Common Genetic Risk for Melanoma Encourages Preventive Behavior Change
Abstract
:1. Introduction
2. Results and Discussion
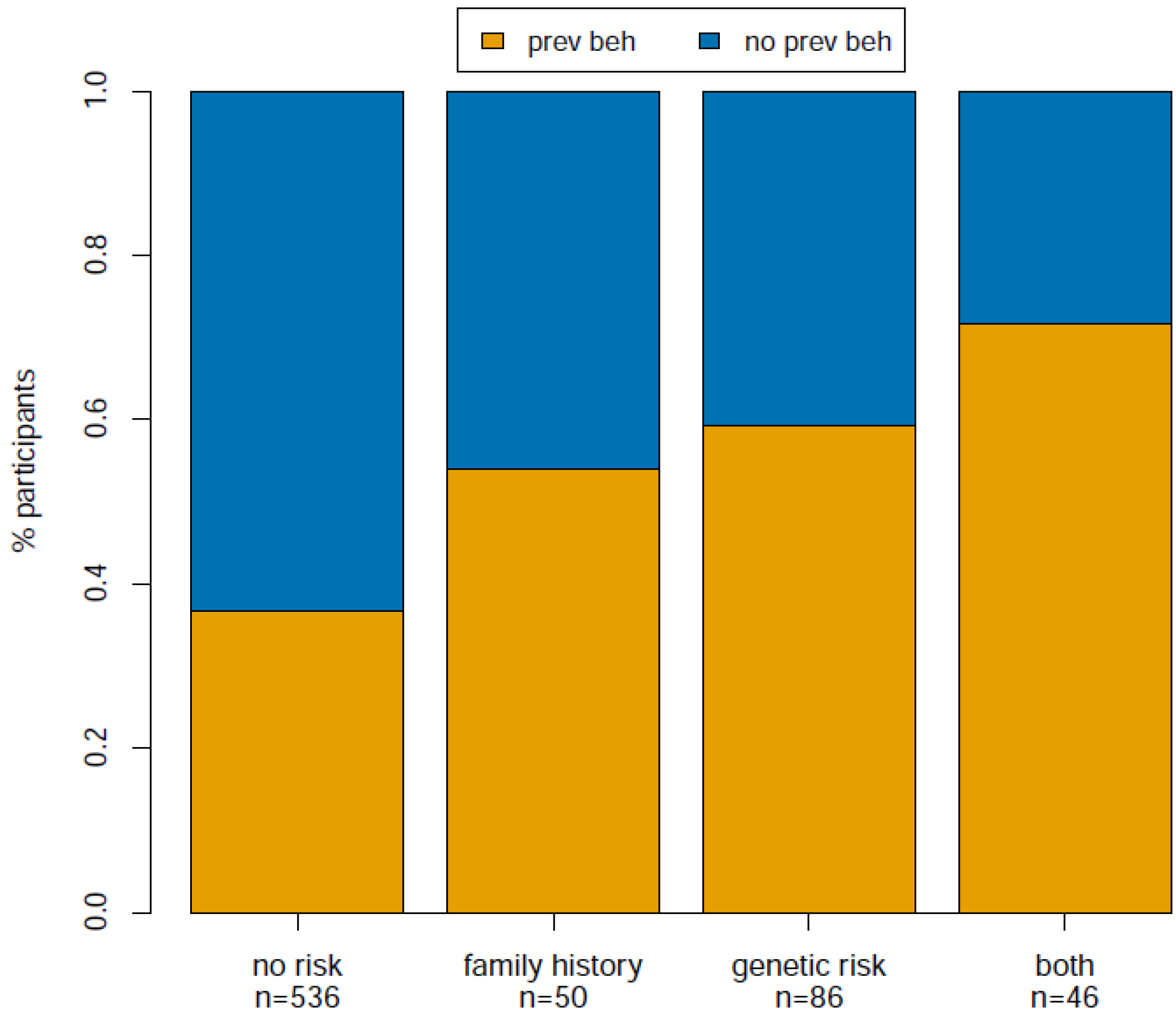
2.1. Results
| n | 718 |
|---|---|
| age in years, mean (range) | 52.86 (21–91) |
| male, n (%) | 245 (34.12) |
| female, n (%) | 473 (65.88) |
| Air Force Medical Service, n (%) | 118 (16.43) |
| Coriell Personalized Medicine Collaborative community, n (%) | 498 (69.36) |
| The Ohio State University community, n (%) | 102 (14.21) |
| OR | eta | SE | z Value | p Value | |
|---|---|---|---|---|---|
| (Intercept) | 0.20 | −1.61 | 0.36 | −4.43 | 9.25 e-06 |
| family history (vs. no risk) | 2.04 | 0.71 | 0.30 | 2.36 | 0.02 |
| genetic (vs. no risk) | 2.79 | 1.03 | 0.25 | 4.18 | 2.86 e-05 |
| both (vs. no risk) | 4.06 | 1.40 | 0.34 | 4.07 | 4.67 e-05 |
| Column1 | OR | eta | SE | z Value | p Value |
|---|---|---|---|---|---|
| (Intercept) | 0.07 | −2.62 | 0.41 | −6.34 | 2.36 e-10 |
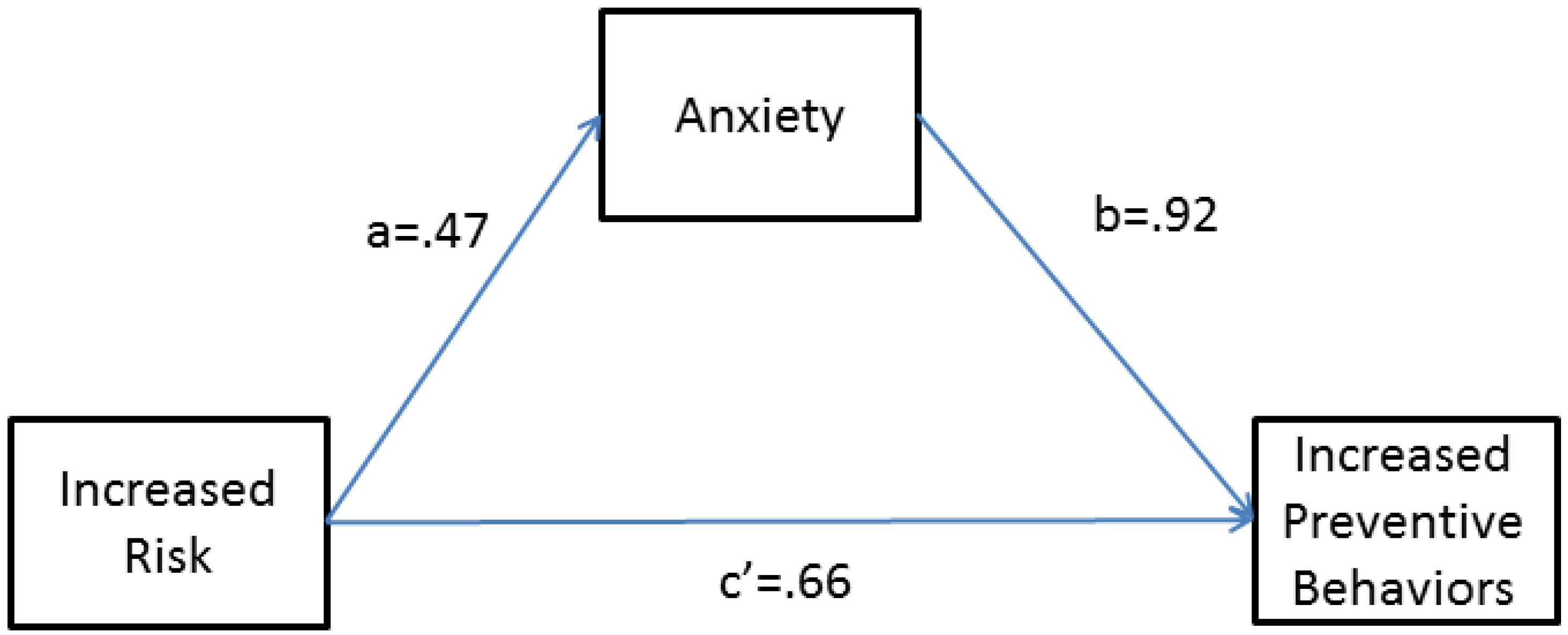
| anxiety | NA | 0.91 | 0.16 | 5.74 | 9.64 e-09 |
| family history (vs. no risk) | 1.71 | 0.53 | 0.31 | 1.71 | 0.09 |
