Biobased Lubricating Oil Prepared from Ethyl Cellulose/Montmorillonite Additives and Waste Cooking Oil
Abstract
1. Introduction
| Type | Ingredient | Characteristic |
|---|---|---|
| Synthetic base oil | Synthetic base stocks such as poly-α-olefin, synthetic esters, polyethers, fluorosilicones, and phosphate esters. | Key Properties: excellent viscosity index, superior low-temperature fluidity (low pour point), high flash point for safety, minimal volatility, oxidation stability and so on; Drawbacks: poor economic performance, elaborate process required. |
| Mineral oil base oil | Elevated boiling point HMW hydrocarbons, non-hydrocarbon constituents, and related compounds. | Key Properties: consistent performance, broad applicability, and cost-effective manufacturing; Drawbacks: finite, and great harm to the environment. |
| Plant oil base oil [24] | Botanical lipids and their modified constituents. Unrefined plant oils and refined vegetable oil products. | Key Properties: renewable, easy to degrade, low environmental harm, elevated flash point, exceptional lubricity, and minimal evaporation loss; Drawbacks: exhibits a limited kinematic viscosity range and demonstrates poor resistance to oxidation. |
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Methods
2.2.1. Waste Oil Lipid Pretreatment
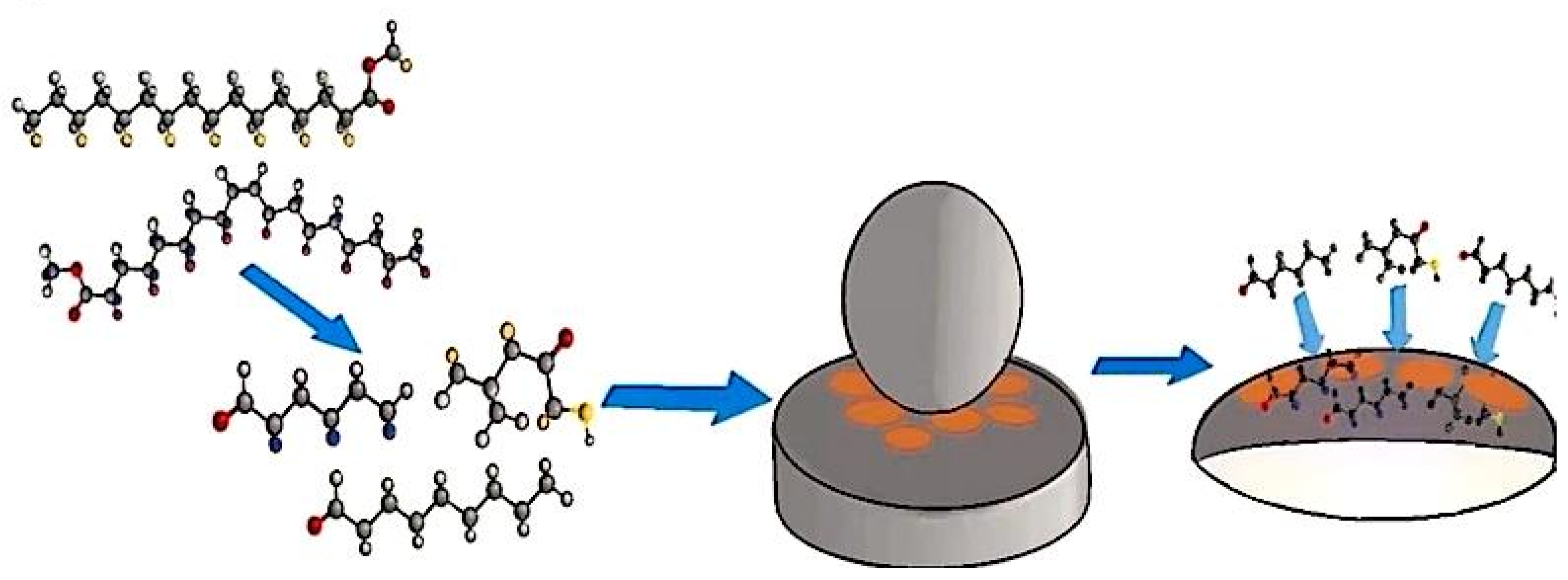
2.2.2. Preparation of CTAB-MMT/EC Additive
2.2.3. Preparation of Bio-Based Lubricating Oil Through CTAB-MMT/KH560-EC Modification of Waste Oil
2.3. Determination of Physical and Chemical Properties of Waste Oil
- A is the absorbance of waste oil, and
- A1 is the absorbance of waste oil after pretreatment.
2.4. Structural Performance Characterization of CTAB-MMT/KH560-EC
Fourier-Transform Infrared (FTIR) Spectroscopy
2.5. Measurement of Rheological Properties of Lubricating Oil
2.5.1. Analysis of Lubricating Oil Kinematic Viscosity
2.5.2. Determine the Viscosity Index
- U represents the kinematic viscosity of the sample at 40 °C, and the unit is mm2/s;
- Y represents the kinematic viscosity of the sample at 100 °C, and the unit is mm2/s.
