Impact of Donor Age on Graft Failure After Deceased Donor Liver Transplantation by Donor-Recipient Sex Combinations: An Analysis of the UNOS OPTN Database
Abstract
1. Introduction
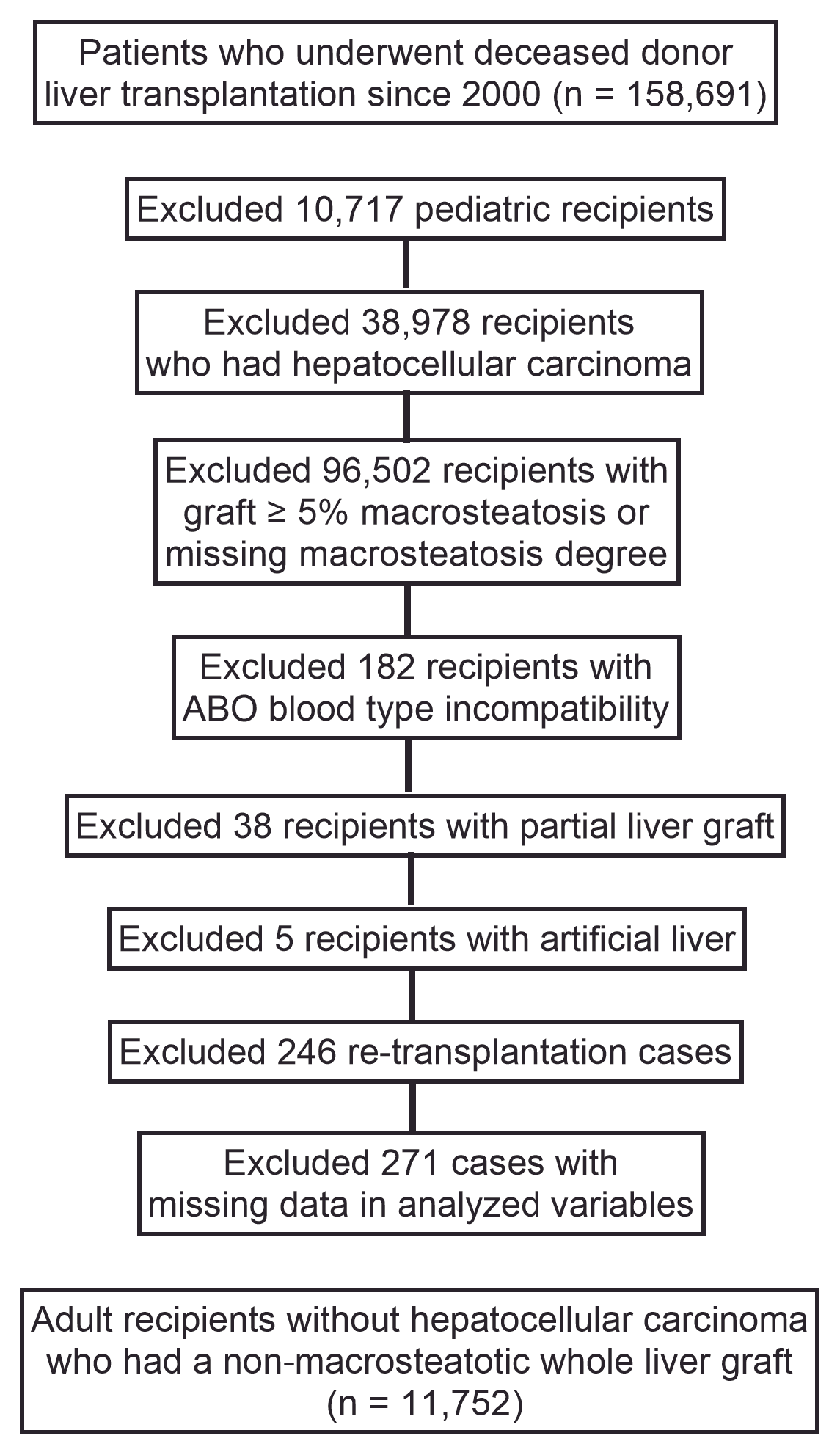
2. Materials and Methods
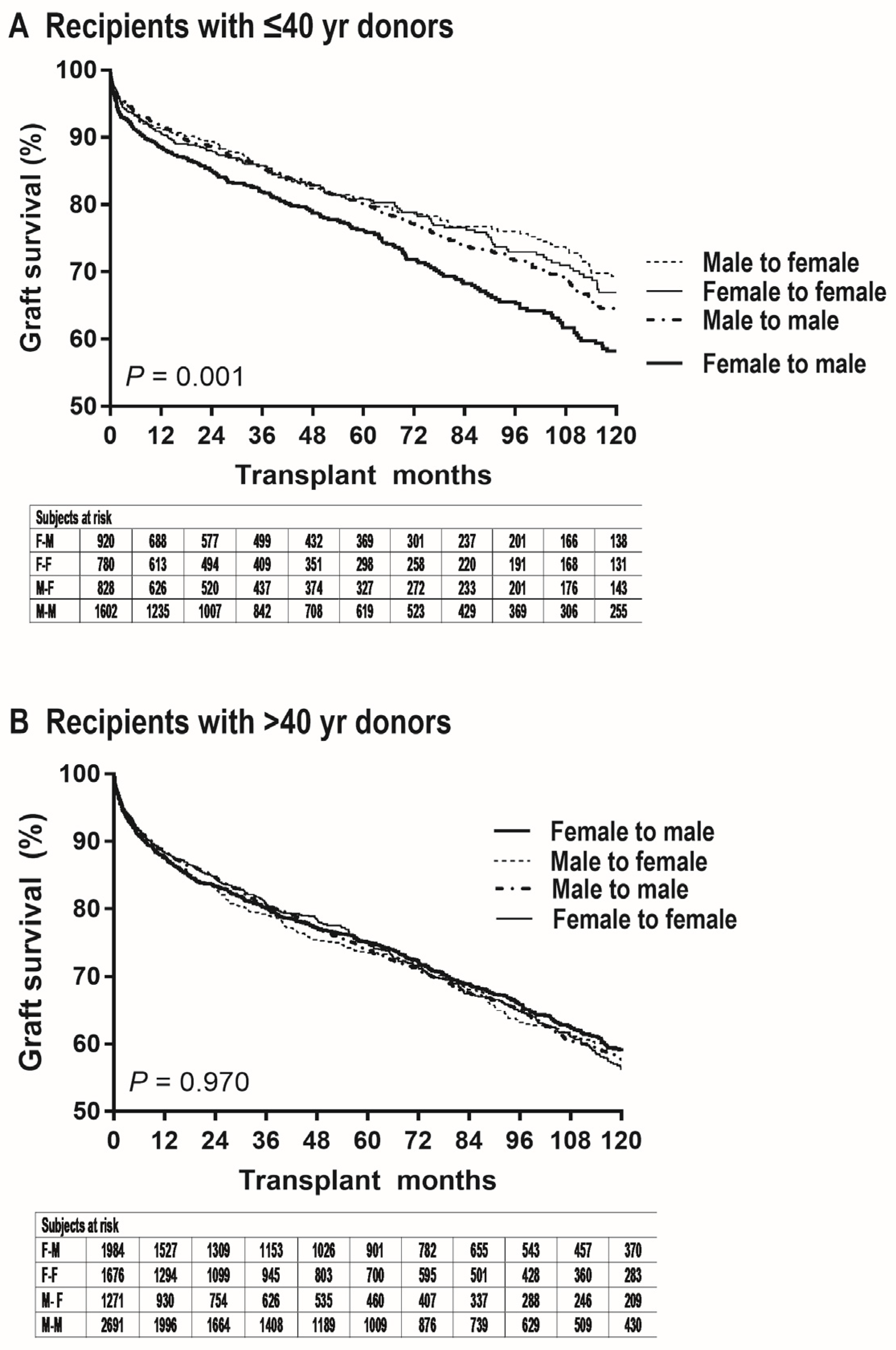
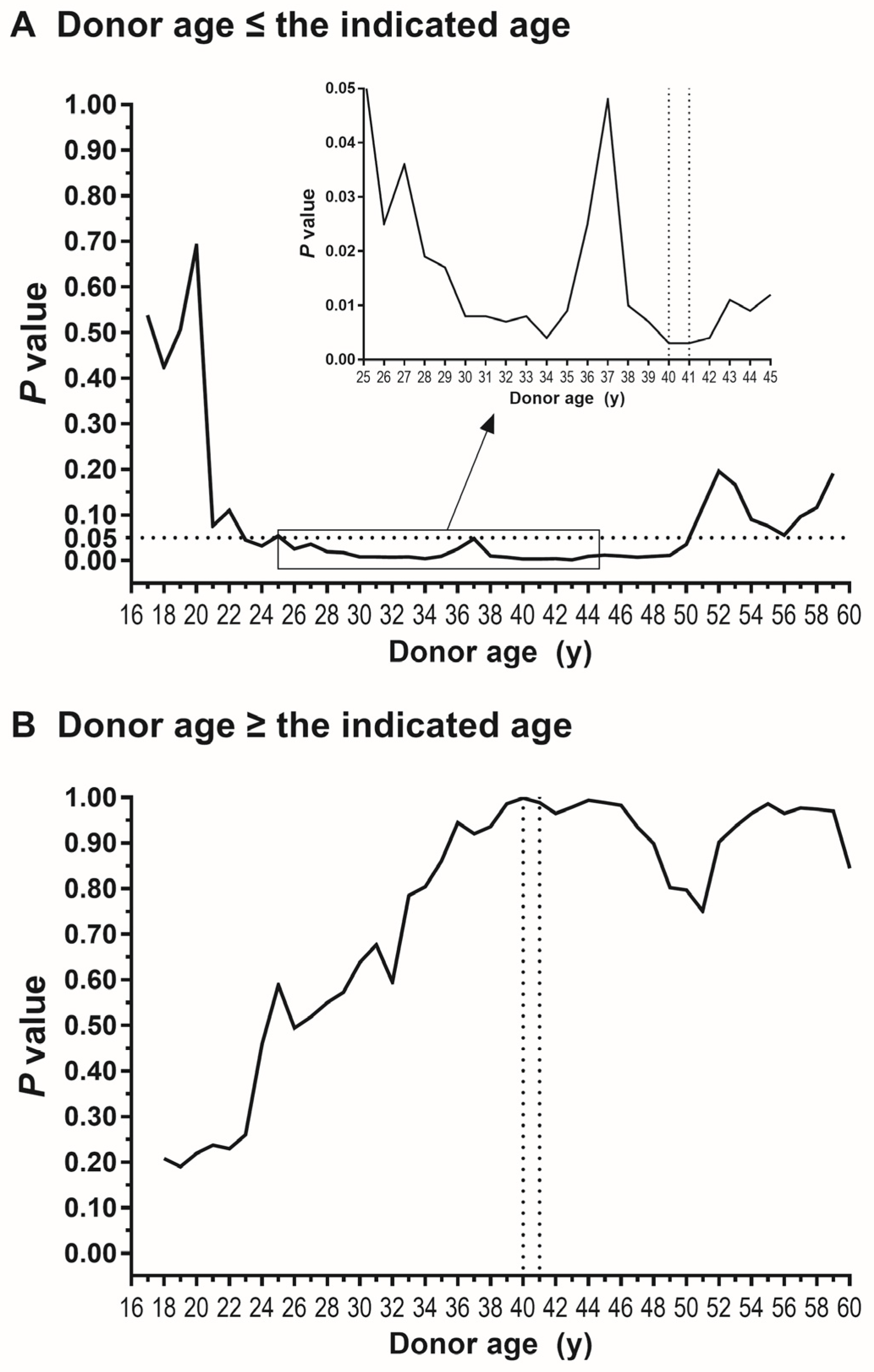
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ELTR | European liver transplant registry |
| HCC | hepatocellular carcinoma |
| HR | hazard ratio |
| LT | liver transplantation |
| MASH | metabolic dysfunction-associated steatohepatitis |
| OPTN | Organ Procurement and Transplantation Network |
| UNOS | United Network for Organ Sharing |
References
- Shi, Y.; Ma, J.; Li, S.; Liu, C.; Liu, Y.; Chen, J.; Liu, N.; Liu, S.; Huang, H. Sex difference in human diseases: Mechanistic insights and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 238. [Google Scholar] [CrossRef]
- Feng, Z.; Liao, M.; Zhang, L. Sex differences in disease: Sex chromosome and immunity. J. Transl. Med. 2024, 22, 1150. [Google Scholar] [CrossRef]
- Massey, S.C.; Whitmire, P.; Doyle, T.E.; Ippolito, J.E.; Mrugala, M.M.; Hu, L.S.; Canoll, P.; Anderson, A.R.A.; Wilson, M.A.; Fitzpatrick, S.M.; et al. Sex differences in health and disease: A review of biological sex differences relevant to cancer with a spotlight on glioma. Cancer Lett. 2021, 498, 178–187. [Google Scholar] [CrossRef]
- Cattaneo, A.; Bellenghi, M.; Ferroni, E.; Mangia, C.; Marconi, M.; Rizza, P.; Borghini, A.; Martini, L.; Luciani, M.N.; Ortona, E.; et al. Recommendations for the Application of Sex and Gender Medicine in Preclinical, Epidemiological and Clinical Research. J. Pers. Med. 2024, 14, 908. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Han, S.; Cho, J.; Wi, W.; Won Lee, K.; Hwa Cha, H.; Lee, S.; Hyun Ahn, J.; Kim, S.; Sung Choi, G.; Man Kim, J.; et al. Sex difference in the tolerance of hepatic ischemia-reperfusion injury and hepatic estrogen receptor expression according to age and macrosteatosis in healthy living liver donors. Transplantation 2022, 106, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Yang, J.D.; Sinn, D.H.; Kim, J.M.; Choi, G.S.; Jung, G.; Ahn, J.H.; Kim, S.; Ko, J.S.; Gwak, M.S.; et al. Risk of post-transplant hepatocellular carcinoma recurrence is higher in recipients of livers from male than female living donors. Ann. Surg. 2018, 268, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Kasarinaite, A.; Sinton, M.; Saunders, P.T.K.; Hay, D.C. The Influence of Sex Hormones in Liver Function and Disease. Cells 2023, 12, 1604. [Google Scholar] [CrossRef]
- Harada, H.; Pavlick, K.P.; Hines, I.N.; Hoffman, J.M.; Bharwani, S.; Gray, L.; Wolf, R.E.; Grisham, M.B. Effects of gender on reduced-size liver ischemia and reperfusion injury. J. Appl. Physiol. 2001, 91, 2816–2822. [Google Scholar] [CrossRef]
- Lee, K.W.; Han, S.; Lee, S.; Cha, H.H.; Ahn, S.; Ahn, H.S.; Ko, J.S.; Gwak, M.S.; Kim, G.S.; Joh, J.W.; et al. Higher risk of posttransplant liver graft failure in male recipients of female donor grafts might not be due to anastomotic size disparity. Transplantation 2018, 102, 1115–1123. [Google Scholar] [CrossRef]
- Kahn, D.; Gavaler, J.S.; Makowka, L.; van Thiel, D.H. Gender of donor influences outcome after orthotopic liver transplantation in adults. Dig. Dis. Sci. 1993, 38, 1485–1488. [Google Scholar] [CrossRef]
- Brooks, B.K.; Levy, M.F.; Jennings, L.W.; Abbasoglu, O.; Vodapally, M.; Goldstein, R.M.; Husberg, B.S.; Gonwa, T.A.; Klintmalm, G.B. Influence of donor and recipient gender on the outcome of liver transplantation. Transplantation 1996, 62, 1784–1787. [Google Scholar] [CrossRef]
