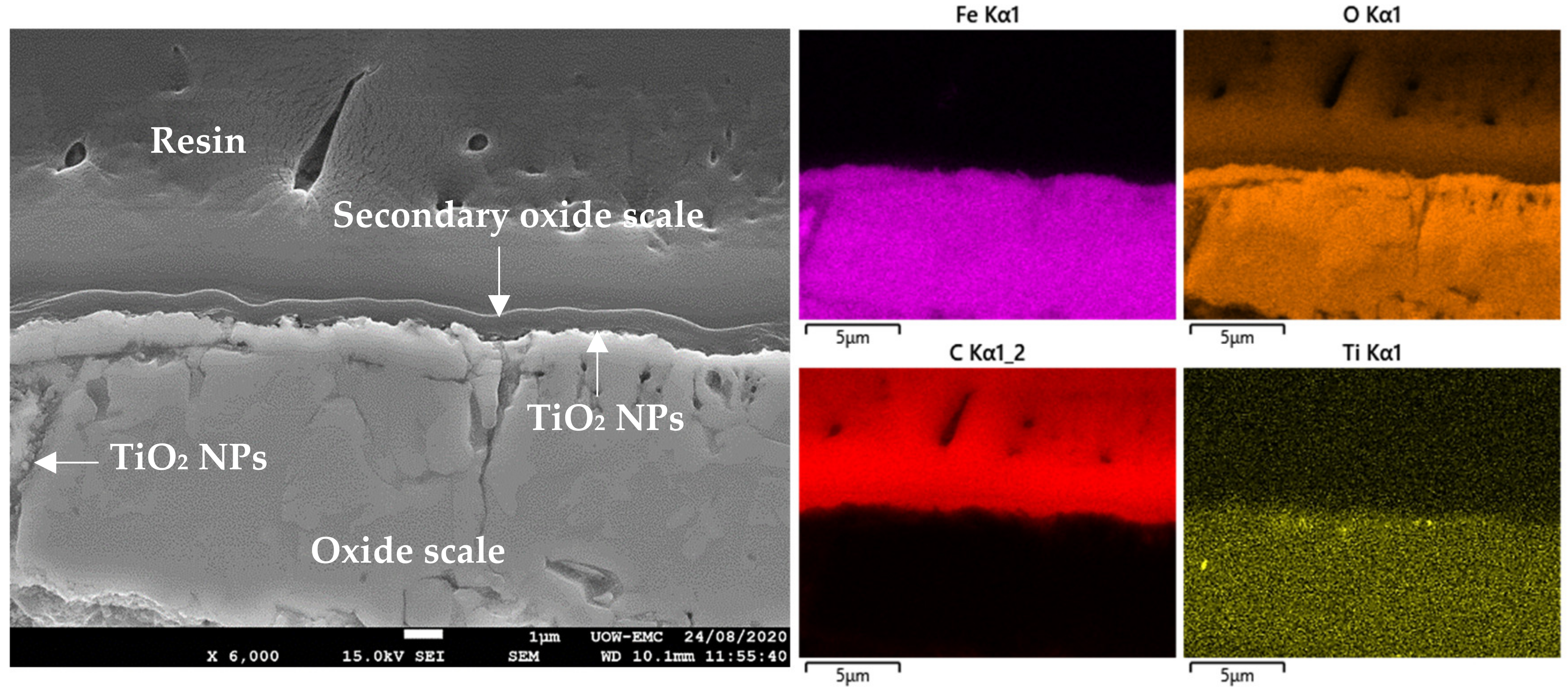
4.2. Analyses of Surface and Resin/Oxide Scale Interface
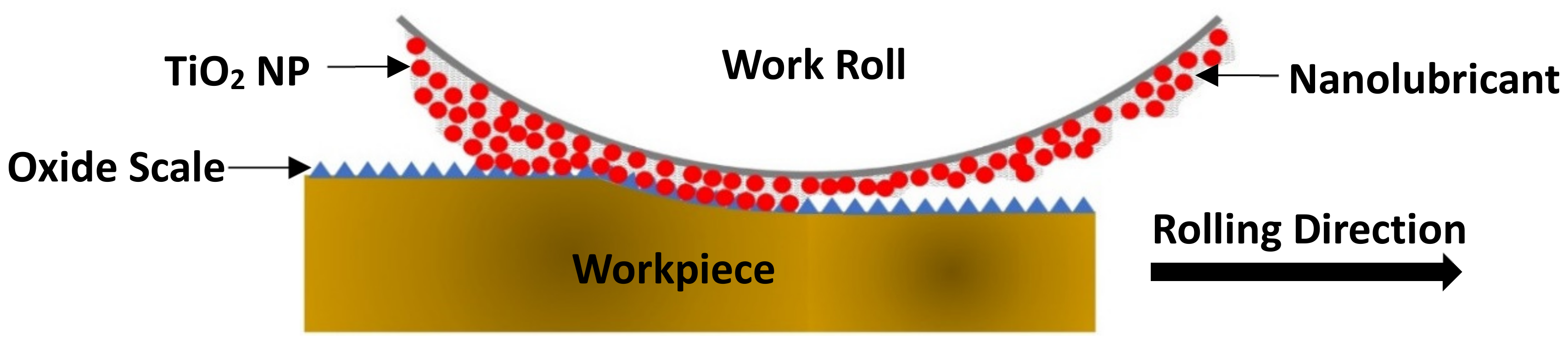
4.3. Lubrication Mechanisms
5. Conclusions
- (1)
- The as-synthesised eco-friendly water-based nanolubricants presented excellent dispersion stability, wettability and thermal conductivity.
- (2)
- The rolling force, surface roughness, and oxide scale thickness obtained under pure water condition would be decreased by up to 8.1%, 53.7% and 50%, respectively, by the use of the optimal nanolubricant (4 wt % TiO2 + 10 wt % glycerol + 0.2 wt % SDBS + 1 wt % Snailcool).
- (3)
- The use of the optimal nanolubricant tended to increase the surface hardness by 4.4%, compared to that of pure water.
- (4)
- The lubrication mechanisms of water-based nanolubricants were ascribed to the synergistic effect of ball bearing, laminae and mending of nano-TiO2.
- (5)
- The eco-friendly water-based nanolubricants can be obtained using much lower cost additives via a simpler synthesis process, compared to other eco-friendly lubricants.
- (6)
- The optimal water-based nanolubricant will be a promising candidate that can be used in industrial-scale hot steel rolling.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Jiang, Z. Thermomechanical processing of advanced high strength steels. Prog. Mater. Sci. 2018, 94, 174–242. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, J.; Sun, W.; Tieu, A.; Wei, D. Analysis of tribological feature of the oxide scale in hot strip rolling. Tribol. Int. 2010, 43, 1339–1345. [Google Scholar] [CrossRef]
- Matsubara, Y.; Hiruta, T.; Kimura, Y. Effect of Oil Film Thickness on Lubrication Property in Hot Rolling. ISIJ Int. 2015, 55, 632–636. [Google Scholar] [CrossRef]
- Azushima, A.; Xue, W.; Yoshida, Y. Influence of Lubricant Factors on Coefficient of Friction and Clarification of Lubrication Mechanism in Hot Rolling. ISIJ Int. 2009, 49, 868–873. [Google Scholar] [CrossRef]
- Shirizly, A.; Lenard, J.G. The effect of lubrication on mill loads during hot rolling of low carbon steel strips. J. Mater. Process. Technol. 2000, 97, 61–68. [Google Scholar] [CrossRef]
- Xia, W.; Zhao, J.; Wu, H.; Zhao, X.; Zhang, X.; Xu, J.; Jiao, S.; Wang, X.; Zhou, C.; Jiang, Z. Effects of oil-in-water based nanolubricant containing TiO2 nanoparticles in hot rolling of 304 stainless steel. J. Mater. Process. Technol. 2018, 262, 149–156. [Google Scholar] [CrossRef]
- Li, Y.-L.; Cao, J.-G.; Kong, N.; Wen, D.; Ma, H.-H.; Zhou, Y.-S. The effects of lubrication on profile and flatness control during ASR hot strip rolling. Int. J. Adv. Manuf. Technol. 2017, 91, 2725–2732. [Google Scholar] [CrossRef]
