Environmentally Friendly Phosphating Treatment for Wear-Resistant and Anti-Corrosion Coating on Steel Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
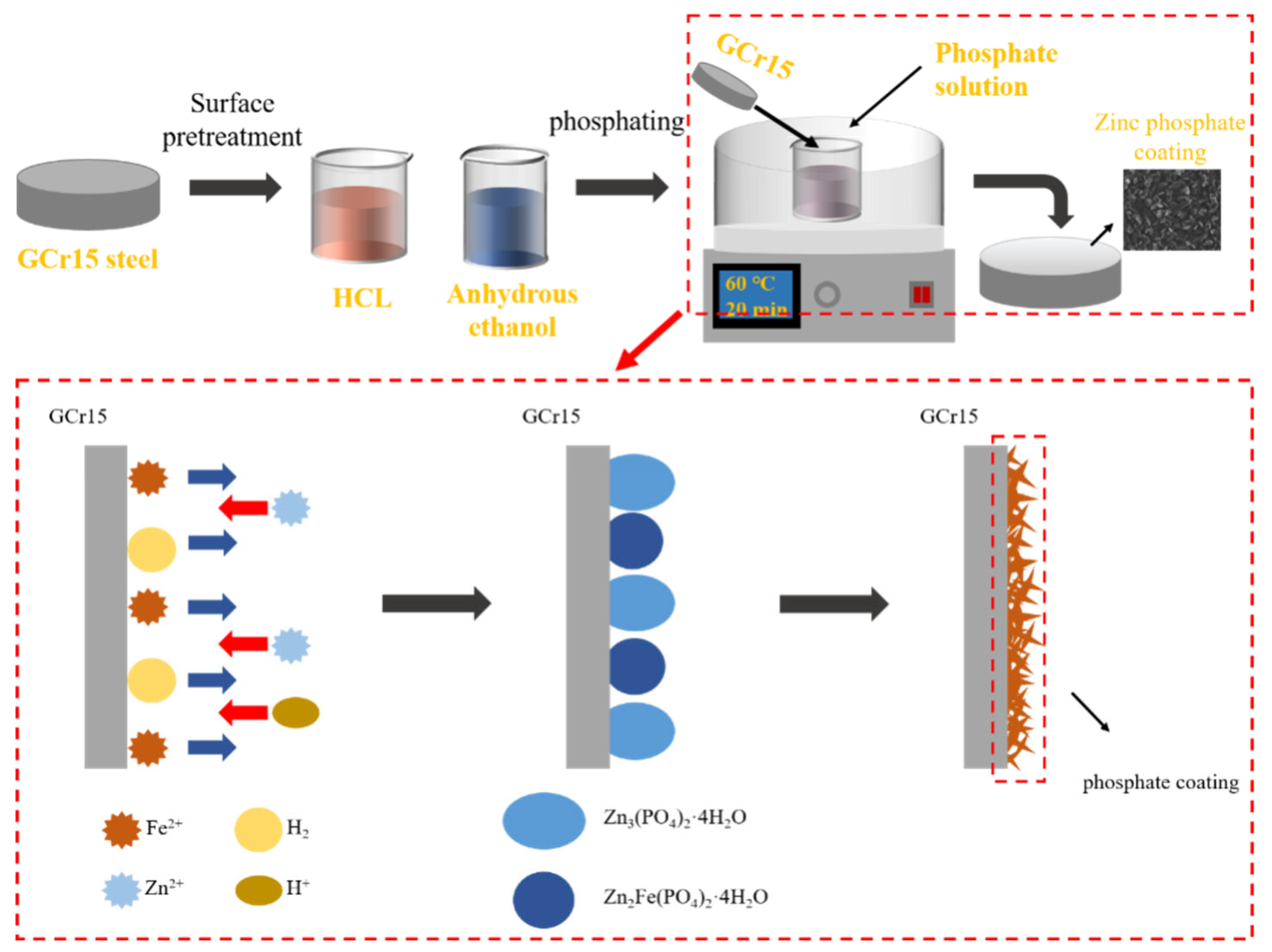
2.2. Preparation of Coating
2.3. Zinc Phosphate Coating Performance Test
2.3.1. Surface Morphology and Composition
2.3.2. Thickness and Unit Weight Measurement
2.3.3. Corrosion Resistance Tests
2.3.4. Friction and Wear Tests
3. Results and Discussion
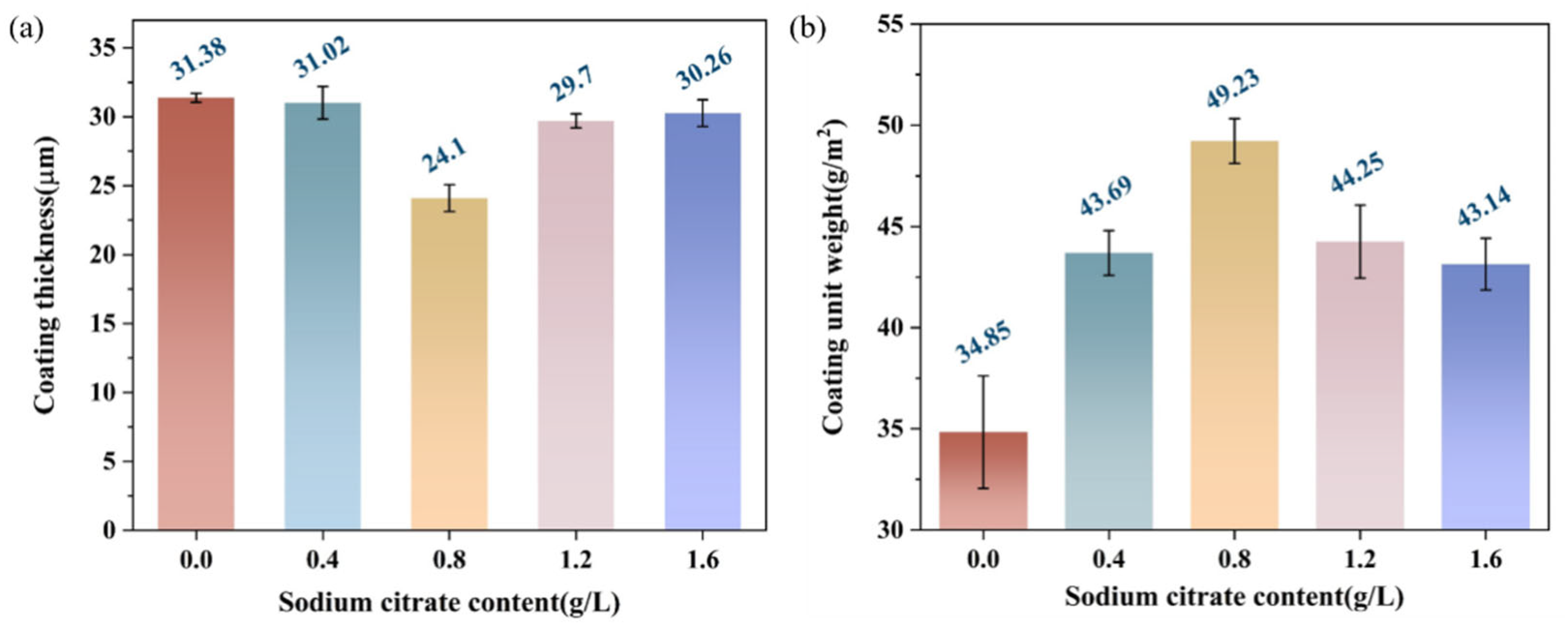
3.1. Thickness and Unit Weight of Different Zinc Phosphate Coatings
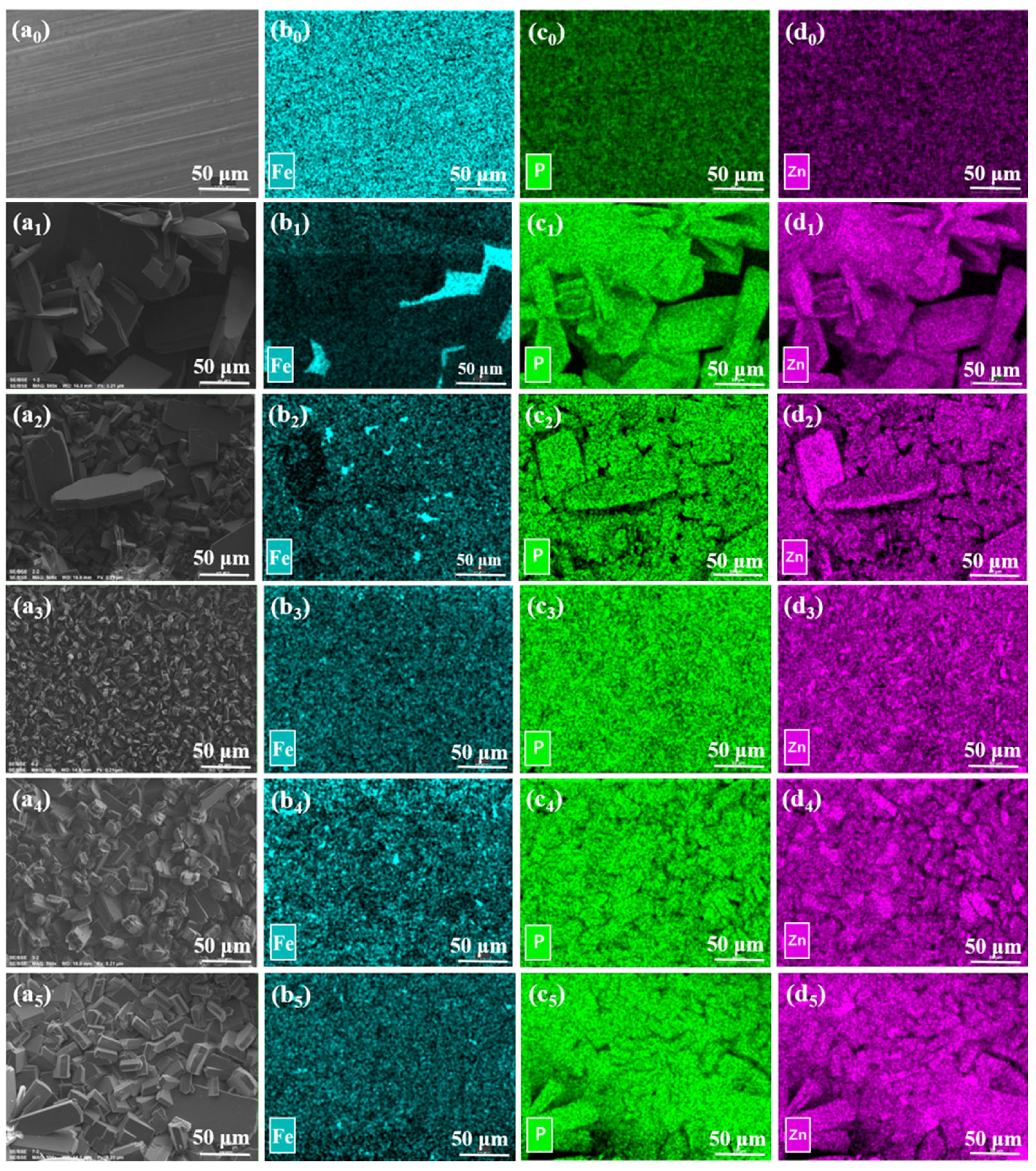
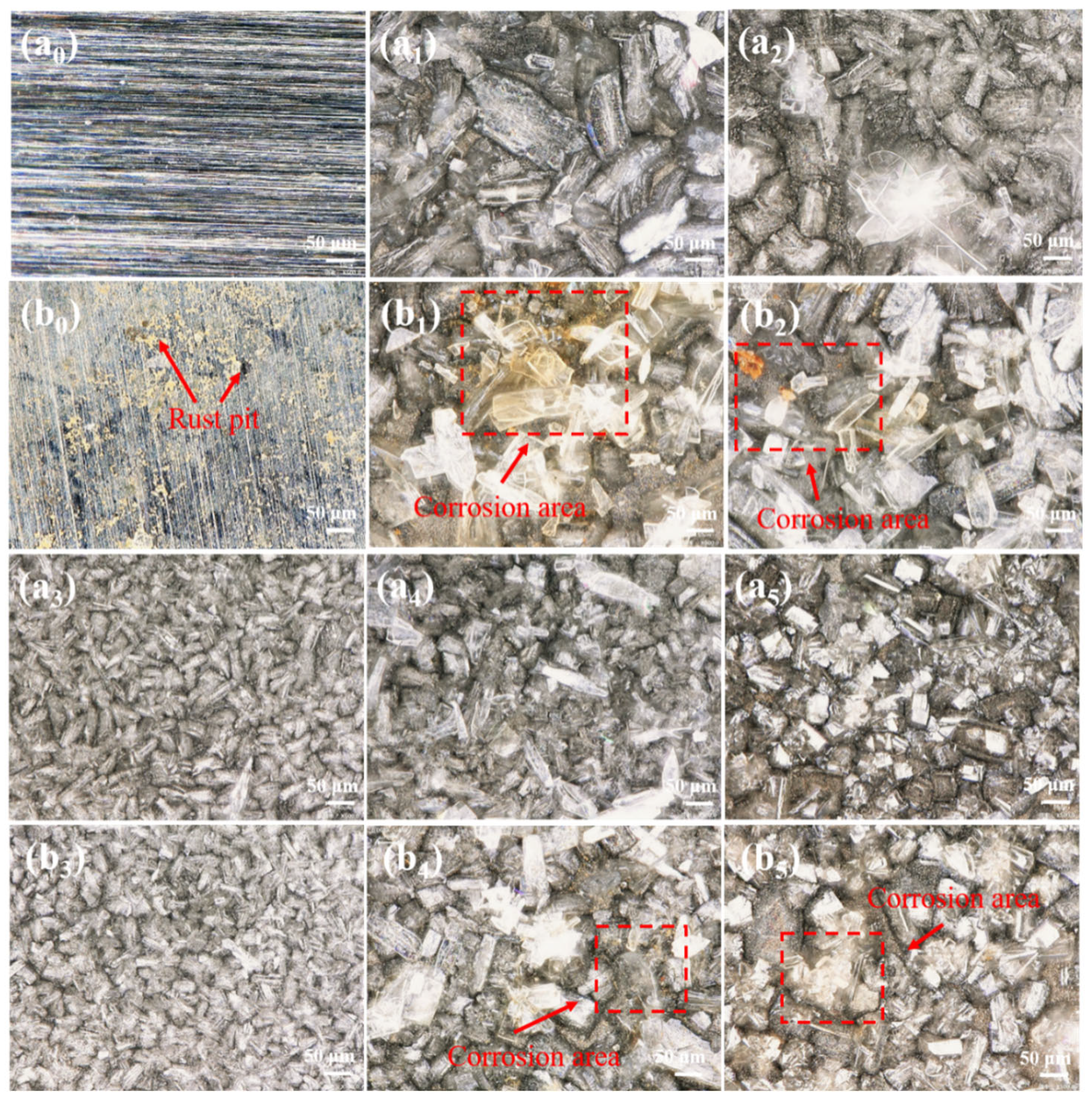
3.2. Surface Morphology of the Zinc Phosphate Coatings
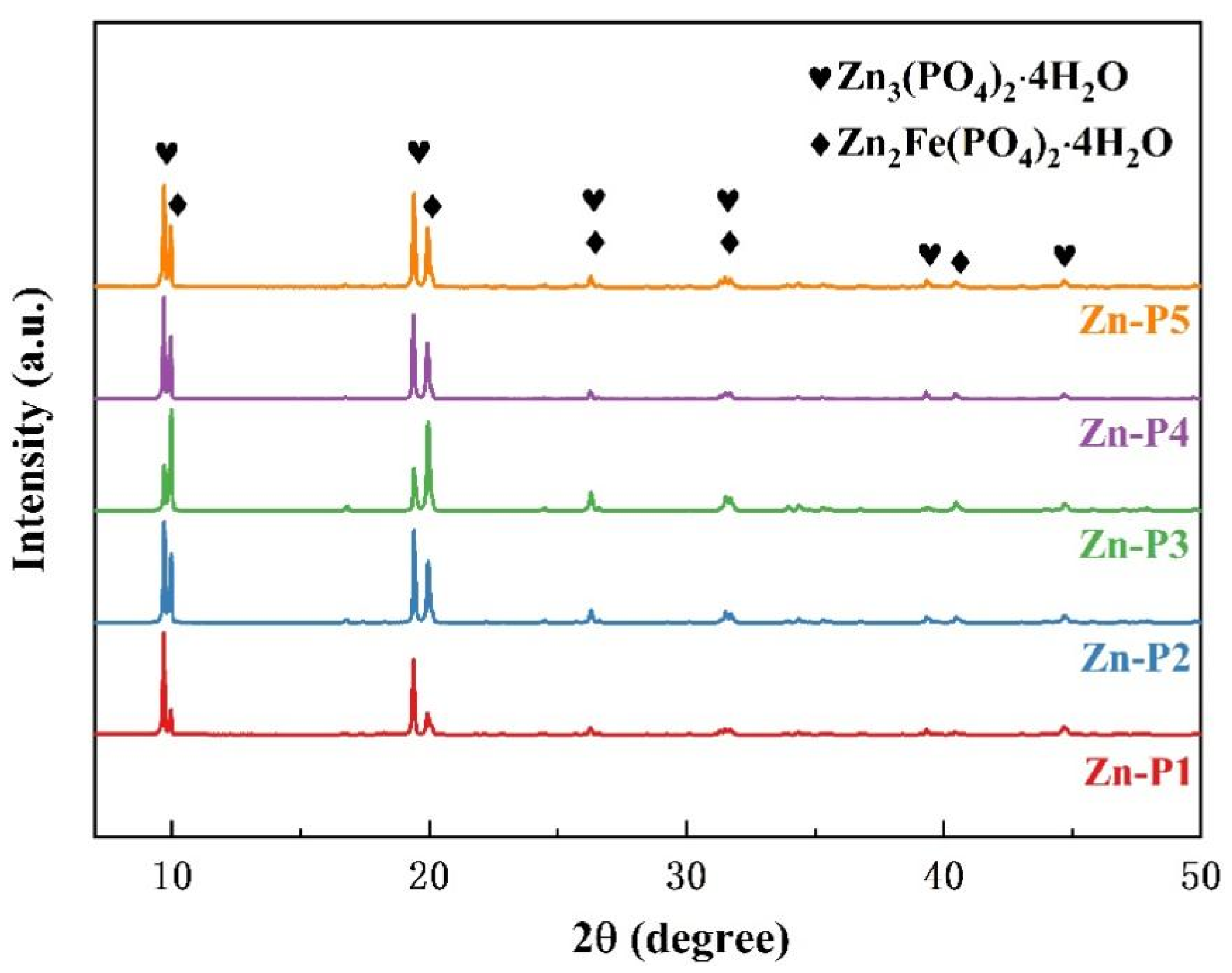
3.3. Crystal Phase
3.4. Corrosion Property of the Zinc Phosphate Coatings
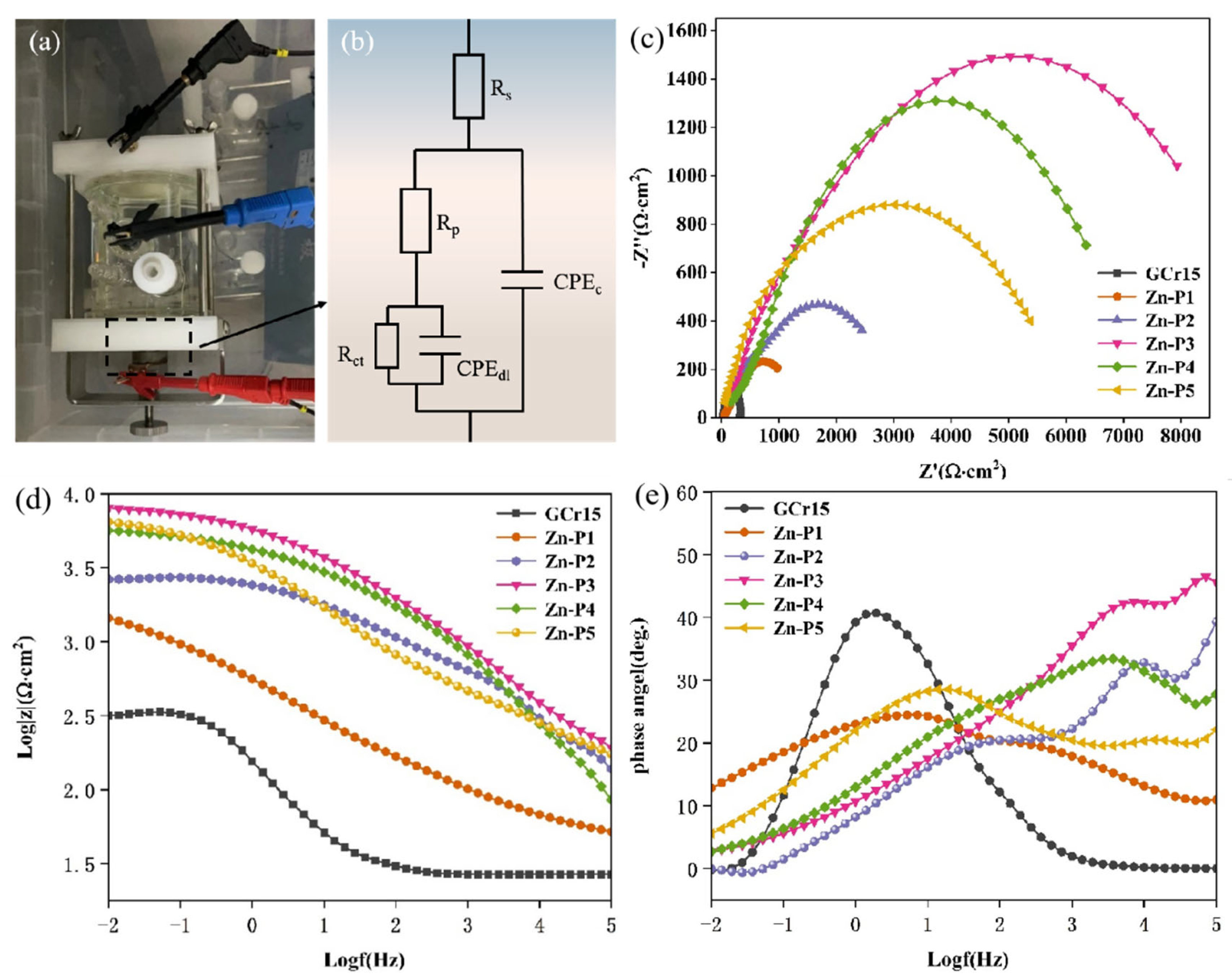
3.4.1. EIS Analysis
3.4.2. Surface Corrosion Morphology
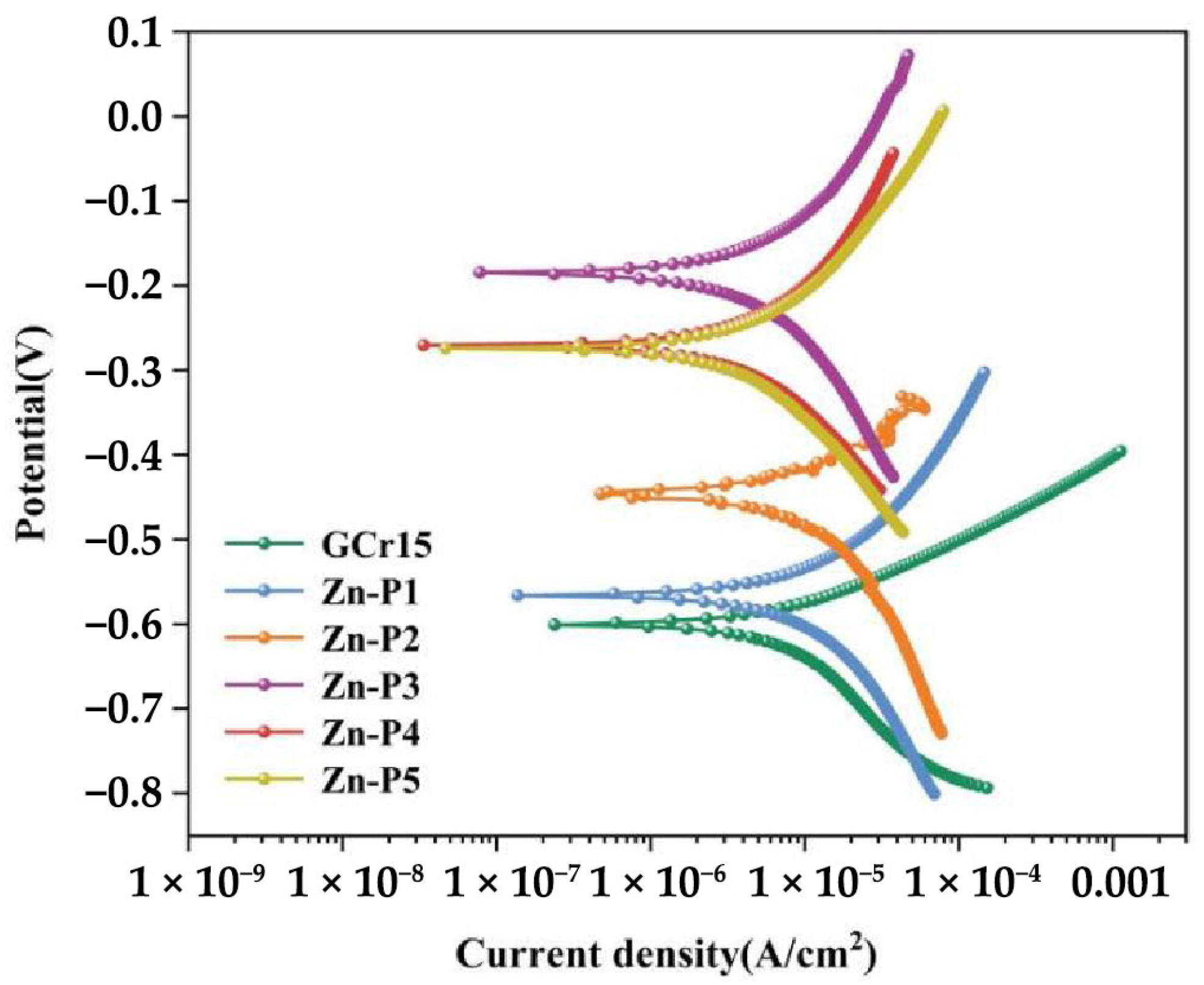
3.4.3. Potentiodynamic Polarization (PDP) Testing
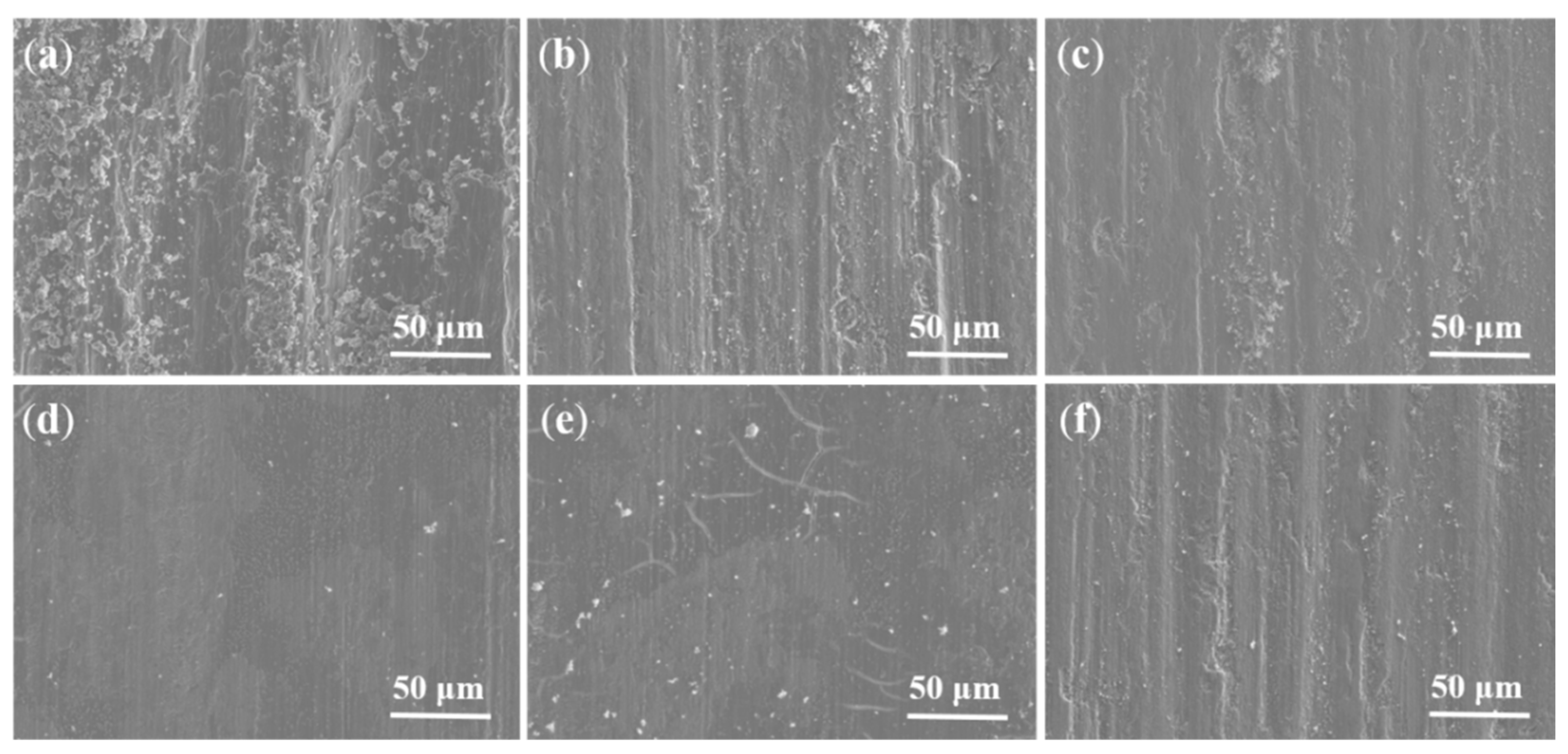
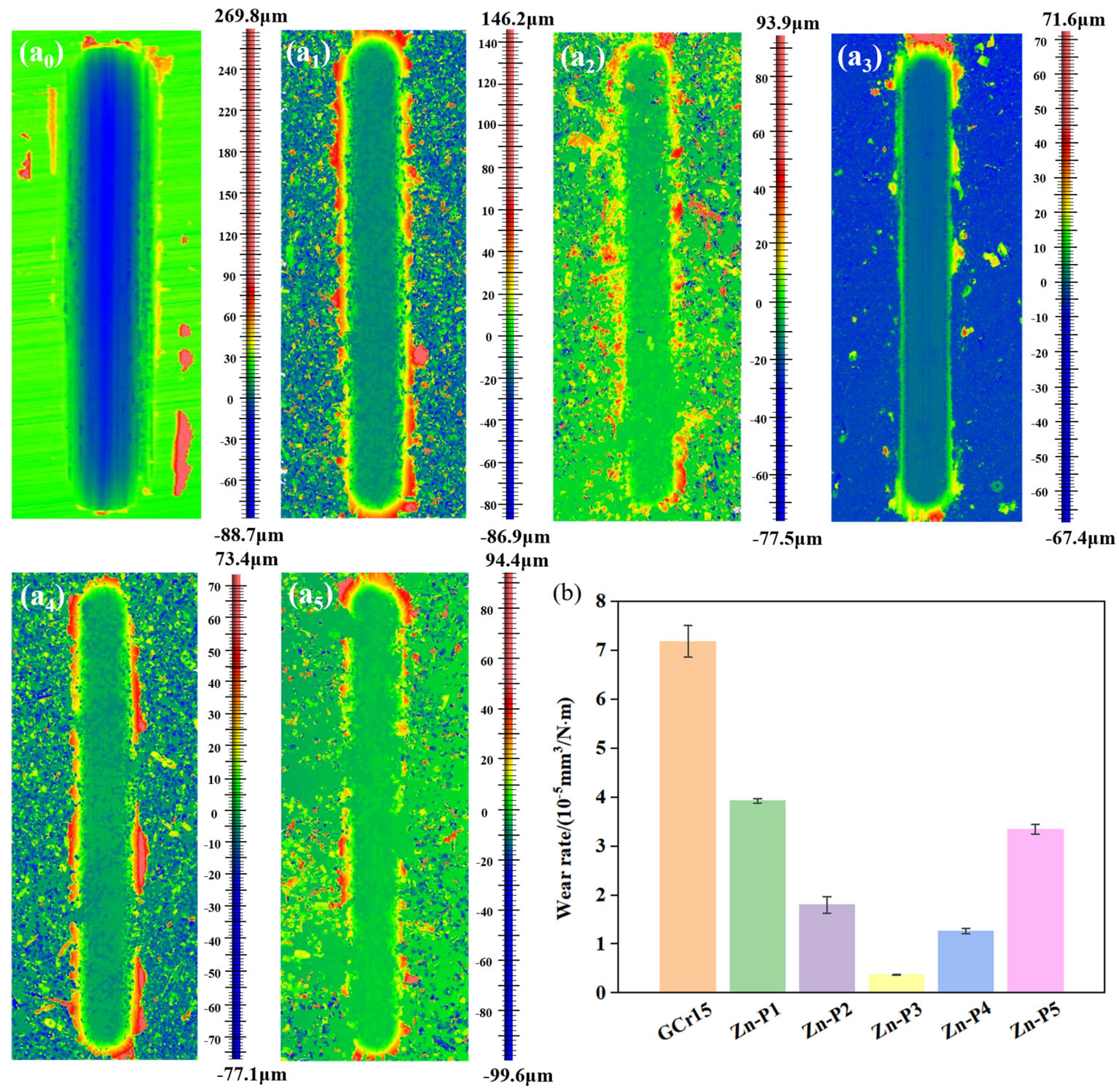
3.5. Friction and Wear
3.6. Analysis of Phosphate Crystal Formation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cai, H.; Li, X.; Zhang, Y.; Yang, C.; Cui, S.; Sheng, L.; Wu, Z. A high corrosion-resistant waterborne epoxy resin coating improved by addition of multi-interface structured zinc phosphate particles. J. Mater. Res. Technol. 2023, 26, 7829–7844. [Google Scholar] [CrossRef]
- Tong, H.T.; Liu, X.H.; Liu, Y.P.; Zhang, H.X.; Li, X.B. Electrochemical investigation of the corrosion inhibiting effect of organic paints doped with benzotriazole coated on steel substrates. Corros. Eng. Sci. Technol. 2021, 56, 618e25. [Google Scholar]
