Theoretical Study and Adsorption Behavior of Urea on Mild Steel in Automotive Gas Oil (AGO) Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Mild Steel
2.3. Gasometric Measurement
2.4. Surface Examination Study
2.5. Computational Investigation
2.5.1. DFT Calculations
2.5.2. MC Simulation
2.6. Property Test of Automotive Gas Oil (AGO)
3. Results and Discussion
3.1. Corrosive Properties of AGO
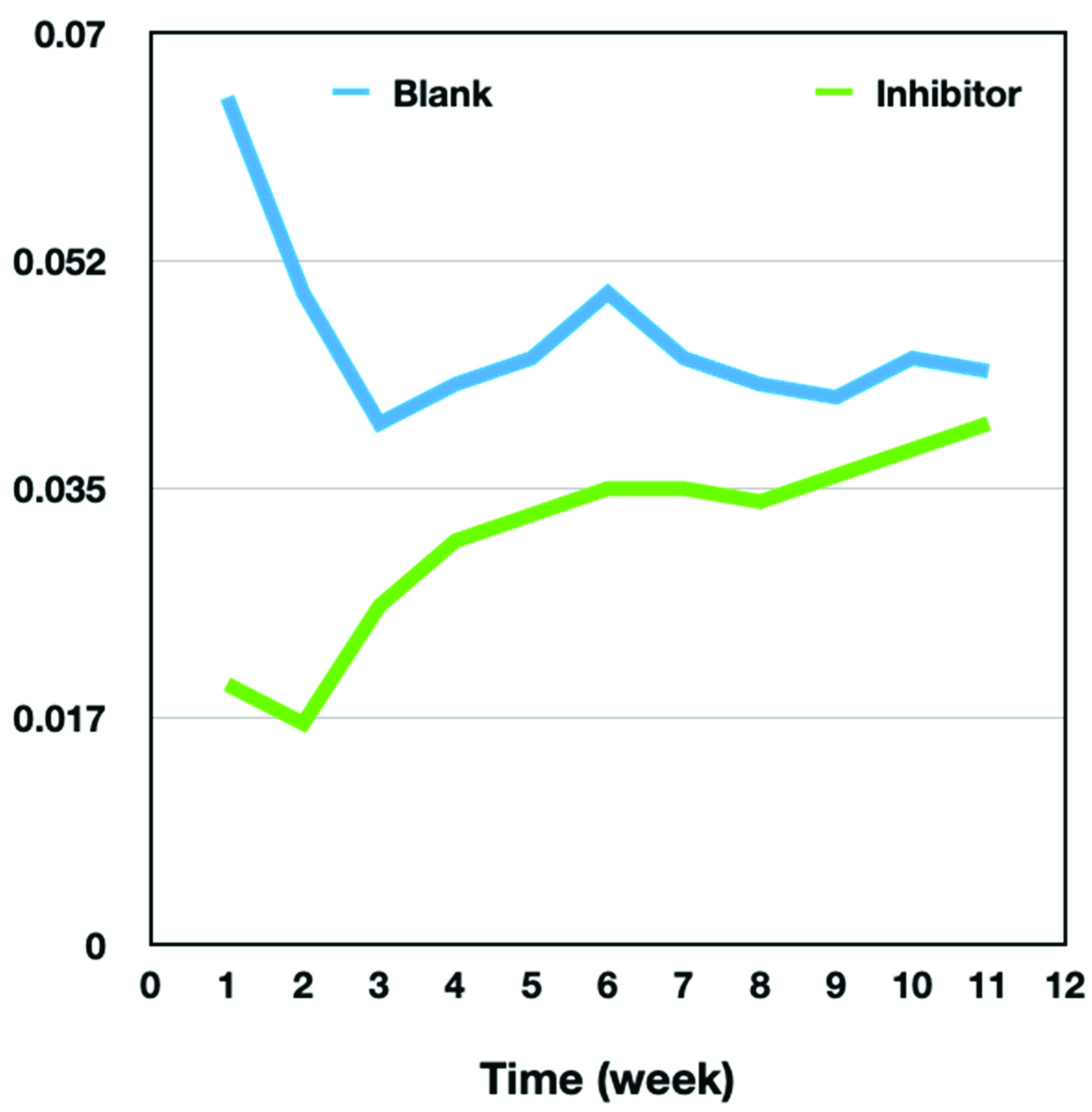
3.2. Corrosion Study
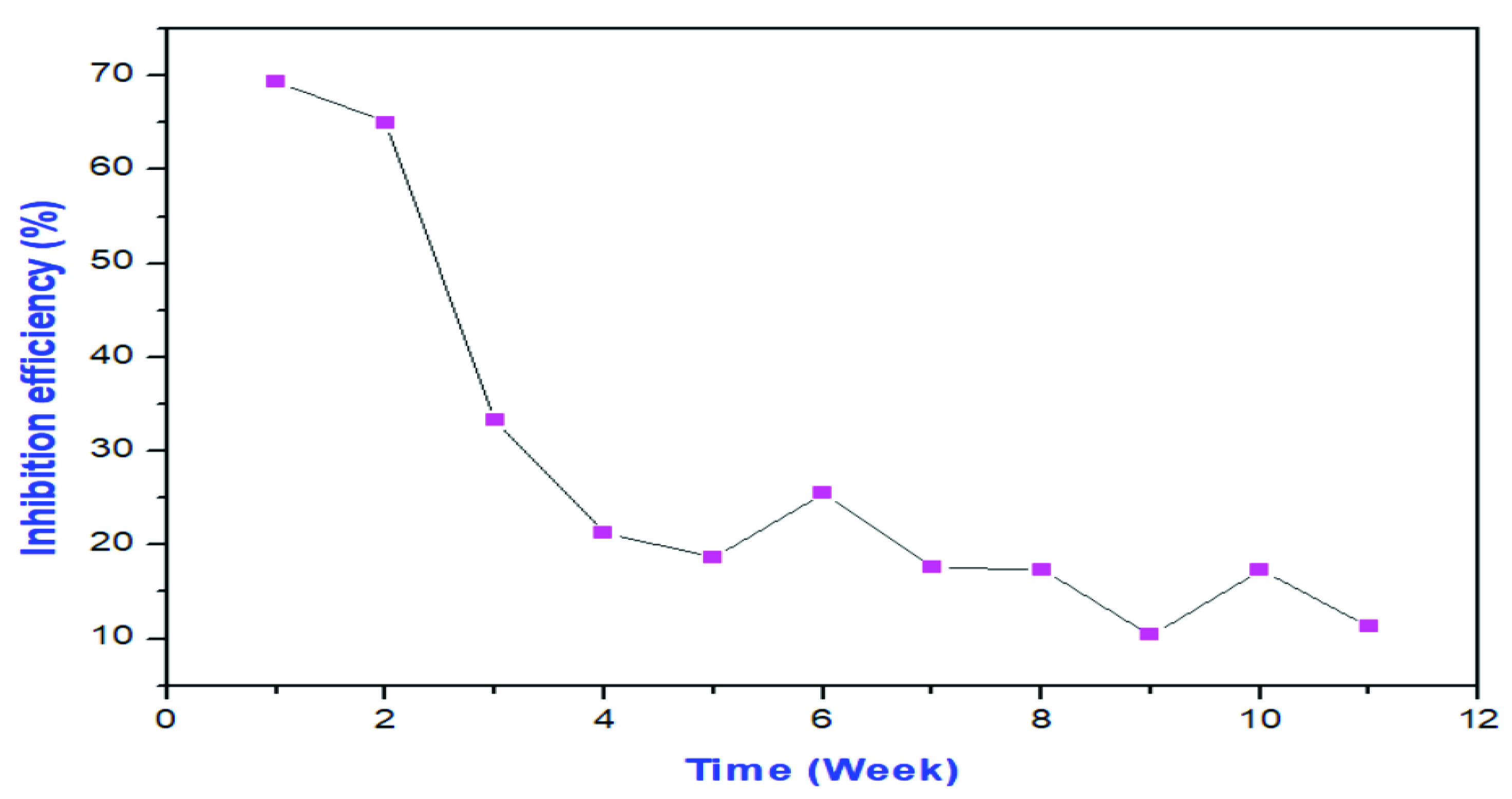
3.3. Adsorption Study
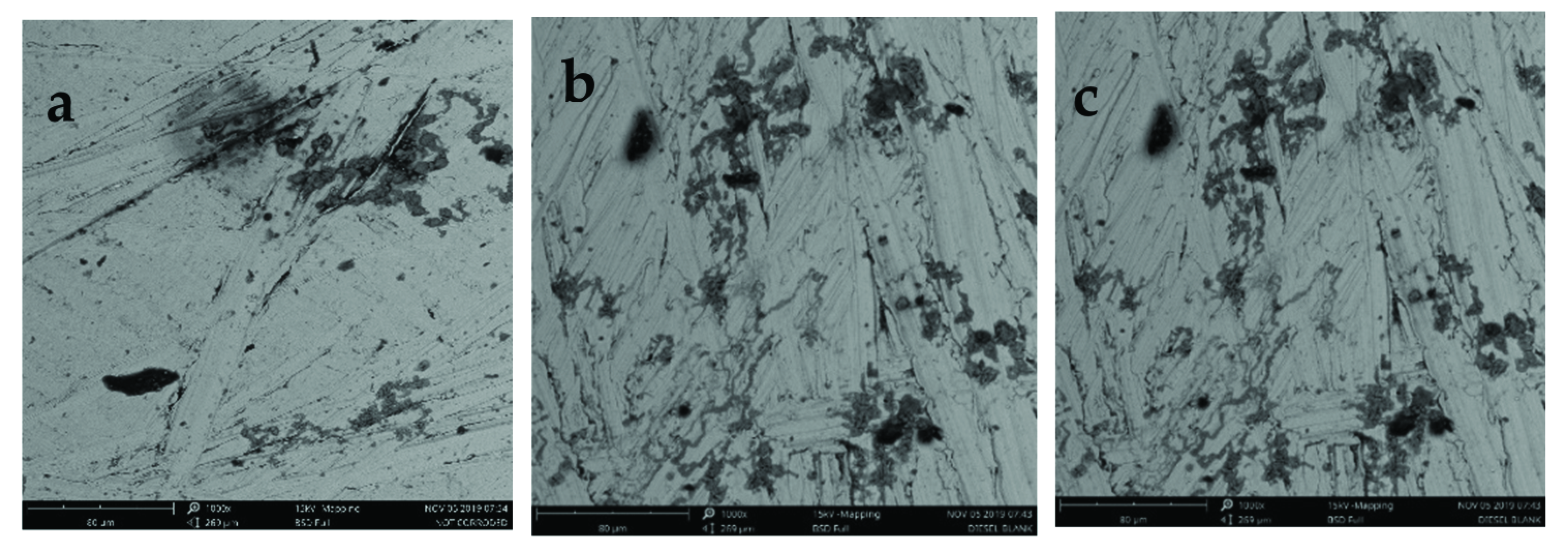
3.4. Surface Analysis
3.5. Theoretical Approach
3.6. Global Molecular Reactivity of Urea Molecule
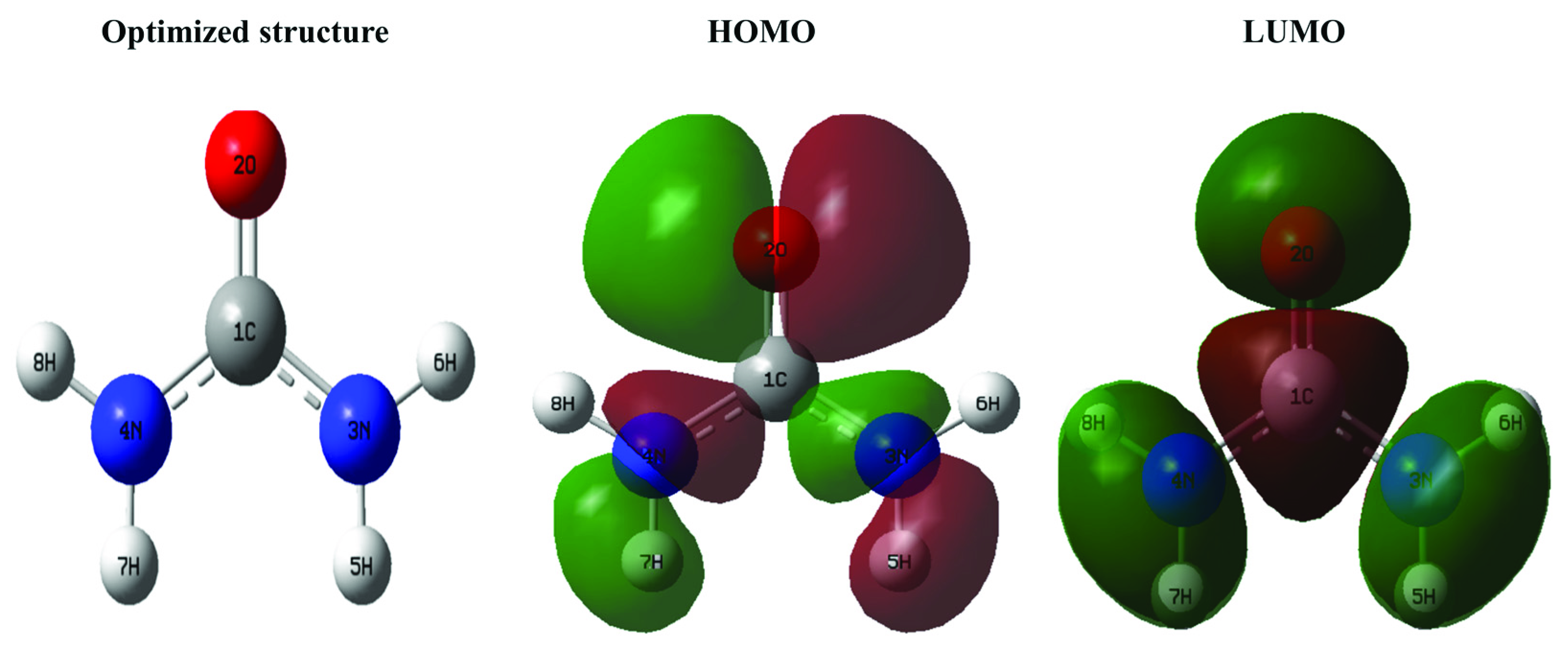
3.6.1. HOMO and LUMO
3.6.2. Fukui Function of Urea Inhibitor in Aqueous Phase
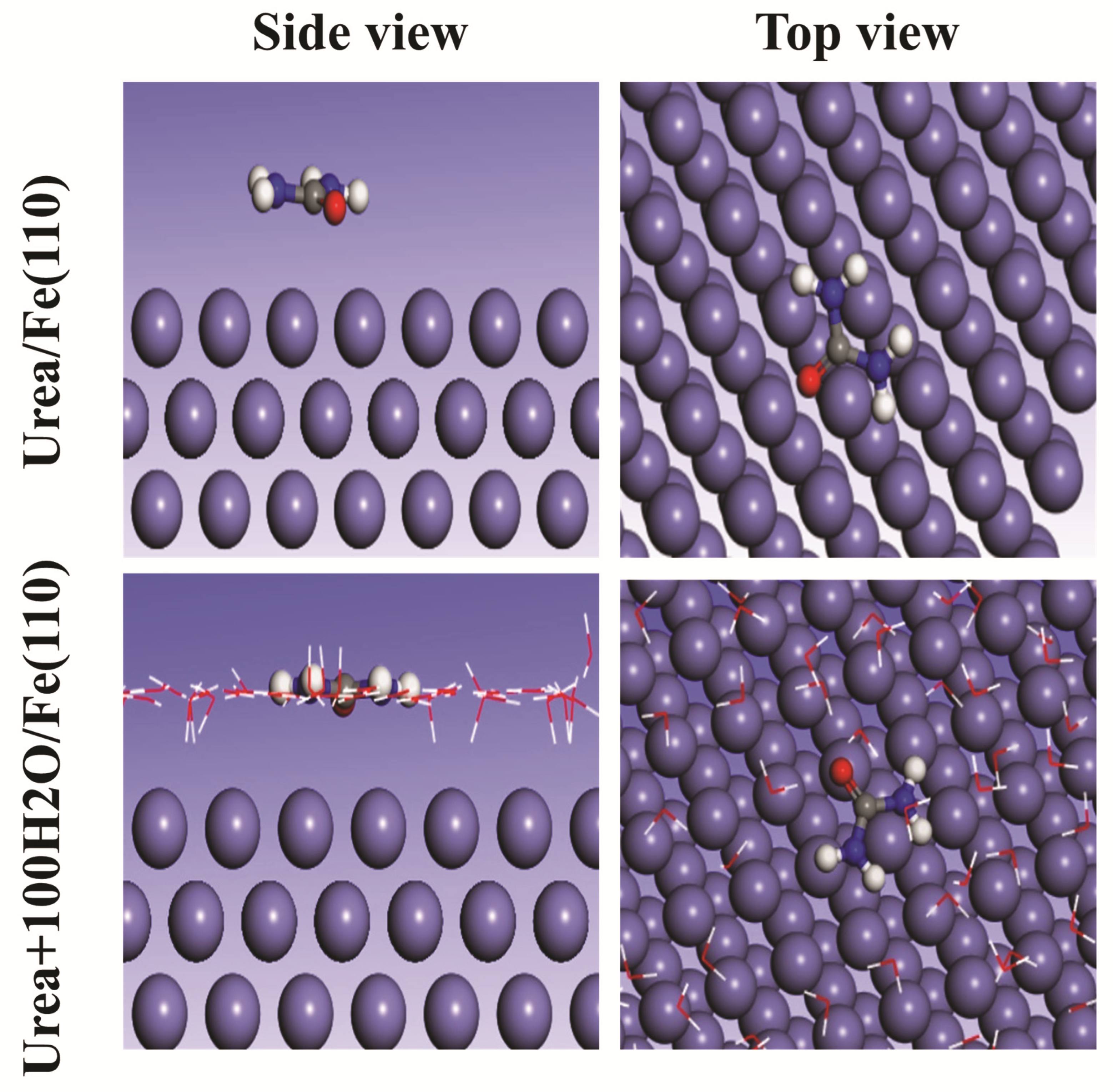
3.6.3. Monte Carlo Simulation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolawale, F.O.; Kolawale, S.K.; Agunsonye, J.O.; Adebisi, J.A.; Bello, S.A.; Hassan, S.B. Mitigation of corrosion problems I API5L steel pipeline: A review. J. Mater. Environ. Sci. 2018, 9, 2397–2410. [Google Scholar]
- Fajobi, M.A.; Loto, T.R.; Oluwole, O.O. Corrosion in crude distillation overhead system: A review. J. Bio-Tribo-Corros. 2019, 5, 67. [Google Scholar] [CrossRef]
- Fajobi, M.A.; Loto, T.R.; Oluwole, O.O. Austentic 316L stainless steel; corrosion and organic inhibitor: A review. Key Eng. Mater. 2021, 886, 126–132. [Google Scholar] [CrossRef]
- Tamalmani, K.; Husin, H. Review on corrosion inhibitors for oil and gas corrosion issues. Appl. Sci. 2020, 10, 3389. [Google Scholar]
- Obanijesu, E.O.; Pareek, V.; Gubner, R.; Tade, M.O. Corrosion education as a tool for the survival of natural gas industry. NAFTA 2010, 61, 541–554. [Google Scholar]