- Matsuoka, S.; Morita, M.; Furukimi, O.; Obara, T. Recrystallization and Related Phenomena. Effect of Lubrication Condition on Recrystallization Texture of Ultra-low C Sheet Steel Hot-rolled in Ferrite Region. ISIJ Int. 1998, 38, 633–639. [Google Scholar] [CrossRef]
- Barrett, C. Influence of lubrication on through thickness texture of ferritically hot rolled interstitial free steel. Ironmak. Steelmak. 1999, 26, 393–397. [Google Scholar] [CrossRef]
- Etou, M.; Fukushima, S.; Sasaki, T.; Haraguchi, Y.; Miyata, K.; Wakita, M.; Tomida, T.; Imai, N.; Yoshida, M.; Okada, Y. Super Short Interval Multi-pass Rolling Process for Ultrafine-grained Hot Strip. ISIJ Int. 2008, 48, 1142–1147. [Google Scholar] [CrossRef]
- Wen, P.; Lei, Y.; Li, W.; Fan, M. Synergy between Covalent Organic Frameworks and Surfactants to Promote Water-Based Lubrication and Corrosion Resistance. ACS Appl. Nano Mater. 2020, 3, 1400–1411. [Google Scholar] [CrossRef]
- Su, F.; Chen, G.; Huang, P. Lubricating performances of graphene oxide and onion-like carbon as water-based lubricant additives for smooth and sand-blasted steel discs. Friction 2020, 8, 47–57. [Google Scholar] [CrossRef]
- Chen, W.; Amann, T.; Kailer, A.; Rühe, J. Macroscopic Friction Studies of Alkylglucopyranosides as Additives for Water-Based Lubricants. Lubricants 2020, 8, 11. [Google Scholar] [CrossRef]
- He, A.; Huang, S.; Yun, J.-H.; Jiang, Z.; Stokes, J.R.; Jiao, S.; Wang, L.; Huang, H. Tribological Characteristics of Aqueous Graphene Oxide, Graphitic Carbon Nitride, and Their Mixed Suspensions. Tribol. Lett. 2018, 66, 42. [Google Scholar] [CrossRef]
- He, A.; Huang, S.; Yun, J.-H.; Jiang, Z.; Stokes, J.R.; Jiao, S.; Wang, L.; Huang, H. The pH-dependent structural and tribological behaviour of aqueous graphene oxide suspensions. Tribol. Int. 2017, 116, 460–469. [Google Scholar] [CrossRef]
- Huo, M.; Wu, H.; Xie, H.; Zhao, J.; Su, G.; Jia, F.; Li, Z.; Lin, F.; Li, S.; Zhang, H.; et al. Understanding the role of water-based nanolubricants in micro flexible rolling of aluminium. Tribol. Int. 2020, 151, 106378. [Google Scholar] [CrossRef]
- He, J.; Sun, J.; Meng, Y.; Pei, Y. Superior lubrication performance of MoS2-Al2O3 composite nanofluid in strips hot rolling. J. Manuf. Process. 2020, 57, 312–323. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; Wu, P.; Dong, C.; Yan, X. The Role of Nano-TiO2 Lubricating Fluid on the Hot Rolled Surface and Metallographic Structure of SS41 Steel. Nanomaterials 2018, 8, 111. [Google Scholar] [CrossRef]