- Wang, J.; Wu, M.; Huang, J.; Miao, X.; Chen, Y.; Zhao, Y. In Situ Polymerized Self-Healing Microcapsules as Multifunctional Fillers toward Phosphate Ceramic Coatings. ACS Appl. Mater. Interfaces 2024, 16, 26768–26786. [Google Scholar] [CrossRef]
- Aswathy, S.P.; Kumar, A.S.; Saji, V.S.; Shibli, S.M. A Near-Infrared Reflectance, Thermal Shielding, and Corrosion Resistance of Neodymium-Modified Zinc Aluminate Spinel-Based Zinc Phosphate Coatings. Adv. Eng. Mater. 2024, 27, 2401351. [Google Scholar] [CrossRef]
- Zhang, P.J.; Liu, Q.; Huang, J.; Cui, J.W.; Sun, W.; Li, B.S.; Xu, G.Q. Phosphate conversion of electroplated Ni coatings on NdFeB magnets improving the anticorrosion Property. J. Alloys Compd. 2022, 922, 166206. [Google Scholar] [CrossRef]
- Zang, L.B.; Zhong, Q.L.; Chen, Y.; Hou, W.J.; Zhao, B.S.; Wu, Y.M. Effect of coating thickness on tribological properties of manganese phosphate conversion coating in different motion conditions. Tribol. Int. 2022, 176, 107894. [Google Scholar] [CrossRef]
- Chai, H.; Wang, L.F.; Cao, X.Q.; Zhang, Q.; Arthanari, S.; Lee, H.; Huang, G.S.; Xing, B.; Zheng, L.W.; Zhang, H.; et al. The effects of chemical conversion parameters on morphology and corrosion performance of calcium phosphate coating on AZ31 alloy, Mater. Chem. Phys. 2023, 296, 127338. [Google Scholar]
- Soroush, E.; Davarpanah, A.; Keramatinia, M.; Nouri, N.; Ramezanzadeh, B. Synergistic impact of the functionalized graphene oxide (fGO) nano-sheets and Mn2+-doped zinc phosphate conversion film on the polyester coating corrosion protection properties. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132510. [Google Scholar] [CrossRef]
- Han, X.C.; Li, N.; Wu, B.; Li, D.Y.; Pan, Q.M.; Wang, R. Microstructural characterization and corrosion resistance evaluation of chromate-phosphate/water-soluble resin composite conversion coating on Al surfaces. Prog. Org. Coat 2022, 173, 107205. [Google Scholar] [CrossRef]