- Rajeev, P.; Surendranathan, A.O.; Murthy, C.S.N. Corrosion mitigation of the oil well steels using organic inhibitors—A Review. J. Mater. Environ Sci. 2012, 3, 856–869. [Google Scholar]
- Al Sultani, K.F.; Al Roubaiy, A.O.; Duaa, A.A. Characterization of corrosion behavior of low carbon steel oil pipelines by crude oil. Res. J. Recent Sci. 2016, 5, 7–13. [Google Scholar]
- Papoola, L.T.; Grema, A.S.; Latinwo, G.K.; Gutti, B.; Balogun, A.S. Corrosion problems during oil and gas production and its mitigation. Int. J. Ind. Chem. 2013, 4, 1–15. [Google Scholar]
- Perez, T.E. Corrosion in the oil and gas industry: An increasing challenge for materials. JOM 2013, 65, 1033–1042. [Google Scholar] [CrossRef] [Green Version]
- Olvera-Martinez, M.E.; Mendoza-Flores, J.; Genesca, J. CO2 corrosion control in steel pipelines: Influence of turbulent flow on the performance of corrosion inhibitors. J. Loss Prev. Process Ind. 2015, 35, 19–28. [Google Scholar]
- Asmara, Y.P. The roles of H2S gas in betiaviour of carbon steel corrosion in oil and gas environment: A review. Jurnal Tek. Mesin. 2018, 7, 37–43. [Google Scholar]
- Kahyarian, A.; Achour, M.; Nesic, S. CO2 corrosion of mild steel. In Trends in Oil and Gas Corrosion Research and Technologies; Elsevier: Amsterdam, The Netherlands, 2015; pp. 149–190. [Google Scholar]
- Ezeibe, A.U.; Nleonu, E.C.; Ahumonye, A.M. Thermodynamics study of inhibitory action of lignin extract from Gmelina arborea on the corrosion of mild steel in dilute hydrochloric acid. Int. J. Sci. Eng. Res. 2019, 7, 133–136. [Google Scholar]
- Ezeibe, A.U.; Onyemenonu, C.C.; Nleonu, E.C.; Onyema, A.V. Corrosion inhibition performance of safranine towards mild steel in acidic corrosion. Int. J. Sci. Eng. Res. 2019, 10, 1539–1544. [Google Scholar]