| genetic risk (vs. no risk) | 1.93 | 0.66 | 0.26 | 2.51 | 0.01 |
| both (vs. no risk) | 2.35 | 0.86 | 0.37 | 2.33 | 0.02 |
2.2. Discussion
3. Experimental Section
3.1. Study Population
3.2. CPMC Survey
3.3. Exclusion Criteria
3.4. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Appendix
| OR | eta | SE | z Value | p Value | |
|---|---|---|---|---|---|
| (Intercept) | 0.18 | −1.70 | 0.37 | −4.58 | 4.55 e-06 |
| family history (vs. no risk) | 2.04 | 0.71 | 0.30 | 2.35 | 0.02 |
| genetic (vs. no risk) | 1.90 | 0.64 | 0.24 | 2.65 | 7.99 e-03 |
| both (vs. no risk) | 3.44 | 1.24 | 0.33 | 3.78 | 1.57 e-04 |
| OR | eta | SE | z Value | p Value | |
|---|---|---|---|---|---|
| (Intercept) | 0.12 | −2.14 | 0.43 | −5.00 | 5.66 e-07 |
| family history (vs. no risk) | 2.10 | 0.74 | 0.33 | 2.26 | 0.02 |
| genetic (vs. no risk) | 3.43 | 1.23 | 0.25 | 4.90 | 9.41 e-07 |
| both (vs. no risk) | 5.23 | 1.67 | 0.32 | 5.14 | 2.78 e-07 |
- 1
- 2
- 3
- 4
- 5
- Do not want to answer
- Did your CPMC result show that you have a genetic risk variant for melanoma?