- Bao, Y.; Sun, J.; Kong, L. Effects of nano-SiO2 as water-based lubricant additive on surface qualities of strips after hot rolling. Tribol. Int. 2017, 114, 257–263. [Google Scholar] [CrossRef]
- Kong, L.; Sun, J.; Bao, Y.; Meng, Y. Effect of TiO2 nanoparticles on wettability and tribological performance of aqueous suspension. Wear 2017, 376-377, 786–791. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, M.M. Experimental investigation of flank wear in end milling of aluminum alloy with water-based TiO2 nanofluid lubricant in minimum quantity lubrication technique. Int. J. Adv. Manuf. Technol. 2016, 86, 2527–2537. [Google Scholar] [CrossRef]
- Ohenoja, K.; Saari, J.; Illikainen, M.; Niinimäki, J. Effect of molecular weight of sodium polyacrylates on the particle size distribution and stability of a TiO2 suspension in aqueous stirred media milling. Powder Technol. 2014, 262, 188–193. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, X.; Liu, Y.; Lv, Y. Preparation and Tribological Properties of Dual-Coated TiO2 Nanoparticles as Water-Based Lubricant Additives. J. Nanomater. 2014, 2014, 2. [Google Scholar] [CrossRef]
- Wu, H.; Jia, F.; Zhao, J.; Huang, S.; Wang, L.; Jiao, S.; Huang, H.; Jiang, Z. Effect of water-based nanolubricant containing nano-TiO2 on friction and wear behaviour of chrome steel at ambient and elevated temperatures. Wear 2019, 426, 792–804. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, J.; Cheng, X.; Xia, W.; He, A.; Yun, J.-H.; Huang, S.; Wang, L.; Huang, H.; Jiao, S.; et al. Friction and wear characteristics of TiO2 nano-additive water-based lubricant on ferritic stainless steel. Tribol. Int. 2018, 117, 24–38. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, J.; Xia, W.; Cheng, X.; He, A.; Yun, J.-H.; Wang, L.; Huang, H.; Jiao, S.; Huang, L.; et al. A study of the tribological behaviour of TiO2 nano-additive water-based lubricants. Tribol. Int. 2017, 109, 398–408. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, J.; Xia, W.; Cheng, X.; He, A.; Yun, J.H.; Wang, L.; Huang, H.; Jiao, S.; Huang, L.; et al. Analysis of TiO2 nano-additive water-based lubricants in hot rolling of microalloyed steel. J. Manuf. Process. 2017, 27, 26–36. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, J.; Luo, L.; Huang, S.; Wang, L.; Zhang, S.; Jiao, S.; Huang, H.; Jiang, Z. Performance Evaluation and Lubrication Mechanism of Water-Based Nanolubricants Containing Nano-TiO2 in Hot Steel Rolling. Lubricants 2018, 6, 57. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, C.; Zhang, J.; Huang, S.; Wang, L.; Jiao, S.; Huang, H.; Jiang, Z. Oxidation Behaviour of Steel During hot Rolling by Using TiO2-Containing Water-Based Nanolubricant. Oxid. Met. 2019, 92, 315–335. [Google Scholar] [CrossRef]