- Verma, D.K.; Aslam, R.; Aslam, J.; Quraishi, M.A.; Ebenso, E.E.; Verma, C. Computational modeling: Theoretical predictive tools for designing of potential organic corrosion inhibitors. J. Mol. Struct. 2021, 1236, 1–22. [Google Scholar]
- Rosli, N.R.; Yusuf, S.M.; Sauki, A.; Razali, W.M.R.W. Musa sapientum (Banana) peels as green corrosion inhibitor for mild steel. Key Eng. Mater. 2019, 797, 230–239. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A.; Obot, I.B. Adsorption behavior of glucosamine—Based, pyrimidine fused heterocycles as green corrosion inhibitors for mild steel: Experimental and theomitical studies. J. Phys. Chem. C 2016, 120, 11598–11611. [Google Scholar]
- Charitha, B.P.; Rao, P. An ecofriendly approach for corrosion control of 6061 Al-15%(v) SiC(P) composite and its base alloy. Chin. J. Chem. Eng. 2017, 25, 363–372. [Google Scholar]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar]
- Ubaka, K.G.; Mong, o.o.; Nleonu, E.C. Investigation of inhibitory effect of thiourea on corrosion of mild steel in dual purpose kerosene (DPK) and premium motor spirit (PMS). Int. J. Adv. Eng. Manag. 2020, 2, 162–168. [Google Scholar]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E. organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 563–573. [Google Scholar]
- Ayuba, A.M.; Abubakar, M. Computational study for molecular properties of some of the isolated chemicals from leaves extract of Guiera senegalensis as aluminium corrosion inhibitor. J. Sci. Technol. 2021, 13, 47–56. [Google Scholar] [CrossRef]
- Arrousse, N.; Mabrouk, E.; Ismaily Alaoui, K.; El hajjaji, F.; Rais, Z.; Taleb, M. Economical and new synthesis strategy, characterization and theoretical study of 3-oxo-3H-spiro [isobenzofuran-1,9’-xanthene]-3’,6’-diyl dibenzoate. SN Appl. Sci. 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Verma, C.; Saji, V.S.; Quraishi, M.A.; Ebenso, E.E. Pyrazole derivatives as environmental benign acid corrosion inhibitors for mild steel: Experimental and computational studies. J. Mol. Liq. 2020, 298, 111943. [Google Scholar] [CrossRef]