- Francavilla, R.; Hadzic, N.; Heaton, N.D.; Rela, M.; Baker, A.J.; Dhawan, A.; Mieli-Vergani, G. Gender matching and outcome after pediatric liver transplantation. Transplantation 1998, 66, 602–605. [Google Scholar] [CrossRef]
- Croome, K.P.; Segal, D.; Hernandez-Alejandro, R.; Adams, P.C.; Thomson, A.; Chandok, N. Female donor to male recipient gender discordance results in inferior graft survival: A prospective study of 1,042 liver transplants. J. Hepatobiliary Pancreat. Sci. 2014, 21, 269–274. [Google Scholar] [CrossRef]
- Gao, F.; Dong, L.; Chen, J.; Xu, S.; Wang, Z.; Xu, H.; Qiu, X.; Wu, Y.; Shao, C.; Wei, X.; et al. Impact of donor-recipient sex-matching patterns on liver transplantation outcomes: A cohort study based on United Network of Organ Sharing data. Hepatobiliary Surg. Nutr. 2025. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Shirabe, K.; Taketomi, A.; Uchiyama, H.; Harada, N.; Ijichi, H.; Yoshimatsu, M.; Ikegami, T.; Soejima, Y.; Maehara, Y. Risk factors that increase mortality after living donor liver transplantation. Transplantation 2012, 93, 93–98. [Google Scholar] [CrossRef]
- Kahn, D.; Zeng, Q.H.; Makowka, L.; Murase, N.; Nakajima, Y.; Eagon, P.K.; Francavilla, A.; Starzl, T.E.; Van Thiel, D.H. Orthotopic liver transplantation and the cytosolic estrogen-androgen receptor status of the liver: The influence of the sex of the donor. Hepatology 1989, 10, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Kwon, J.H.; Lee, K.W.; Lee, S.; Choi, G.S.; Kim, J.M.; Ko, J.S.; Gwak, M.S.; Kim, G.S.; Ha, S.Y.; et al. Abrogation of greater graft failure risk of female-to-male liver transplantation with donors older than 40 years or graft macrosteatosis greater than 5%. Sci. Rep. 2023, 13, 12914. [Google Scholar] [CrossRef] [PubMed]