- Wu, H.; Jia, F.; Li, Z.; Lin, F.; Huo, M.; Huang, S.; Sayyar, S.; Jiao, S.; Huang, H.; Jiang, Z. Novel water-based nanolubricant with superior tribological performance in hot steel rolling. Int. J. Extreme Manuf. 2020, 2, 025002. [Google Scholar] [CrossRef]
- Hanaor, D.; Assadi, M.H.N.; Li, S.; Yu, A.; Sorrell, C.C. Ab initio study of phase stability in doped TiO2. Comput. Mech. 2012, 50, 185–194. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Xia, W.; Zhao, J.; Wu, H.; Zhao, X.; Zhang, X.; Xu, J.; Hee, A.C.; Jiang, Z. Effects of Nano-TiO2 Additive in Oil-in-Water Lubricant on Contact Angle and Antiscratch Behavior. Tribol. Trans. 2017, 60, 362–372. [Google Scholar] [CrossRef]
- Anderson, W. Wettability Literature Survey—Part 2: Wettability Measurement. J. Pet. Technol. 1986, 38, 1246–1262. [Google Scholar] [CrossRef]
- Özerinç, S.; Kakaç, S.; Yazıcıoğlu, A.G. Enhanced thermal conductivity of nanofluids: A state-of-the-art review. Microfluid. Nanofluidics 2010, 8, 145–170. [Google Scholar] [CrossRef]
- Cannio, M.; Ponzoni, C.; Gualtieri, A.F.; Lugli, E.; Leonelli, C.; Romagnoli, M. Stabilization and thermal conductivity of aqueous magnetite nanofluid from continuous flows hydrothermal microwave synthesis. Mater. Lett. 2016, 173, 195–198. [Google Scholar] [CrossRef]
- Kedzierski, M.; Brignoli, R.; Quine, K.; Brown, J. Viscosity, density, and thermal conductivity of aluminum oxide and zinc oxide nanolubricants. Int. J. Refrig. 2017, 74, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Warrier, P.; Teja, A. Effect of particle size on the thermal conductivity of nanofluids containing metallic nanoparticles. Nanoscale Res. Lett. 2011, 6, 247. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Zabihi, F. Thermal conductivity studies of novel nanofluids based on metallic silver decorated mesoporous silica nanoparticles. Mater. Res. Bull. 2013, 48, 4150–4156. [Google Scholar] [CrossRef]
- Mishra, S.; Nayak, M.; Misra, A. Thermal Conductivity of Nanofluids-A Comprehensive Review. Int. J. Thermofluid Sci. Technol. 2020, 7, 7. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, P.C.; Chaudhuri, P. Enhancing Thermo-Economic Performance of TiO2-Water Nanofluids: An Experimental Investigation. JOM 2020, 1–10. [Google Scholar] [CrossRef]
- Wu, Y.-X.; Fu, J.-X.; Zhang, H.; Xu, J.; Zhai, Q. Effects of Rolling and Cooling Conditions on Microstructure of Umbrella-Bone Steel. High Temp. Mater. Process. 2017, 36, 947–953. [Google Scholar] [CrossRef]