- Shehu, N.U.; Gaya, U.I.; Muhammad, A.A. Influence of side chain on the inhibition of aluminium corrosion in HCl by α-amino acids. Appl. Sci. Eng. Progr. 2019, 12, 186–197. [Google Scholar] [CrossRef]
- Abdellaoui, O.; Skalli, M.K.; Haoudi, A.; Kandri Rodi, Y.; Arrousse, N.; Taleb, M.; Ghibate, R.; Senhaji, O. Study of the inhibition of corrosion of mild steel in a 1M HCl solution by a new quaternary ammonium surfactant. Mor. J. Chem. 2021, 9, 44–56. [Google Scholar] [CrossRef]
- Alaoui Mrani, S.; Ech-chihbi, E.; Arrousse, N.; Rais, Z.; El Hajjaji, F.; El Abiad, C.; Radi, S.; Mabrouki, J.; Taleb, M.; Jodeh, S. DFT and Electrochemical Investigations on the Corrosion Inhibition of Mild Steel by Novel Schiff’s Base Derivatives in 1M HCl Solution. Arab. J. Sci. Eng. 2021, 46, 5691–5707. [Google Scholar] [CrossRef]
- Ayuba, A.M.; Uzairu, A.; Abba, H.; Shallangwa, G.A. Hydroxycarboxylic acids as corrosion inhibitors on aluminium metal: A computational study. J. Mater. Environ. Sci. 2018, 9, 3026–3034. [Google Scholar]
- Aluvihara, S.; Premachandra, J.K. Fundamental corrosive properties of crude oils and their effects on the ferrous metals. J. Chem. Eng. Process Technol. 2018, 9, 1–7. [Google Scholar]
- Undiandeye J, A.; Choir, T.J.; Mohammed, A.; Offurum, J.C. Kinetics of the corrosion of mild steel in petroleum-water mixtures using ethyl ester of lard as inhibitor. AU J. Technol. 2014, 17, 129–136. [Google Scholar]