- Liu, H.L.; Tong, Z.P.; Yang, Y.; Zhou, W.F.; Chen, J.N.; Pan, X.Y.; Ren, X.D. Preparation of phosphate conversion coating on laser surface textured surface to improve corrosion performance of magnesium alloy. J. Alloys Compd. 2021, 865, 158701. [Google Scholar] [CrossRef]
- Narayanan, T.S.N.S. Surface pretretament by phosphate conversion Coatings a review. Rev. Adv. Mater. Sci. 2005, 9, 130–177. [Google Scholar]
- Li, G.Y.; Lian, J.; Niu, L.; Jiang, H. Influence of pH of Phosphating Bath on the Zinc Phosphate Coating on AZ91D Magnesium Alloy. Adv. Eng. Mater. 2006, 8, 123–127. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Akbarian, M.; Ramezanzadeh, M.; Mahdavian, M.; Alibakhshi, E.; Kardar, P. Corrosion protection of steel with zinc phosphate conversion coating and post-treatment by hybrid organic-inorganic sol gel based silane film. J. Electrochem. Soc. 2017, 164, 224. [Google Scholar] [CrossRef]
- Su, H.Y.; Lin, C.S. Effect of additives on the properties of phosphate conversion coating on electrogalvanized steel sheet. Corros. Sci. 2014, 83, 137–146. [Google Scholar] [CrossRef]
- Lin, B.L.; Lu, J.T.; Kong, G. Effect of Molybdate Post Sealing on the Corrosion Resistance of Zinc Phosphate Coatings on Hot-Dip Galvanized Steel. Corros. Sci. 2008, 50, 962–967. [Google Scholar] [CrossRef]
- Deepa, L.C. Effect of divalent cations in low zinc ambient temperature phosphating bath. Anticorros. Methods Mater. 2003, 502, 86–90. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Chen, X.H.; Tan, H.Y. Effect of ultrasonic treatment on the morphology and corrosion resistance of zinc-manganese phosphate coatings on 16Mn steel in 3.5% sodium chloride. Int. J. Electrochem. Sci. 2023, 18, 100274. [Google Scholar] [CrossRef]