2.5.3. Characterization of the Freezing Point Behavior of Lubricating Oils
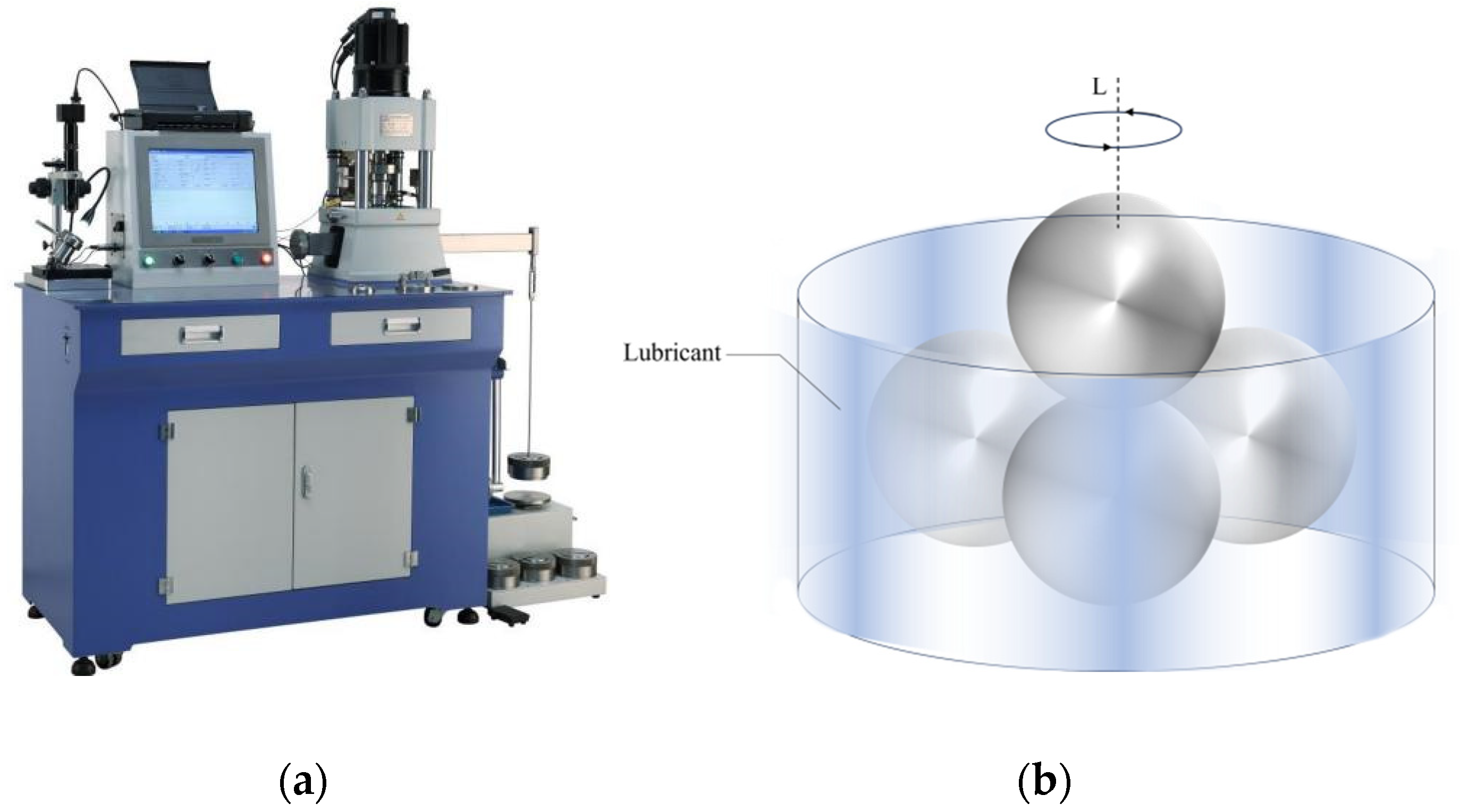
2.5.4. Friction Wear Test
3. Results and Discussion
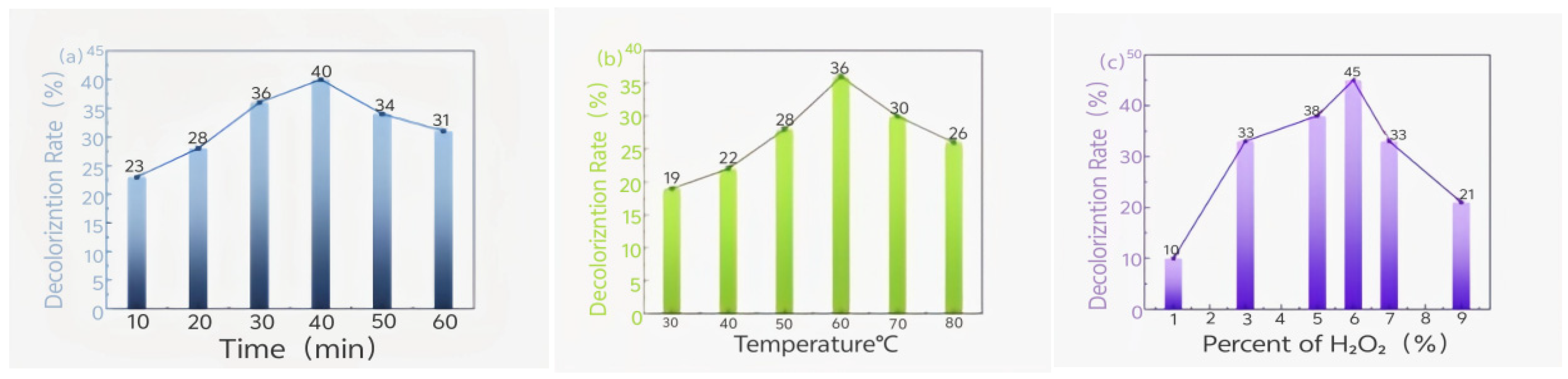
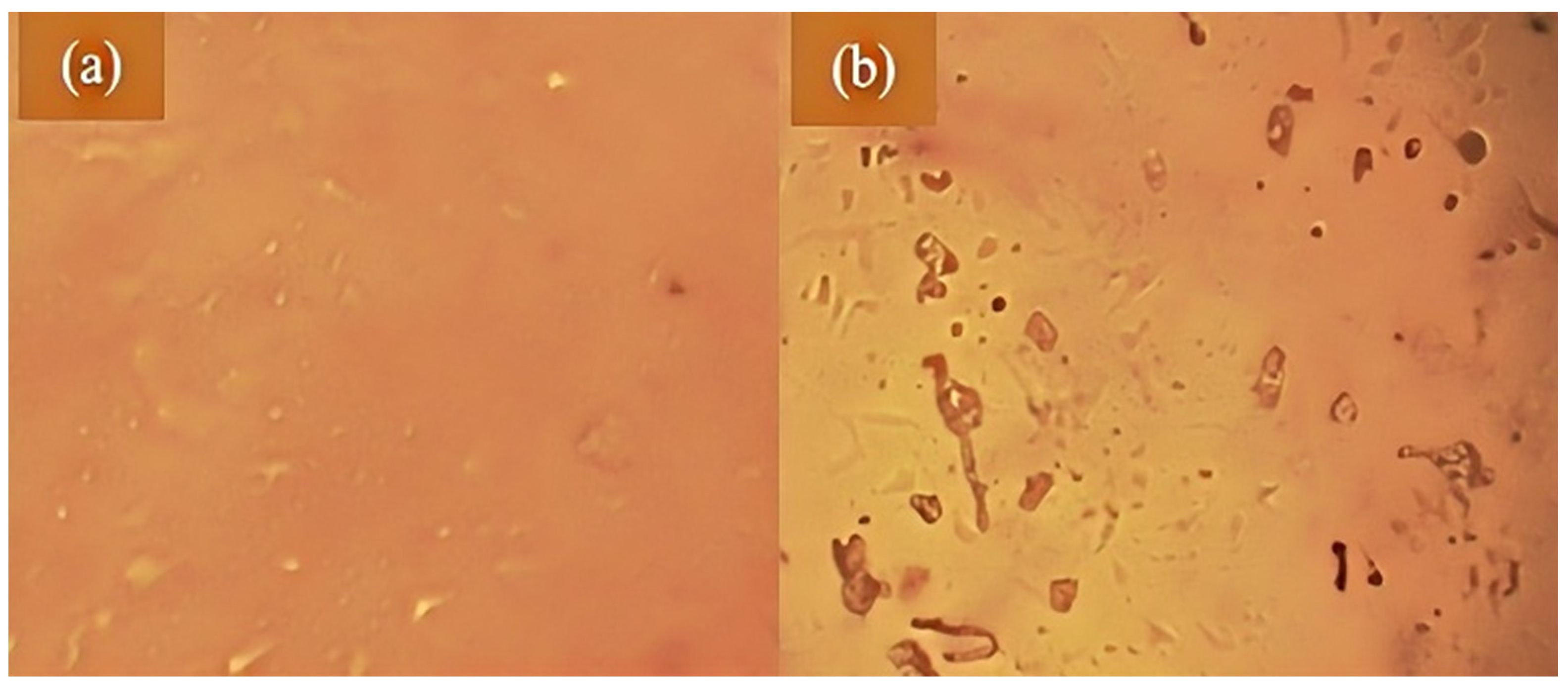
3.1. Effect of Hydrogen Peroxide on the Pretreatment of Waste Oil
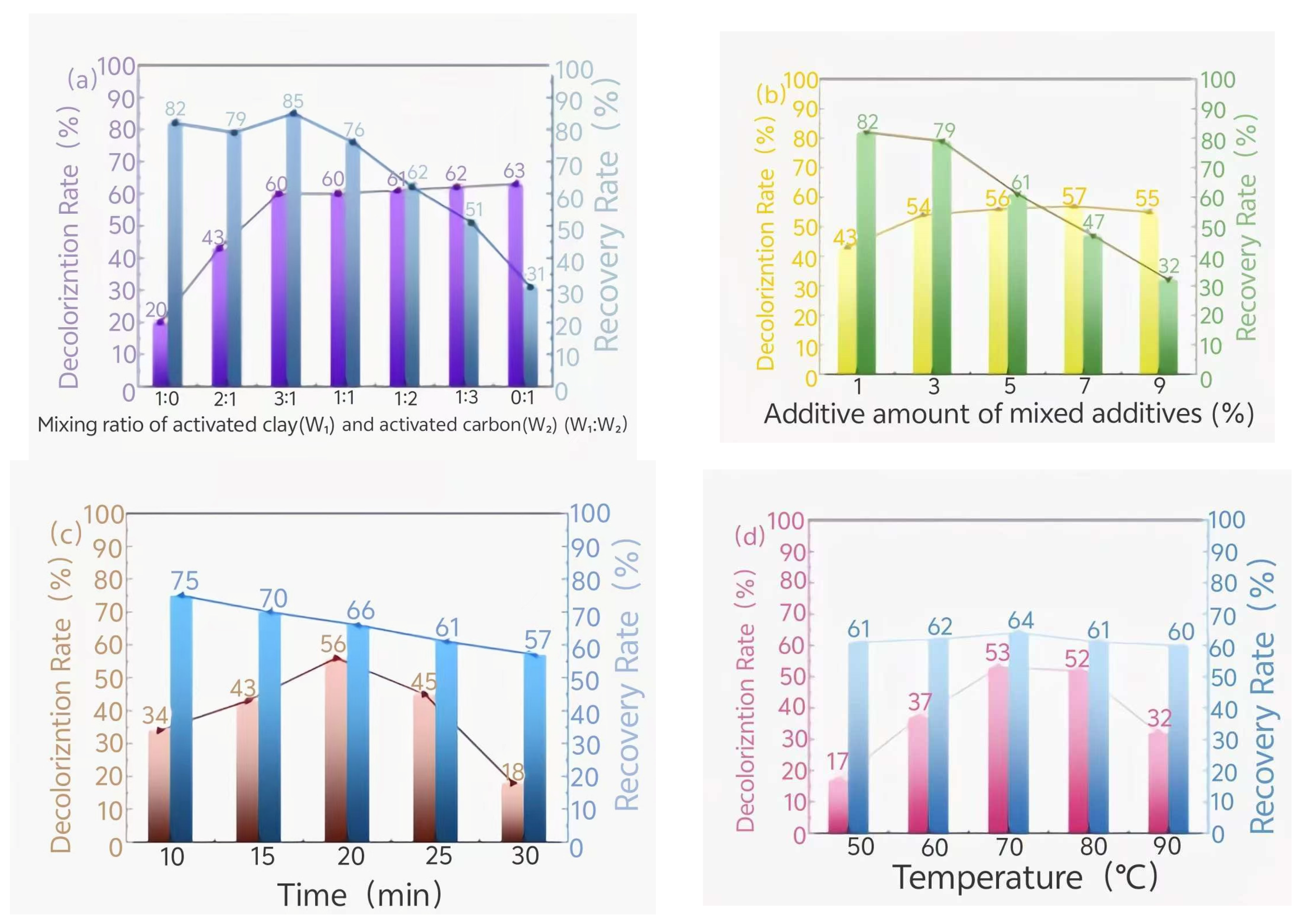
3.2. Influence of Activated Clay and Activated Carbon on the Treatment Effect of Waste Oil
3.3. Comparison of Main Indexes Before and After Waste Oil Treatment