- Arrousse, N.; Nahlé, A.; Mabrouk, E.; Salim, R.; El hajjaji, F.; Rais, Z.; Taleb, M. An Intelligent and Efficient Synthesis of New Inhibitors against Corrosion of Mild Steel in Acidic Media. Surf. Eng. Appl. Electrochem. 2021, 57, 136–147. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Al-Hadeethi, M.R.; Ali, I.H.; Mohamed SKToumiat, K.; Salghi, R. Naproxen-based hydrazones as effective corrosion inhibitors for mild steel in 1.0 M HCl. Coatings 2020, 10, 700. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Isahak, W.N.R.W.; Takriff, M.S. Inhibition of mild steel corrosion by 4-benzyl-1-94-oxo-4-phenylbutanoyl)thiosemicarbazide: Gravimetrical, adsorption and theoretical studies. Lubricants 2021, 9, 93. [Google Scholar] [CrossRef]
- Arrousse, N.; Mabrouk, E.; Hammouti, B.; El hajjaji, F.; Rais, Z.; Taleb, M. Synthesis, Characterization, anti corrosionbehaviour and theoritical study of the new organic dye: 3-oxo-3Hspiro[isobenzofuran-1,9′-xanthene]-3′,6′-diyl bis(3-methylbenzenesulfonate). Int. J. Corros. Scale Inhib. 2020, 9, 661–687. [Google Scholar]
- Fernine, Y.; Ech-chihbi, E.; Arrousse, N.; El Hajjaji, F.; Bousraf, F.; EbnTouhami, M.; Rais, Z.; Taleb, M. Ocimum basilicium seeds extract as an environmentally friendly antioxidant and corrosion inhibitor for aluminium alloy 2024 -T3 corrosion in 3 wt % NaCl medium. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127232. [Google Scholar] [CrossRef]
- Saady, A.; El-Hajjaji, F.; Taleb, M.; Ismaily Alaoui, K.; El Biache, A.; Mahfoud, A.; Alhouari, G.; Hammouti, B.; Chauhan, D.S.; Quraishi, M.A. Experimental and theoretical tools for corrosion inhibition study of mild steel in aqueous hydrochloric acid solution by new Indanones derivatives. Mater. Discov. 2019, 217, 1230–1242. [Google Scholar]
- Lazrak, J.; Salim, R.; Arrousse, N.; Ech-chihbi, E.; El-Hajjaji, F.; Taleb, M.; Farah, A.; Ramzi, A. Mentha viridis oil as a green effective corrosion inhibitor for mild steel in 1 M HCl medium. Int. J. Corros. Scale Inhib. 2020, 9, 1580–1606. [Google Scholar] [CrossRef]