- Pokorny, P.; Szelag, P.; Novak, M.; Mastny, L.; Brozek, V. Thermal stability of phosphate coatings on steel. Metalurgija 2015, 54, 489–492. [Google Scholar]
- Qi, Z.H.; Zhao, Y.H.; Ji, M.B.; Wang, G.X.; Ying, L.X.; Wang, Z.D.; Krit, B. Preparation of chitosan/phosphate composite coating on Mg alloy (AZ31B) via one step chemical conversion method. Resour. Chem. Mater. 2023, 2, 39–48. [Google Scholar] [CrossRef]
- Jiang, C.C.; Zhang, X.Z.; Wang, D.; Zhang, L.; Cheng, X. Phosphate conversion coatings on 35CrMnSi steels subjected to different heat treatments. Electrochem. Commun. 2020, 110, 106636. [Google Scholar] [CrossRef]
- Tamilselvi, M.; Kamaraj, P.; Arthanareeswari, M.; Devikala, S. Nano zinc phosphate coatings for enhanced corrosion resistance of mild steel. Appl. Surf. Sci. 2015, 327, 218–225. [Google Scholar] [CrossRef]
- Tamilselvi, M.; Kamaraj, P.; Arthanareeswari, M.; Devikala, S.; Arockiaselvi, J.; Pushpamalini, T. Effect of nano ZrO2 on nano zinc phosphating of mild steel. Mater. Today Proc. 2018, 5, 8880–8888. [Google Scholar] [CrossRef]
- He, X.L.; Wu, J.X.; Chen, Y.; Zhang, L.; Sheng, X.X. A trace amount of MXene@PDA nanosheets for low-temperature zinc phosphating coatings with superb corrosion resistance. Appl. Surf. Sci. 2022, 603, 154455. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, H.; Dai, H.; Jin, W. Composition and expansion coefficient of rust based on X-ray diffraction and thermal analysis. Corros. Sci. 2011, 53, 1646–1658. [Google Scholar] [CrossRef]