3.4. Structural Characterization of CTAB-MMT/EC Composites
3.4.1. FTIR Characterization
3.4.2. Scanning Electron Microscope Analysis
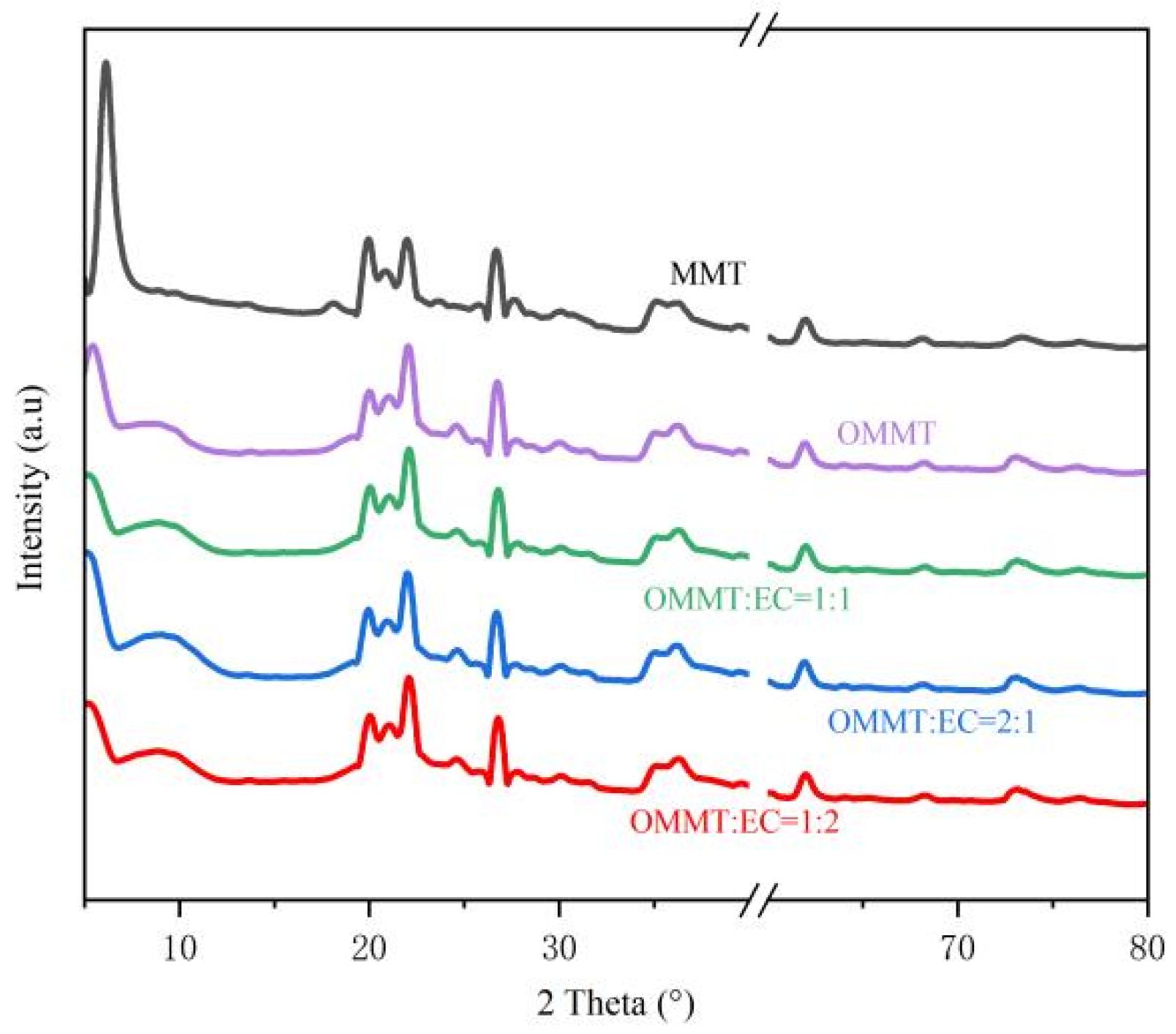
3.4.3. XRD Analysis
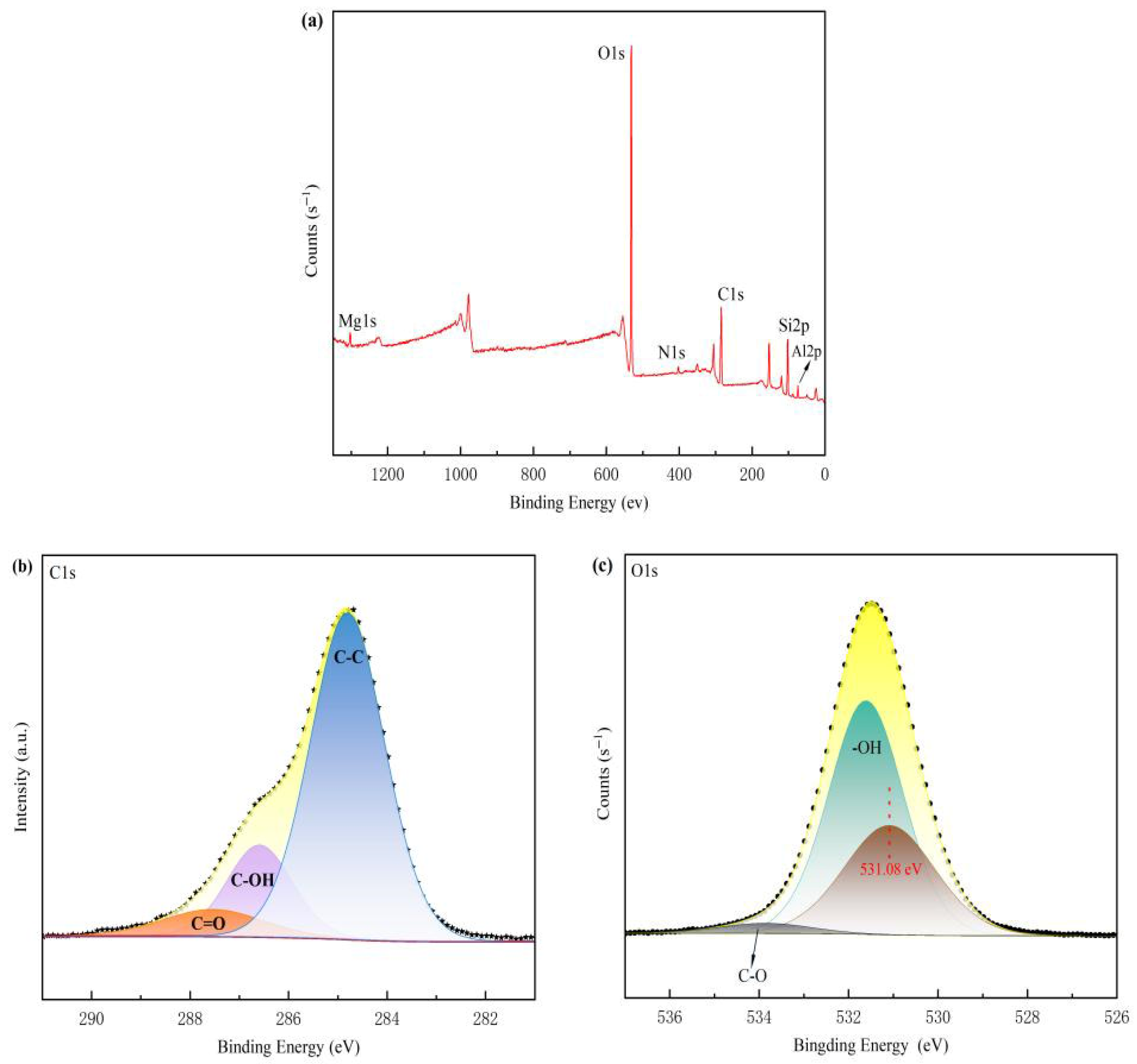
3.4.4. XPS Analysis
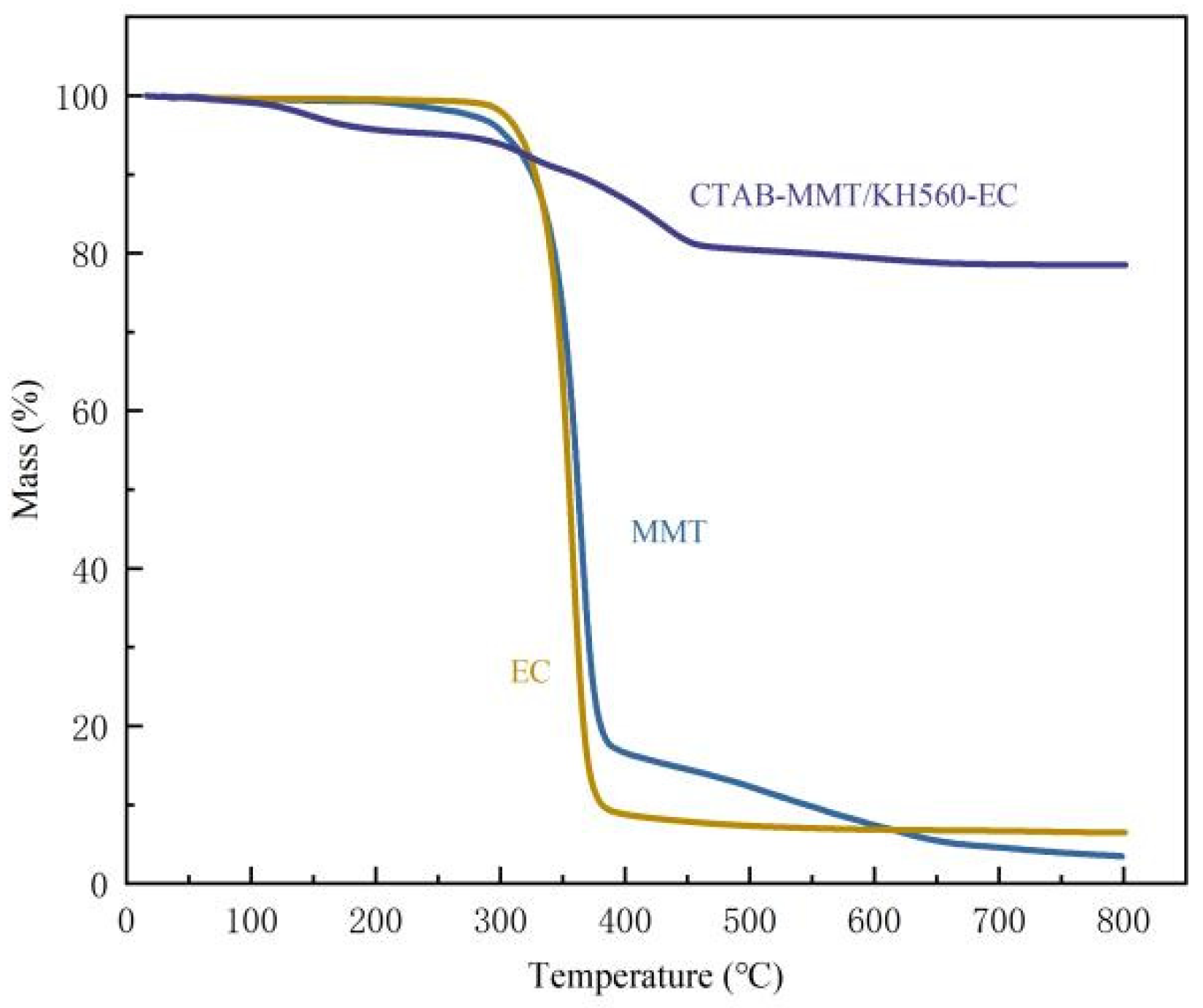
3.4.5. TG Analysis
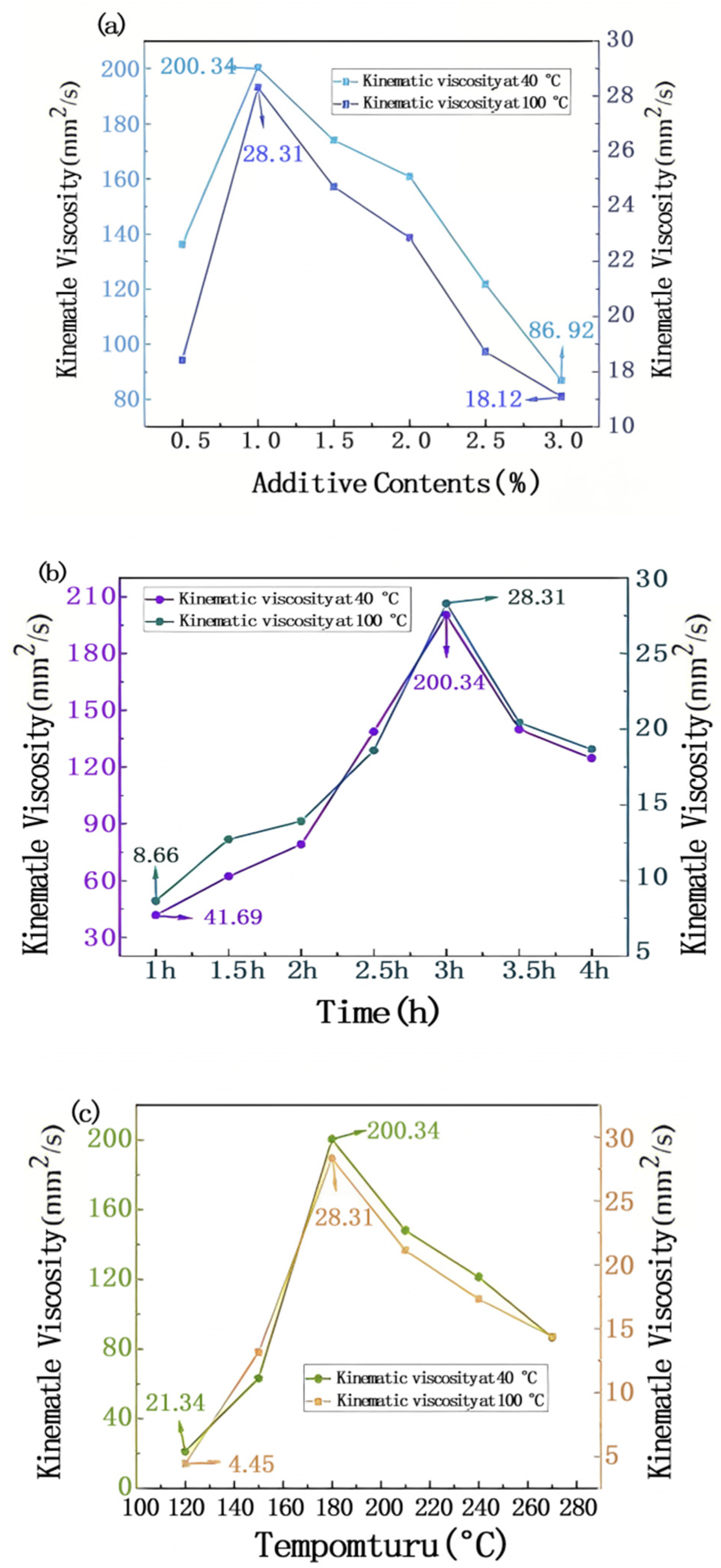
3.5. Determination of Kinematic Viscosity
3.5.1. Analysis of Rheological Properties of Lubricating Fluids
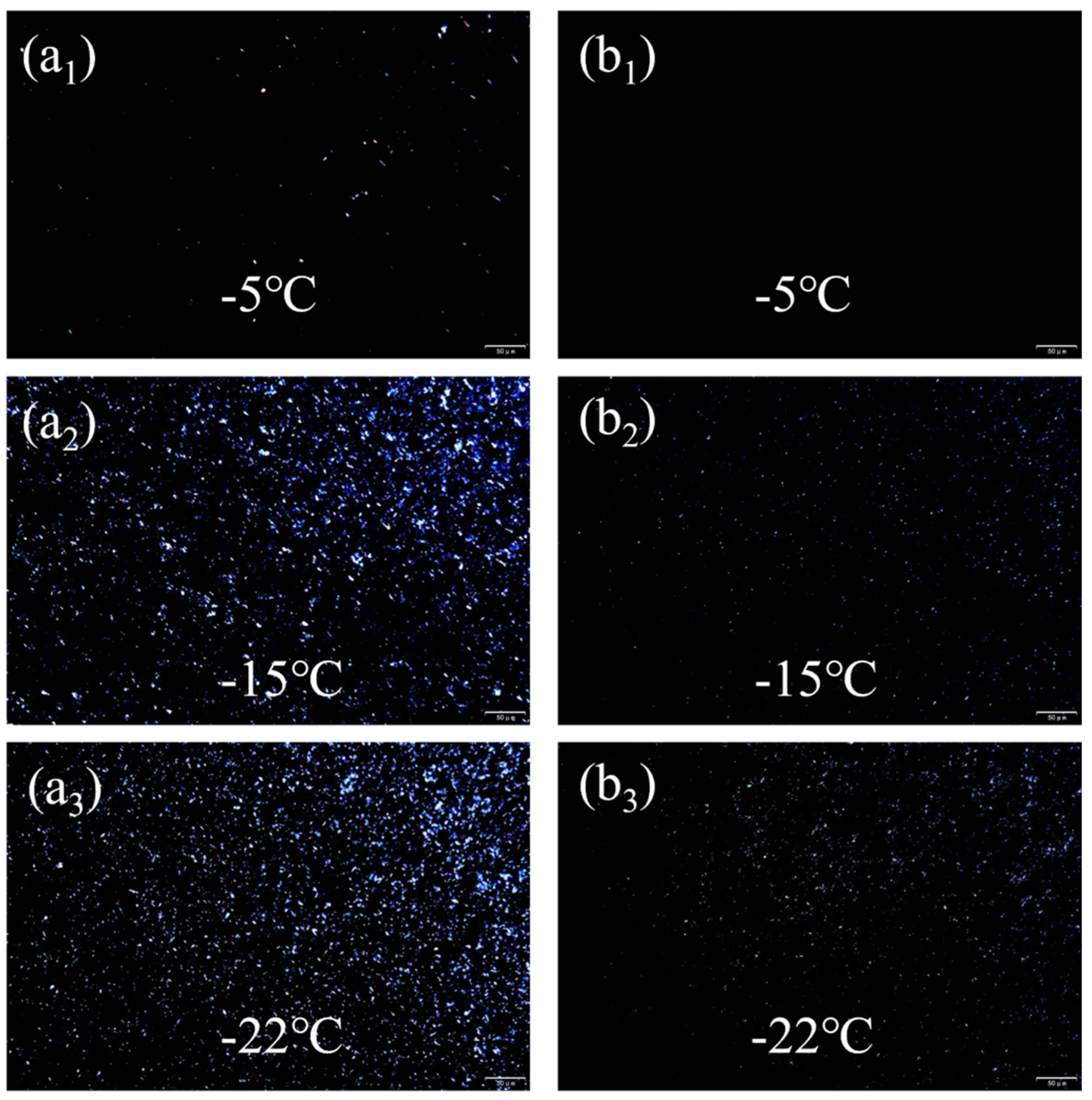
3.5.2. Lubricating Oil Freezing Point Analysis