- Mohamed, A.M.M.; Elmsellem, H.; Benchidmi, M.; Sebbar, N.K.; Belghiti, M.A.; El ouasif, L.; Jilalat, A.E.; Kadmi, Y.; Essassi, E.M. A DFT and Molecular Dynamics Study on Inhibitory Action of indazole Derivative on Corrosion of Mild Steel. JMES 2017, 8, 1860–1876. [Google Scholar]
- Zaidi, S.Z.; Hassan, S.; Raza, M.; Walsh, F.C. Transition metal oxide as possible electrode materials for Li-ion battereies: A DFT Analysis. Int. J. Electrochem. Sci. 2021, 16, 1–10. [Google Scholar]
- Arrousse, N.; Mabrouk, E.; Salim, R.; Ismaily alaoui, K.; El Hajjaji, F.; Rais, Z.; Taleb, M.; Hammouti, B. Fluorescein as commercial and environmentally friendly inhibitor against corrosion of mild steel in molar hydrochloric acid medium. Mater. Today Proc. 2020, 27, 3184–3292. [Google Scholar]
- Obot, I.B.; Haruna, K.; Saleh, T.A. Atomistic Simulation: A Unique and Powerful Computational Tool for Corrosion Inhibition Research. Arab. J. Sci. Eng. 2019, 44, 1–32. [Google Scholar]
- Arrousse, N.; Salim, R.; Kaddouri, Y.; Abdelkader, Z.D.Z.; El Hajjaji, F.; Touzani, R.; Taleb, M.; Jodeh, S. The inhibition behavior of Two pyrimidine-pyrazole derivatives against corrosion in hydrochloric solution: Experimental, surface analysis and in silico approach studies. Arab. J. Chem. 2020, 12, 5949–5965. [Google Scholar]
- Arrousse, N.; Salim, R.; Benhiba, F.; Mabrouk, E.H.; Abdelaoui, A.; El-Hajjaji, F.; Warad, I.; Zarrouk, A.; Taleb, M. Insight into the corrosion inhibition property of two new soluble and non-toxic xanthenbenzoate derivatives. J. Mol. Liq. 2021, 338, 116610. [Google Scholar]