- Tamilselvi, M.; Kamaraj, P.; Arthanareeswari, M.; Devikala, S.; Selvi, J.A. Development of nano SiO2 incorporated nano zinc phosphate coatings on mild steel. Appl. Surf. Sci. 2015, 332, 12–21. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Zhang, M.; Yang, L.; Li, J.; Yang, P.; Cao, D. characterization and anticorrosion performance of molybdate pillared hydrotalcite in situ created ZnO composite as pigment for Mg–Li alloy protection. Surf. Coat. Technol. 2008, 203, 250–255. [Google Scholar] [CrossRef]
- Gimeno, M.J.; Puig, M.; Chamorro, S.; Molina, J.; March, R.; Oro, E.; P’erez, P.; Gracenea, J.J.; Suay, J.J. Improvement of the anticorrosive properties of an alkyd coating with zinc phosphate pigments assessed by NSS and ACET. Prog. Org. Coat. 2016, 95, 46–53. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Tian, Y.; Xie, Y.; Sheng, X.; Jiang, X.; Zhang, X. Exfoliation and functionalization of α-zirconium phosphate in one pot for waterborne epoxy coatings with enhanced anticorrosion performance. Prog. Org. Coat. 2020, 138, 105390. [Google Scholar] [CrossRef]
- Thompson, I.; Campbell, D. Interpreting Nyquist responses from defective coatings on steel substrates. Corros. Sci. 1994, 36, 187–198. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Keramatinia, M.; Ramezanzadeh, M.; Ramezanzadeh, B. Detailed experimental investigation of the highly active corrosion inhibitive green molecules based on zinc cations/Nepeta Pogonosperma extract and toward the corrosion mitigation of mild steel in the saline solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128613. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, H.; Zhu, R.; Sheng, X.; Xie, D.; Mei, Y. Facile modification of graphene oxide with Lysine for improving anti-corrosion performances of waterborne epoxy coatings. Prog. Org. Coat. 2019, 136, 105200. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Z.; Han, E.H.; Wang, S.; Liu, Q. Corrosion performance of nano-ZrO2 modified coatings in hot mixed acid solutions. Materials 2018, 11, 934. [Google Scholar] [CrossRef]