3.5.3. Friction and Wear Performance of Lubricating Oil
Lubricity Property
Wear Resistance
3.5.4. Thermal Weight Analysis of Lubricating Oil
3.5.5. Rheological Property Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Chen, H.; Ding, M.J.; Li, Y.; Xu, H.M.; Li, Y.; Wei, Z. Feedstocks, environmental effects and development suggestions for biodiesel in China. J. Traffic Transp. Eng. (Engl. Ed.) 2020, 7, 791–807. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, W. China’s Energy Transition Pathway in a Carbon Neut. Engineering 2022, 14, 64–76. [Google Scholar] [CrossRef]
- Muthurathinam, S.G.; Perumal, A.V. Synthesis, characterization and tribological investigation of vegetable oil methyl esters based bio-lubricants. Ind. Crop. Prod. 2023, 203, 117098. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Duan, Q.H.; Liu, Y.N.; Yu, K.; Wang, L.H.; Zeng, J.L. Structure Innovation and Commercialization Progress of Bio-Based Lube Base Oil. Pet. J. (Pet. Process.) 2018, 34, 2. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, J.; Li, C.; Xu, H.; Sun, M.; Xu, L.; Yu, L. Synthesis and Lubricating Properties of Bio-Based Lubricants from Palm Oil. Chempluschem 2025, 90, e202500013. [Google Scholar] [CrossRef]
- Fan, N.N.; Luo, W.; Bai, Z.X. Lubricating oil additives and their development trends. Aging Appl. Synth. Mater. 2021, 50, 140. [Google Scholar] [CrossRef]
- NB/SH/T 0822-2010; Determination of Nitrogen Content in Lubricating Oils and Additives—Boat-Inlet Chemiluminescence Method. Petroleum Industry Press: Beijing, China, 2010.