- Yes, 1 copy
- Yes, 2 copies
- No
- Do not know
- Do not want to answer
- Did your CPMC result show that you have an increased risk for melanoma based on your family history?
- Yes
- No
- Do not know
- Do not want to answer
- Have you shared your CPMC result for melanoma with a health care provider?
- Yes
- No
- Not yet, but I am planning to
- Do not want to answer
| Increased | Did not Change | Decreased | Do not Want to Answer | |
|---|---|---|---|---|
| My exposure to the sun | ||||
| My use of sunscreen | ||||
| The amount of protective clothing I wear | ||||
| The number of skin self-exams I perform |
- What motivated you to change your level of exposure to the sun?
- My CPMC genetic variant result for melanoma
- My CPMC family history result for melanoma
- I had symptoms of melanoma
- My CPMC results for other conditions
- My health care provider's recommendations
- I have/had another type of skin cancer (basal cell, squamous cell, etc.)
- Do not want to answer
- Other
- What motivated you to change your use of sunscreen?
- My CPMC genetic variant result for melanoma
- My CPMC family history result for melanoma
- I had symptoms of melanoma
- My CPMC results for other conditions
- My health care provider’s recommendations
- I have/had another type of skin cancer (basal cell, squamous cell, etc.)
- Do not want to answer
- Other
- What motivated you to change the amount of protective clothing you wear?
- My CPMC genetic variant result for melanoma
- My CPMC family history result for melanoma
- I had symptoms of melanoma
- My CPMC results for other conditions
- My health care provider’s recommendations
- I have/had another type of skin cancer (basal cell, squamous cell, etc.)
- Do not want to answer
- Other
- What motivated you to change the number of skin self exams you perform?
- My CPMC genetic variant result for melanoma
- My CPMC family history result for melanoma
- I had symptoms of melanoma
- My CPMC results for other conditions
- My health care provider’s recommendations
- I have/had another type of skin cancer (basal cell, squamous cell, etc.)
- Do not want to answer
- Other
- Please indicate on the following scale the level of anxiety, if any, you felt immediately after viewing your CPMC result report for melanoma:
- None
- Low
- Moderate
- High
- Very High
- Do not want to answer
Conflicts of Interest
References
- Haga, S.B.; Khoury, M.J.; Burke, W. Genomic profiling to promote a healthy lifestyle: Not ready for prime time. Nat. Genet. 2003, 34, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P.; Skrzynia, C.; Burke, W. The complexities of predictive genetic testing. Br. Med. J. 2001, 322, 1052–1056. [Google Scholar] [CrossRef]
- Burke, W.; Atkins, D.; Gwinn, M.; Guttmacher, A.; Haddow, J.; Lau, J.; Palomaki, G.; Press, N.; Richards, C.S.; Wideroff, L.; et al. Genetic test evaluation: Information needs of clinicians, policy makers, and the public. Am. J. Epidemiol. 2002, 156, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Scheuner, M.T.; Sieverding, P.; Shekelle, P.G. Delivery of genomic medicine for common chronic adult diseases: A systematic review. JAMA 2008, 299, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- CDC. Skin Cancer. Available online: http://www.cdc.gov/cancer/skin/statistics/behavior.htm (accessed on 17 Octorber 2014).