- Keramatinia, M.; Majidi, R.; Ramezanzadeh, B. La-MOF coordination polymer: An effective environmentally friendly pH-sensitive corrosion inhibitive-barrier nanofiller for the epoxy polyamide coating reinforcement. J. Environ. Chem. Eng. 2022, 10, 108246. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ghasemi, E.; Mahdavian, M.; Changizi, E.; MohamadzadehMoghadam, M.H. Covalently-grafted graphene oxide nanosheets to improve barrier and corrosion protection properties of polyurethane coatings. Carbon 2015, 93, 555–573. [Google Scholar] [CrossRef]
- Duraipandian, M. Effects of Fe2O3 and B4C Addition on the Mechanical Properties and Corrosion Resistance of Al–Mg–Si Alloy in 3.5% Brine Solution. Met. Mater. Int. 2021, 27, 1506–1518. [Google Scholar] [CrossRef]
- Al-Mamun, N.S.; Haider, W.; Shabib, I. Corrosion resistance of additively manufactured 316L stainless steel in chloride-thiosulfate environment. Electrochim. Acta 2020, 362, 137039. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, Z.; Bai, S. Corrosion resistance of bis-si-lane-modified epoxy coatings on an Al-Zn-Mg-Cu alloy. J. Mater. Eng. Perform. 2020, 288, 5282–5290. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Q.J.; An, L.Y. Effects of volt-age on electrochemical corrosion behavior of micro-arc oxida-tion coatings. J. Lanzhou Univ. Technol. 2020, 46, 1. [Google Scholar]
- Rui, S.J.; Shao, S.; Li, Z.X. Study on prepa-ration and anticorrosion of silane sealing film on carbon steel. New Chem. Mater. 2019, 47, 267–271. [Google Scholar]
- Tian, Y.; Qiu, W.; Xie, Y.; Huang, H.; Hu, J.; Zhong, L.; Jiang, X.; Zhang, X. Melatonin as an Accelerating Agent for Phosphate Chemical Conversion Coatings on Mild Steel with Enhanced Corrosion Resistance. J. Electrochem. Soc. 2020, 167, 101505. [Google Scholar] [CrossRef]