- Basabe, V.V.; Szpunar, J.A. Growth Rate and Phase Composition of Oxide Scales during Hot Rolling of Low Carbon Steel. ISIJ Int. 2004, 44, 1554–1559. [Google Scholar] [CrossRef]
- Chen, R. Review of the High-Temperature Oxidation of Iron and Carbon Steels in Air or Oxygen. Oxid. Met. 2003, 59, 433–468. [Google Scholar] [CrossRef]
- Hayashi, S.; Mizumoto, K.; Yoneda, S.; Kondo, Y.; Tanei, H.; Ukai, S. The Mechanism of Phase Transformation in Thermally-Grown FeO Scale Formed on Pure-Fe in Air. Oxid. Met. 2014, 81, 357–371. [Google Scholar] [CrossRef]

| Authors | NP Phase | NP Size | Dispersants | Application |
|---|---|---|---|---|
| Meng et al. [18] | Anatase | 90 nm | Glycerin, Triethanolamine (TEA), Sodium polyacrylate (PAAS), Sodium hexametaphosphate (SHMP) etc. | Hot rolling |
| Kong et al. [20] | Anatase | 20 nm | PAAS, SHMP | Tribology |
| Najiha et al. [21] | N/A | 40 nm | Confidential | End milling |
| Ohenoja et al. [22] | Rutile | 200 nm | PAAS | Grinding |
| Gu et al. [23] | Anatase | 20 nm | Silane coupling agent, OP-10 | Drilling |
| Lubrication Type | Description |
|---|---|
| W | Water |
| A | 2 wt % TiO2 + 0.1 wt % SDBS + 1 wt % Snailcool |
| B | 2 wt % TiO2 + 0.2 wt % SDBS + 1 wt % Snailcool |
| C | 2 wt % TiO2 + 10 wt % glycerol + 0.2 wt % SDBS + 1 wt % Snailcool |
| D | 4 wt % TiO2 + 0.2 wt % SDBS + 1 wt % Snailcool |
| E | 4 wt % TiO2 + 10 wt % glycerol + 0.2 wt % SDBS + 1 wt % Snailcool |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Kamali, H.; Huo, M.; Lin, F.; Huang, S.; Huang, H.; Jiao, S.; Xing, Z.; Jiang, Z. Eco-Friendly Water-Based Nanolubricants for Industrial-Scale Hot Steel Rolling. Lubricants 2020, 8, 96. https://doi.org/10.3390/lubricants8110096
Wu H, Kamali H, Huo M, Lin F, Huang S, Huang H, Jiao S, Xing Z, Jiang Z. Eco-Friendly Water-Based Nanolubricants for Industrial-Scale Hot Steel Rolling. Lubricants. 2020; 8(11):96. https://doi.org/10.3390/lubricants8110096
Chicago/Turabian StyleWu, Hui, Hamidreza Kamali, Mingshuai Huo, Fei Lin, Shuiquan Huang, Han Huang, Sihai Jiao, Zhao Xing, and Zhengyi Jiang. 2020. "Eco-Friendly Water-Based Nanolubricants for Industrial-Scale Hot Steel Rolling" Lubricants 8, no. 11: 96. https://doi.org/10.3390/lubricants8110096
APA StyleWu, H., Kamali, H., Huo, M., Lin, F., Huang, S., Huang, H., Jiao, S., Xing, Z., & Jiang, Z. (2020). Eco-Friendly Water-Based Nanolubricants for Industrial-Scale Hot Steel Rolling. Lubricants, 8(11), 96. https://doi.org/10.3390/lubricants8110096