- HS/T 75-2023; Determination of Glucocorticoids in Import and Export Cosmetics—Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry Method. China Customs Press: Beijing, China, 2023.
- Perer, M.H.; Yan, J.Y.; Xu, L.; Yang, M.; Yan, Y.J. Bioprocess development for biolubricant production using non-edible oils, agro-industrial byproducts and wastes. J. Clean. Prod. 2022, 357, 131956. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xu, N.; Li, W.M.; Zhang, M.; Lou, W.J.; Wang, X.B. Viscosity modification of lubricating oil based on high concentration silica nanoparticle colloidal system. J. Dispers. Sci. Technol. 2016, 12, 319. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.S.; Erhan, S.Z. Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 2006, 83, 129. [Google Scholar] [CrossRef]
- Liu, P.; Wang, X.; Wu, J.; Lin, W.; Feng, Y.H.; Chen, B.S.; Fang, J.H.; Jiang, Z.Q. Effect of boron-nitrogen modified soybean oil additive on biodegradability, anti-oxidation property, and lubricity of rapeseed oil. Pet. Refin. Chem. Ind. 2019, 50, 88. [Google Scholar] [CrossRef]
- Li, N.; Mao, G.; Wu, W.; Liu, Y. Effect Evaluation of Ethylene Vinyl Acetate/Nano-Montmorillonite Pour-Point Depressant on Improving the Flow Properties of Model Oil. In Colloids and Surfaces A: Physicochemical and Engineering Aspects; Elsevier: Amsterdam, The Netherlands, 2018; Volume 555, pp. 296–303. [Google Scholar]
- Yao, B.; Li, C.; Yang, F.; Sjoblom, J.; Zhang, Y.; Norrman, J.; Paso, K.; Xiao, Z.Q. Organically modified nano-clay facilitates pour point depressing activity of polyoctadecylacrylate. Fuel 2016, 166, 96. [Google Scholar] [CrossRef]
- Alves, B.F.; Silva, B.K.; Silva, C.A.; Celestino, G.G.; Nunes, R.C.; Lucas, E.F. Preparation and evaluation of polymeric nanocomposites based on EVA/montmorillonite, EVA/palygorskite and EVA/halloysite as pour point depressants and flow improvers of waxy systems. Fuel 2023, 333 Pt 2, 126540. [Google Scholar] [CrossRef]
- Liu, Q.; Mo, L.; Yan, F.; Nie, C. Study on the Effect of Nano Pour Point Depressant on Wax Oil Crystallization. E3S Web Conf. 2024, 573, 01010. [Google Scholar] [CrossRef]
- Zhang, K.; Li, Z.X.; Duan, R.; Li, M.Y.; Wang, J.W.; Sheng, J.; Ren, H.; Wang, S.; Wang, X.M.; Hao, Y.N. Rheological Effects of Montmorillonite and Ethyl Cellulose for Xanthoceras sorbifolium Bunge Oil-based Lubricants. Bioresources 2024, 32, 1377. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, G.; He, B.; Chen, L.H.; Huang, L.L. Immobilization of pectinase and lipase on macroporous resin coated with chitosan for treatment of whitewater from papermaking. Bioresour. Technol. 2012, 123, 616. [Google Scholar] [CrossRef]
- Adam, P.H.; Malcolm, R.M.; Thomas, S. Process intensification of biodiesel production using a continuous oscillatory flow reactor. J. Chem. Technol. Biotechnol. 2003, 78, 87. [Google Scholar] [CrossRef]
- Shara, S.; Eissa, E.; Basta, J. Polymers additive for improving the flow properties of lubricating oil. Egypt. J. Pet. 2017, 27, 795–799. [Google Scholar] [CrossRef]
- Shimada, Y.; Watanabe, Y.; Sugihara, A.; Tominaga, Y. Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. J. Mol. Catal. B Enzym. 2002, 17, 133. [Google Scholar] [CrossRef]
- Hao, Y.N.; Fu, Y.J.; Zhang, Y.Q.; Wang, X.M.; Xie, P.F.; Xue, Z.H. Study on preparation and properties of Xanthoceras sorbifolia oil-based lubricating oil. J. Chin. Cereals Oils 2023, 38. [Google Scholar] [CrossRef]
- Dhar, A.; Agarwal, A.K. Experimental investigations of effect of Karanja biodiesel on tribological properties of lubricating oil in a compression ignition engine. Fuel 2014, 130, 112. [Google Scholar] [CrossRef]
- Love, K.; Ankush, R. Friction and wear performance of olive oil containing nanoparticles in boundary and mixed lubrication regimes. Wear 2019, 426, 819. [Google Scholar] [CrossRef]
- Jiang, Q.F.; Liu, W.G.; Liao, Y.X.; Guo, J.C.; Li, H.N.; Li, J.H.; Chen, Y.X.; Su, X.B.; Hua, R. CTAB modification bentonite for enhanced Re adsorption and diffusion suppression. Kerntechnik 2024, 89, 340–350. [Google Scholar] [CrossRef]
- Garusinghe, U.M.; Varanasi, S.; Raghuwanshi, V.S.; Garnier, G.; Batchelor, W. Nanocellulose-montmorillonite composites of low water vapour permeability. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 233–241. [Google Scholar] [CrossRef]
- Jin, Y.J.; Min, S.K.; Han, M.J.; Cheol, M.S. Graphite oxide/poly(methyl methacrylate) nanocomposites prepared by a novel method utilizing macroazoinitiator. Compos. Technol. 2008, 69, 186. [Google Scholar] [CrossRef]
- Tapas, K.; Saswata, B.; Partha, K.; Nam, H.K.; Kyong, Y.R.; Joong, H.L. Characterization and properties of in situ emulsion polymerized poly(methylmethacrylate)/graphene nanocomposites. Compos. A: Appl Sci. Manuf. 2011, 42, 1856. [Google Scholar] [CrossRef]
- Sneha, E.; Karthik, G.V.S.; Thampi, A.D.; Krishna, A.; Revikumar, A.; Rani, S. Experimental and quantum chemical investigation of bio-fuels/lubricants for its oxidative stability. J. Mol. Liq. 2021, 340, 117292. [Google Scholar] [CrossRef]
- Colclough, T. Role of additives and transition metals in lubricating oil oxidation. Ind. Eng. Chem. Res. 2002, 26, 1888–1895. [Google Scholar] [CrossRef]
- Fazal, M.A.; Sundus, F.; Masjuki, H.H.; Rubaiee, S.; Quazi, M.M. Tribological assessment of additive doped B30 biodiesel-diesel blend by using high frequency reciprocating rig test. Composites 2021, 6, 995. [Google Scholar] [CrossRef]
- GB/T 265-1988; Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity of Petroleum Products. Standards Press of China: Beijing, China, 1988.