- Magyar, C.T.J.; Arteaga, N.F.; Germani, G.; Karam, V.H.; Adam, R.; Romagnoli, R.; De Simone, P.; Robin, F.; Cherqui, D.; Bosca, A.; et al. Recipient-donor sex constellation in liver transplantation for hepatocellular carcinoma-an ELTR study. Liver Int. Off. J. Int. Assoc. Study Liver 2024, 45, e16178. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Tannapfel, A.; Denk, H.; Dienes, H.P.; Langner, C.; Schirmacher, P.; Trauner, M.; Flott-Rahmel, B. Histopathological diagnosis of non-alcoholic and alcoholic fatty liver disease. Virchows Arch. Int. J. Pathol. 2011, 458, 511–523. [Google Scholar] [CrossRef]
- Erkan, G.; Yilmaz, G.; Konca Degertekin, C.; Akyol, G.; Ozenirler, S. Presence and extent of estrogen receptor-alpha expression in patients with simple steatosis and NASH. Pathol. Res. Pract. 2013, 209, 429–432. [Google Scholar] [CrossRef]
- Han, S.; Kim, G.; Lee, S.K.; Kwon, C.H.; Gwak, M.; Lee, S.; Ha, S.; Park, C.K.; Ko, J.S.; Joh, J. Comparison of the tolerance of hepatic ischemia/reperfusion injury in living donors: Macrosteatosis versus microsteatosis. Liver Transpl. 2014, 20, 775–783. [Google Scholar] [CrossRef]
- Galle, P.R.; Forner, A.; Llovet, J.M.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; European Association for the Study of the Liver. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar]
- Eagon, P.K.; Porter, L.E.; Francavilla, A.; DiLeo, A.; Van Thiel, D.H. Estrogen and androgen receptors in liver: Their role in liver disease and regeneration. Semin. Liver Dis. 1985, 5, 59–69. [Google Scholar] [CrossRef]
- Chen, K.L.; Madak-Erdogan, Z. Estrogens and female liver health. Steroids 2018, 133, 38–43. [Google Scholar] [CrossRef]