- Parhizkar, N.; Ramezanzadeh, B.; Shahrabi, T. Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a solgel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets. Appl. Surf. Sci. 2018, 439, 45–59. [Google Scholar] [CrossRef]
- Mo, S.; Ye, J.; Jia, L.; Chen, Y. Properties and performance of hybrid suspensions of MPCM/nanoparticles for LED thermal management. Energy 2022, 239, 122650. [Google Scholar] [CrossRef]
- Jaber, N.B.; Drevet, R.; Fauré, J.; Demangel, C.; Potiron, S.; Tara, A.; Benhayoune, H. A New Process for the Thermal Treatment of Calcium Phosphate Coatings Electrodeposited on Ti6Al4V Substrate. Adv. Eng. Mater. 2015, 17, 1608–1615. [Google Scholar] [CrossRef]


| Element | C | Si | Mn | Cr | Mo | S | Ni |
|---|---|---|---|---|---|---|---|
| Mass fraction/% | 0.95–1.05 | 0.15–0.35 | 0.25–0.45 | 1.40–1.65 | ≤0.10 | ≤0.025 | ≤0.030 |
| Phosphate Solution | Composition and Content | ||||
|---|---|---|---|---|---|
| ZnO/g·L−1 | H3PO4/mL·L−1 | NaF/g·L−1 | C6H8O7/g·L−1 | C6H5Na3O7/g·L−1 | |
| P1 | 6 | 20 | 4 | 2 | 0 |
| P2 | 6 | 20 | 4 | 2 | 0.4 |
| P3 | 6 | 20 | 4 | 2 | 0.8 |
| P4 | 6 | 20 | 4 | 2 | 1.2 |
| P5 | 6 | 20 | 4 | 2 | 1.6 |
| Sample | Rp (Ω·cm2) | Rct (Ω·cm2) | |Z0.01 Hz| (Ω·cm2) | CPEc (F·cm2·sn−1) | CPEdl (F·cm2·sn−1) |
|---|---|---|---|---|---|
| GCr15 | 15.9 | 257 | 30.57 | 4.361 × 10−3 | 4.883 × 10−3 |
| Zn-P1 | 1.013 × 103 | 1.539 × 103 | 168.06 | 8.276 × 10−4 | 3.55 × 10−5 |
| Zn-P2 | 1.34 × 103 | 1.551 × 103 | 1070.24 | 1.796 × 10−5 | 3.658 × 10−5 |
| Zn-P3 | 5.02 × 103 | 2.957 × 103 | 1987.37 | 2.155 × 10−5 | 4.988 × 10−5 |
| Zn-P4 | 2.976 × 103 | 1.678 × 103 | 1732.36 | 1.547 × 10−5 | 3.652 × 10−5 |