- Arrousse, N.; Salim, R.; Abdellaoui, A.; El hajjaji, F.; Hammouti, B.; Wilson, A.D.; Mabrouk El Taleb, M. Synthesis, characterization, and evaluation of xanthene derivative as highly effective. nontoxic corrosion inhibitor for mild steel immersed in 1 M HCl solution. J. Taiwan Inst. Chem. Eng. 2021, 120, 344–359. [Google Scholar] [CrossRef]
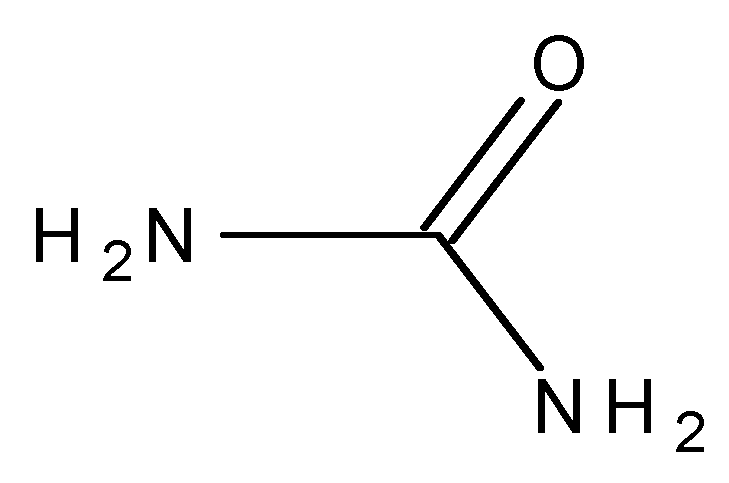

| Atoms | Fe | Ca | P | S | O | K | Mg | Na | Al | Si | Mo | C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 95.23 | 0.20 | 0.20 | 0.23 | 0.26 | 0.25 | 0.25 | 0.35 | 0.35 | 0.39 | 0.69 | 1.61 |
| Parameters | AGO |
|---|---|
| pH | 7.01 |
| Conductivity (μS/cm3) | 0.00 |
| Carbon (ppm) | 85.70 |
| Sulphur (ppm) | 236.41 |
| Density (g/mL) | 0.8191 |
| Frumkin Isotherm | Values | Flory-Huggins Isotherm | Values |
|---|---|---|---|
| R2 | 0.9821 | R2 | 0.8751 |
| d | 2.4077 | Χ | −1.5951 |
| Kads | 17.30 | Kads | 6.26 × 10−2 |
| −17.30 | (KJ/MNI) | 8.00 |
| Elements | Pure Mild Steel | Mild Steel in AGO | Mild Steel in AGO and Inhibitor |
|---|---|---|---|
| Fe | 95.23 | 94.53 | 93.81 |
| C | 1.61 | 1.69 | 3.23 |
| Mo | 0.69 | 0.00 | 0.00 |
| Si | 0.39 | 0.49 | 0.64 |
| Al | 0.35 | 0.22 | 0.42 |
| Na | 0.35 | 0.33 | 0.27 |
| K | 0.26 | 0.50 | 0.21 |
| Mg | 0.25 | 0.17 | 0.09 |
| o | 0.24 | 0.33 | 0.35 |
| S | 0.23 | 0.45 | 0.22 |
| P | 0.20 | 0.36 | 0.19 |
| Ca | 0.20 | 0.59 | 0.25 |
| Cr | 0.00 | 0.15 | 0.18 |
| Ti | 0.00 | 0.20 | 0.13 |
| Descriptors | EHOMO (eV) | ELUMO (eV) | ∆E (eV) | 𝞰 | 𝞼 (eV−1) | ∆N | χ | ω | μ (D) | Total Energy (u.a) |
|---|---|---|---|---|---|---|---|---|---|---|
| Urea (Gas phase) | −6.7187 | −1.5885 | 5.1302 | 5.9244 | 0.1688 | 0.2364 | 4.1536 | 1.4561 | 4.2487 | −225.2716 |
| Urea (Aqueous phase) | −7.0176 | −1.9935 | 5.0241 | 6.0208 | 0.1661 | 0.2071 | 4.5055 | 1.6858 | 5.3282 | −225.2845 |
| Atoms | P(N) | P(N + 1) | P(N − 1) | |||
|---|---|---|---|---|---|---|
| C1 | 5.1960 | 5.2084 | 5.2655 | 0.0124 | −0.0695 | −0.0571 |