- Francavilla, A.; di Leo, A.; Eagon, P.K.; Wu, S.Q.; Ove, P.; van Thiel, D.H.; Starzl, T.E. Regenerating rat liver: Correlations between estrogen receptor localization and deoxyribonucleic acid synthesis. Gastroenterology 1984, 86, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Gunduz, N.; Saffer, E.A.; Zheng, S. Relation of estrogen and its receptor to rat liver growth and regeneration. Cancer Res. 1984, 44, 2410–2415. [Google Scholar] [PubMed]
- Faddy, M.J.; Gosden, R.G.; Gougeon, A.; Richardson, S.J.; Nelson, J.F. Accelerated disappearance of ovarian follicles in mid-life: Implications for forecasting menopause. Hum. Reprod. 1992, 7, 1342–1346. [Google Scholar] [CrossRef]
- Han, S.; Kwon, J.H.; Jung, S.H.; Seo, J.Y.; Jo, Y.J.; Jang, J.S.; Yeon, S.M.; Jung, S.H.; Ko, J.S.; Gwak, M.S.; et al. Perioperative fresh red blood cell transfusion may negatively affect recipient survival after liver transplantation. Ann. Surg. 2018, 267, 346–351. [Google Scholar] [CrossRef] [PubMed]
| Female to Male (n = 920) | Female to Female (n = 780) | Male to Female (n = 828) | Male to Male (n = 1602) | |
|---|---|---|---|---|
| Donor age (y) | 32 (25–37) | 32 (25–36) | 29 (22–35) | 30 (24–35) |
| Donor body mass index (kg/m2) | 28.1 (23.7–33.9) | 26.4 (22.7–31.6) | 25.9 (23.0–29.5) | 27.6 (24.2–31.7) |
| Donor cause of death | ||||
| Anoxia | 516 (58.0) | 431 (57.2) | 398 (49.8) | 771 (49.6) |
| Cerebrovascular/stroke | 190 (21.4) | 165 (21.9) | 76 (9.5) | 174 (11.2) |
| Others * | 214 (23.3) | 184 (23.6) | 354 (42.8) | 657 (41.0) |
| Cold ischemia time (h) | 6.2 (5.0–8.0) | 6.2 (5.0–8.0) | 6.2 (5.0–7.6) | 6.3 (5.0–8.0) |
| Transplant era | ||||
| 2000–2007 | 119 (12.9) | 102 (13.1) | 87 (10.4) | 212 (12.2) |