| Zn-P5 | 2.927 × 103 | 1.307 × 103 | 818.90 | 1.054 × 10−5 | 2.991 × 10−5 |
| Sample | Ecorr (mV) | Icorr (A/cm2) | Rpo (Ω⸱cm2) | βa (mV/dec) | βb (mV/dec) |
|---|---|---|---|---|---|
| GCr15 | −0.541795 | 1.313 × 10−5 | 1.645 × 107 | 441.41 | −3909.87 |
| Zn-P1 | −0.54076 | 5.81 × 10−6 | 2.525 × 109 | 876.74 | −3939.58 |
| Zn-P2 | −0.4496181 | 1.619 × 10−6 | 3.0243 × 109 | 1823.03 | −3958.54 |
| Zn-P3 | −0.19803 | 1.1635 × 10−7 | 1.495 × 1010 | 5204.36 | −7656.94 |
| Zn-P4 | −0.26248 | 1.159 × 10−6 | 1.064 × 1010 | 5940.98 | −7511.82 |
| Zn-P5 | −0.265375 | 1.762 × 10−6 | 4.004 × 109 | 7951.98 | −6635.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, T.; Li, L.; Zhang, L.; Bai, D.; Xie, G.; Wei, B.; Xiao, Y.; Pan, C.; Chen, G. Environmentally Friendly Phosphating Treatment for Wear-Resistant and Anti-Corrosion Coating on Steel Substrate. Lubricants 2025, 13, 367. https://doi.org/10.3390/lubricants13080367
Yan T, Li L, Zhang L, Bai D, Xie G, Wei B, Xiao Y, Pan C, Chen G. Environmentally Friendly Phosphating Treatment for Wear-Resistant and Anti-Corrosion Coating on Steel Substrate. Lubricants. 2025; 13(8):367. https://doi.org/10.3390/lubricants13080367
Chicago/Turabian StyleYan, Tengfeng, Ling Li, Lin Zhang, Dan Bai, Guoxin Xie, Bin Wei, Yang Xiao, Chenyang Pan, and Guoxing Chen. 2025. "Environmentally Friendly Phosphating Treatment for Wear-Resistant and Anti-Corrosion Coating on Steel Substrate" Lubricants 13, no. 8: 367. https://doi.org/10.3390/lubricants13080367
APA StyleYan, T., Li, L., Zhang, L., Bai, D., Xie, G., Wei, B., Xiao, Y., Pan, C., & Chen, G. (2025). Environmentally Friendly Phosphating Treatment for Wear-Resistant and Anti-Corrosion Coating on Steel Substrate. Lubricants, 13(8), 367. https://doi.org/10.3390/lubricants13080367