- SY/T 5651-1993; Technical Conditions for Petroleum Product Kinematic Viscosity Tester. Petroleum Industry Press: Beijing, China, 1993.
- ASTM D445-23; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2023.
- José, M.L.R.; María, J.G.G.; Vanesa, S. Tribological Performance of a Paraffinic Base Oil Additive with Coated and Uncoated SiO2 Nanoparticles. Materials 2024, 17, 1993. [Google Scholar] [CrossRef]
- GB/T 1995-1998; Calculation of Viscosity Index of Petroleum Products. Standards Press of China: Beijing, China, 1998.
- NB/SH/T 0248-2019; Viscosity Index Improvers for Lubricating Oils—Part 1: General Rules. Petroleum Industry Press: Beijing, China, 2019.
- Wang, Y.; Ou, S.; Liu, P.; Zhang, Z. Preparation of biodiesel from waste cooking oil via two-step catalyzed process. Energy Convers. Manag. 2007, 48, 184–188. [Google Scholar] [CrossRef]
- Faraguna, F.; Kraguljac, K.; Vidović, E.; Jukić, A. Crystallization and Pour Point of Solutions of the Dispersant Poly(Styrene-Co-Methacrylate) Viscosity Modifiers in Lubricating Base Oil. Tribol. Trans. 2017, 60, 1063–1069. [Google Scholar] [CrossRef]
- Nestor, U.S.; Veronica, P.M.; Kiyotaka, S.; Masatoshi, M. Crystallization behavior of neat biodiesel and biodiesel treated with ozonized vegetable oil. Eur. J. Lipid Sci. Technol. 2005, 107, 689. [Google Scholar] [CrossRef]
- Li, F.S.; Liu, Z.W.; Ni, Z.H.; Wang, H. Effect of biodiesel components on its lubrication performance. J. Mater. Res. Technol. 2019, 8, 3681. [Google Scholar] [CrossRef]
- Kokhanovsky, V.A.; Maiba, I.A.; Glazunov, D.V. Properties of Lubricant Additives in Slip with Boundary Friction. Russ. Eng. Res. 2023, 43, 135–137. [Google Scholar] [CrossRef]
- Liu, Z.W.; Li, F.S.; Shen, J.X.; Wang, H. Effect of oxidation of Jatropha curcas-derived biodiesel on its lubricating properties. Energy Sustain. Dev. 2019, 52, 33. [Google Scholar] [CrossRef]
- Marcia, M.M.; Clara, M.A.; Rui, F.S.; Carlos, A.A. Assessment of boundary lubrication in biodiesels by nanotribological tests. Energy 2013, 55, 273. [Google Scholar] [CrossRef]



| Acid Value (mg/g) | Saponification Value (mg/g) | Peroxide Value (meq/kg) | Shuifen (%) | Phospholipid Content (%) | |
|---|---|---|---|---|---|
| Prior to pretreatment | 26.31 | 207.84 | 58.61 | 0.65 | 1.07 |
| After the preliminary reasoning | 28.34 | 200.94 | 21.66 | <0.01 | 0.58 |
| Name | 40 °C KV | 100 °C KV |
|---|---|---|
| raw oil | 11.4 mm2/s | 3.0 mm2/s |
| Name | Friction Coefficient |
|---|---|
| Waste oil | 0.099 |
| 1 wt% CTAB-MMT/KH560-EC | 0.081 |
| Friction Regime | Coefficient of Friction μ |
|---|---|
| coulomb friction | 0.15–0.40 |
| boundary lubrication | 0.08–0.10 |
| mixed lubrication | 0.02–0.08 |
| fluid lubrication | 0.001–0.005 |
| Name | No. 1 | No. 2 | No. 3 | Mean Diameter of Pits (mm) | |||
|---|---|---|---|---|---|---|---|
| X1 | Y1 | X2 | Y2 | X3 | Y3 | ||
| Waste oil | 0.8 | 0.83 | 0.78 | 0.82 | 0.84 | 0.81 | 0.81 |
| 1 wt% CTAB-MMT/KH560-EC | 0.96 | 0.93 | 0.90 | 0.93 | 0.98 | 0.94 | 0.94 |
| Main Performance | Waste Oil | CTAB-MMT/KH560-EC Content 1% |
|---|---|---|
| Viscosity index | 120.9 | 181.1 |
| Acidity (mgKOHg) | 36.3 | 36.6 |
| Condensation point (°C) | −12 | −22 |
| Flash point (°C) | >290 | >290 |
| Mechanical admixture (%) | not have | not have |
| Ash content (%) | 0.01 | 0.01 |
| Shuifen (%) | 0.05 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, H.; Wang, Z.; Hou, T.; Zhang, K.; Lv, Z.; Zhao, G.; Sun, H.; Li, W.; Hao, Y. Biobased Lubricating Oil Prepared from Ethyl Cellulose/Montmorillonite Additives and Waste Cooking Oil. Lubricants 2025, 13, 417. https://doi.org/10.3390/lubricants13090417
Wang S, Wang H, Wang Z, Hou T, Zhang K, Lv Z, Zhao G, Sun H, Li W, Hao Y. Biobased Lubricating Oil Prepared from Ethyl Cellulose/Montmorillonite Additives and Waste Cooking Oil. Lubricants. 2025; 13(9):417. https://doi.org/10.3390/lubricants13090417
Chicago/Turabian StyleWang, Sha, Haoyue Wang, Zhenpeng Wang, Tao Hou, Kai Zhang, Zhuoyi Lv, Gaole Zhao, Huimin Sun, Wenkai Li, and Yinan Hao. 2025. "Biobased Lubricating Oil Prepared from Ethyl Cellulose/Montmorillonite Additives and Waste Cooking Oil" Lubricants 13, no. 9: 417. https://doi.org/10.3390/lubricants13090417
APA StyleWang, S., Wang, H., Wang, Z., Hou, T., Zhang, K., Lv, Z., Zhao, G., Sun, H., Li, W., & Hao, Y. (2025). Biobased Lubricating Oil Prepared from Ethyl Cellulose/Montmorillonite Additives and Waste Cooking Oil. Lubricants, 13(9), 417. https://doi.org/10.3390/lubricants13090417