| 2008–2015 | 277 (30.1) | 206 (26.4) | 244 (29.5) | 462 (28.8) |
| 2016–2023 | 524 (57.0) | 472 (60.5) | 498 (60.1) | 945 (59.0) |
| Age (y) | 54 (47–60) | 55 (44–62) | 55 (45–61) | 54 (46–60) |
| Body mass index (kg/m2) | 27.5 (23.8–31.3) | 27.4 (23.4–32.3) | 27.9 (23.8–33.0) | 28.1 (24.4–32.1) |
| Diabetes | 236 (25.7) | 202 (25.9) | 192 (23.2) | 392 (24.5) |
| Primary liver disease | ||||
| Viral | 211 (22.9) | 134 (17.2) | 122 (14.7) | 343 (21.4) |
| Alcoholic | 356 (38.7) | 171 (21.9) | 221 (26.7) | 635 (39.6) |
| MASH | 114 (12.4) | 140 (17.9) | 153 (18.5) | 213 (13.3) |
| Others † | 239 (26.0) | 335 (42.9) | 332 (40.1) | 411 (25.7) |
| Hepatic encephalopathy III–IV | 132 (14.4) | 103 (13.2) | 125 (15.1) | 217 (13.6) |
| Refractory ascites | 359 (39.1) | 271 (34.7) | 305 (36.8) | 650 (40.6) |
| Previous upper abdominal surgery | 374 (40.7) | 458 (58.7) | 475 (57.4) | 605 (37.8) |
| Portal vein thrombosis | 100 (10.9) | 87 (11.2) | 94 (11.4) | 162 (10.1) |
| MELD score | 26 (19–33) | 25 (19–33) | 27 (20–34) | 25 (19–33) |
| Pretransplant critical care | ||||
| Dialysis | 180 (19.6) | 154 (19.7) | 194 (23.4) | 289 (18.0) |
| Life-supporting device | 78 (8.5) | 68 (8.7) | 102 (12.3) | 151 (9.4) |
| Mechanical ventilation | 40 (4.3) | 35 (4.5) | 62 (7.5) | 74 (4.6) |
| Pretransplant biochemical status | ||||
| Albumin (g/dL) | 3.0 (2.6–3.4) | 3.0 (2.6–3.5) | 3.1 (2.6–3.6) | 3.0 (2.6–3.5) |
| Total bilirubin (mg/dL) | 5.5 (2.6–15.2) | 5.6 (2.6–14.3) | 7.1 (2.7–17.8) | 5.3 (2.3–13.7) |
| Creatinine (mg/dL) | 1.30 (0.90–2.17) | 1.10 (0.80–1.91) | 1.13 (0.80–2.00) | 1.30 (0.89–2.10) |
| Prothrombin time (INR) | 1.80 (1.50–2.40) | 1.80 (1.40–2.40) | 1.90 (1.40–2.60) | 1.80 (1.40–2.40) |