| O2 | 8.7322 | 8.7640 | 8.6104 | 0.0318 | 0.1218 | 0.1536 |
| N3 | 7.8990 | 7.9370 | 7.5106 | 0.038 | 0.3884 | 0.4264 |
| N4 | 7.8990 | 7.9370 | 7.5106 | 0.038 | 0.3884 | 0.4264 |
| Systems | Adsorption Energy Inhibitor | Adsorption Energy Water |
|---|---|---|
| Fe(110)/urea | −24.78 | - |
| Fe(110)/urea/100H2O | −1567.98 | −15.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chile, N.E.; Haldhar, R.; Godffrey, U.K.; Chijioke, O.C.; Umezuruike, E.A.; Ifeoma, O.P.; Oke, M.O.; Ichou, H.; Arrousse, N.; Kim, S.-C.; et al. Theoretical Study and Adsorption Behavior of Urea on Mild Steel in Automotive Gas Oil (AGO) Medium. Lubricants 2022, 10, 157. https://doi.org/10.3390/lubricants10070157
Chile NE, Haldhar R, Godffrey UK, Chijioke OC, Umezuruike EA, Ifeoma OP, Oke MO, Ichou H, Arrousse N, Kim S-C, et al. Theoretical Study and Adsorption Behavior of Urea on Mild Steel in Automotive Gas Oil (AGO) Medium. Lubricants. 2022; 10(7):157. https://doi.org/10.3390/lubricants10070157
Chicago/Turabian StyleChile, Nleonu Emmanuel, Rajesh Haldhar, Ubaka Kelechi Godffrey, Onyemenonu Christopher Chijioke, Ezeibe Anderson Umezuruike, Okeke Pamela Ifeoma, Mong Oke Oke, Hamza Ichou, Nadia Arrousse, Seong-Cheol Kim, and et al. 2022. "Theoretical Study and Adsorption Behavior of Urea on Mild Steel in Automotive Gas Oil (AGO) Medium" Lubricants 10, no. 7: 157. https://doi.org/10.3390/lubricants10070157
APA StyleChile, N. E., Haldhar, R., Godffrey, U. K., Chijioke, O. C., Umezuruike, E. A., Ifeoma, O. P., Oke, M. O., Ichou, H., Arrousse, N., Kim, S.-C., Dagdag, O., Ebenso, E. E., & Taleb, M. (2022). Theoretical Study and Adsorption Behavior of Urea on Mild Steel in Automotive Gas Oil (AGO) Medium. Lubricants, 10(7), 157. https://doi.org/10.3390/lubricants10070157