- Aspinwall, L.G.; Leaf, S.L.; Dola, E.R.; Kohlmann, W.; Leachman, S.A. CDKN2A/p16 genetic test reporting improves early detection intentions and practices in high-risk melanoma families. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1510–1519. [Google Scholar] [CrossRef]
- Kasparian, N.A.; Meiser, B.; Butow, P.N.; Simpson, J.M.; Mann, G.J. Genetic testing for melanoma risk: A prospective cohort study of uptake and outcomes among australian families. Genet. Med. 2009, 11, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Aspinwall, L.G.; Leaf, S.L.; Kohlmann, W.; Dola, E.R.; Leachman, S.A. Patterns of photoprotection following CDKN2A/p16 genetic test reporting and counseling. J. Am. Acad. Dermatol. 2009, 60, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Aspinwall, L.G.; Taber, J.M.; Kohlmann, W.; Leaf, S.L.; Leachman, S.A. Unaffected family members report improvements in daily routine sun protection 2 years following melanoma genetic testing. Genet. Med. 2014, 16, 846–853. [Google Scholar] [CrossRef] [PubMed]
- 1000 Genomes Project Consortium; Abecasis, G.R.; Auton, A.; Brooks, L.D.; DePristo, M.A.; Durbin, R.M.; Handsaker, R.E.; Kang, H.M.; Marth, G.T.; McVean, G.A. An integrated map of genetic variation from 1092 human genomes. Nature 2012, 491, 56–65. [Google Scholar]
- Brown, K.M.; Macgregor, S.; Montgomery, G.W.; Craig, D.W.; Zhao, Z.Z.; Iyadurai, K.; Henders, A.K.; Homer, N.; Campbell, M.J.; Stark, M.; et al. Common sequence variants on 20q11.22 confer melanoma susceptibility. Nat. Genet. 2008, 40, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.A.; Gordon, E.S.; Stack, C.B.; Gharani, N.; Sill, C.J.; Schmidlen, T.J.; Joseph, M.; Pallies, J.; Gerry, N.P.; Christman, M.F. Coriell personalized medicine collaborative®: A prospective study of the utility of personalized medicine. Pers. Med. 2010, 7, 301–317. [Google Scholar] [CrossRef]
- Stack, C.B.; Gharani, N.; Gordon, E.S.; Schmidlen, T.; Christman, M.F.; Keller, M.A. Genetic risk estimation in the coriell personalized medicine collaborative. Genet. Med. 2011, 13, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kronenthal, C.K.; Delaney, S.K.; Christman, M.F. Broadening research consent in the era of genome-informed medicine. Genet. Med. 2012, 4, 432–436. [Google Scholar] [CrossRef]
- Hayes, A.F. Beyond baron and kenny: Statistical mediation analysis in the new millennium. Commun. Monogr. 2009, 76, 408–420. [Google Scholar] [CrossRef]
- Rich, E.C.; Burke, W.; Heaton, C.J.; Haga, S.; Pinsky, L.; Short, M.P.; Acheson, L. Reconsidering the family history in primary care. J. Gen. Intern. Med. 2004, 19, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Vassy, J.L. Can genetic information change patient behavior to reduce type 2 diabetes risk? Pers. Med. 2013, 10. [Google Scholar] [CrossRef]
- Weinstock, M.A. Early detection of melanoma. JAMA 2000, 284, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Preacher, K.J.; Rucker, D.D.; Hayes, A.F. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivar. Behav. Res. 2007, 42, 185–227. [Google Scholar] [CrossRef]
- Hayes, A.F. Process: A Versatile Computational Tool for Observed Variable Mediation, Moderation, and Conditional Process Modeling; Guilford Press: New York, NY, USA, 2013. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diseati, L.; Scheinfeldt, L.B.; Kasper, R.S.; Zhaoyang, R.; Gharani, N.; Schmidlen, T.J.; Gordon, E.S.; Sessions, C.K.; Delaney, S.K.; Jarvis, J.P.; et al. Common Genetic Risk for Melanoma Encourages Preventive Behavior Change. J. Pers. Med. 2015, 5, 36-49. https://doi.org/10.3390/jpm5010036
Diseati L, Scheinfeldt LB, Kasper RS, Zhaoyang R, Gharani N, Schmidlen TJ, Gordon ES, Sessions CK, Delaney SK, Jarvis JP, et al. Common Genetic Risk for Melanoma Encourages Preventive Behavior Change. Journal of Personalized Medicine. 2015; 5(1):36-49. https://doi.org/10.3390/jpm5010036
Chicago/Turabian StyleDiseati, Lori, Laura B. Scheinfeldt, Rachel S. Kasper, Ruixue Zhaoyang, Neda Gharani, Tara J. Schmidlen, Erynn S. Gordon, Cecili K. Sessions, Susan K. Delaney, Joseph P. Jarvis, and et al. 2015. "Common Genetic Risk for Melanoma Encourages Preventive Behavior Change" Journal of Personalized Medicine 5, no. 1: 36-49. https://doi.org/10.3390/jpm5010036
APA StyleDiseati, L., Scheinfeldt, L. B., Kasper, R. S., Zhaoyang, R., Gharani, N., Schmidlen, T. J., Gordon, E. S., Sessions, C. K., Delaney, S. K., Jarvis, J. P., Gerry, N., & Christman, M. (2015). Common Genetic Risk for Melanoma Encourages Preventive Behavior Change. Journal of Personalized Medicine, 5(1), 36-49. https://doi.org/10.3390/jpm5010036