| Female to Male (n = 1984) | Female to Female (n = 1676) | Male to Female (n = 1271) | Male to Male (n = 2691) | |
|---|---|---|---|---|
| Donor age (y) | 54 (48–63) | 56 (48–64) | 55 (49–63) | 55 (48–62) |
| Donor body mass index (kg/m2) | 28.1 (23.7–33.9) | 26.4 (22.7–31.6) | 25.9 (23.0–29.5) | 27.6 (24.2–31.7) |
| Donor cause of death | ||||
| Anoxia | 651 (33.3) | 491 (29.9) | 392 (31.4) | 859 (32.5) |
| Cerebrovascular/stroke | 1118 (57.2) | 953 (58.1) | 599 (47.9) | 1213 (45.9) |
| Others * | 215 (10.8) | 232 (13.8) | 280 (22.0) | 619 (23.0) |
| Cold ischemia time (h) | 6.3 (5.0–8.0) | 6.1 (4.9–7.8) | 6.0 (4.8–7.7) | 6.2 (5.0–8.0) |
| Transplant era | ||||
| 2000–2007 | 325 (15.4) | 237 (14.1) | 170 (13.4) | 408 (15.2) |
| 2008–2015 | 757 (38.2) | 609 (36.3) | 396 (31.2) | 864 (32.1) |
| 2016–2023 | 902 (45.5) | 830 (49.5) | 705 (55.5) | 1419 (52.7) |
| Age (y) | 55 (48–61) | 56 (48–63) | 56 (48–62) | 55 (48–61) |
| Body mass index (kg/m2) | 27.7 (24.2–32.1) | 27.5 (23.7–32.5) | 28.1 (24.2–32.4) | 28.3 (24.9–32.3) |
| Diabetes | 505 (25.5) | 422 (25.2) | 344 (27.1) | 702 (26.1) |
| Primary liver disease | ||||
| Viral | 412 (20.8) | 246 (14.7) | 199 (15.7) | 544 (20.2) |
| Alcoholic | 722 (36.4) | 372 (22.2) | 301 (23.7) | 1062 (39.5) |
| MASH | 287 (14.5) | 337 (20.1) | 269 (21.2) | 396 (14.7) |
| Others † | 563 (28.4) | 721 (43.0) | 502 (39.5) | 689 (25.6) |
| Hepatic encephalopathy III–IV | 277 (14.0) | 256 (15.3) | 201 (15.8) | 378 (14.1) |
| Refractory ascites | 772 (38.9) | 576 (34.4) | 445 (35.0) | 1064 (39.6) |
| Previous upper abdominal surgery | 784 (39.5) | 975 (58.2) | 770 (60.6) | 1024 (38.1) |
| Portal vein thrombosis | 138 (12.0) | 191 (11.4) | 147 (11.6) | 323 (12.0) |
| MELD score | 24 (18–31) | 24 (18–31) | 24 (18–31) | 23 (18–30) |
| Pretransplant critical care | ||||
| Dialysis | 254 (12.8) | 253 (15.1) | 206 (16.2) | 327 (12.2) |
| Life-supporting device | 135 (6.8) | 160 (9.6) | 106 (8.3) | 193 (7.2) |
| Mechanical ventilation | 86 (4.3) | 103 (6.1) | 64 (5.0) | 92 (3.4) |
| Pretransplant biochemical status | ||||
| Albumin (g/dL) | 3.0 (2.5–3.5) | 3.0 (2.5–3.5) | 3.0 (2.6–3.5) | 3.0 (2.5–3.5) |
| Total bilirubin (mg/dL) | 5.4 (2.6–12.9) | 5.4 (2.5–13.4) | 5.3 (2.5–12.5) | 4.5 (2.3–10.5) |
| Creatinine (mg/dL) | 1.24 (0.90–1.90) | 1.07 (0.72–1.70) | 1.10 (0.79–1.75) | 1.20 (0.90–1.83) |
| Prothrombin time (INR) | 1.80 (1.44–2.30) | 1.80 (1.40–2.40) | 1.80 (1.40–2.32) | 1.78 (1.40–2.30) |
| Hazard Ratio | p-Value | |
|---|---|---|
| Female-to-male transplantation | ||
| vs. Female-to-female | 1.48 (1.17–1.81) | <0.001 |
| vs. Male-to-female | 1.51 (1.21–1.89) | <0.001 |
| vs. Male-to-male | 1.27 (1.06–1.52) | 0.009 |
| Donor age (y) | 1.00 (0.99–1.01) | 0.367 |
| Donor body mass index (kg/m2) | 1.00 (0.99–1.01) | 0.410 |
| Cause of donor death (vs. anoxia) | ||
| Cerebrovascular/stroke | 1.33 (1.09–1.61) | 0.004 |
| Others | 1.02 (0.99–1.04) | 0.184 |
| Cold ischemia time (h) | 1.02 (0.99–1.04) | 0.156 |
| Transplant era (vs. 2000–2007) | 0.001 | |
| 2008–2015 | 0.73 (0.60–0.88) | <0.001 |
| 2016–2023 | 0.70 (0.56–0.86) | 0.001 |
| Age (y) | 1.02 (1.01–1.02) | <0.001 |
| Body mass index (kg/m2) | 0.98 (0.97–0.99) | 0.002 |
| Diabetes | 1.39 (1.19–1.62) | <0.001 |
| Primary liver disease (vs. viral) | ||
| Alcoholic | 1.03 (0.84–1.25) | 0.803 |
| MASH | 1.10 (0.86–1.42) | 0.439 |
| Others | 1.22 (1.01–1.47) | 0.037 |
| Hepatic encephalopathy III–IV | 1.16 (0.94–1.42) | 0.163 |
| Refractory ascites | 1.13 (0.97–1.32) | 0.105 |
| Previous upper abdominal surgery | 1.20 (1.04–1.38) | 0.013 |
| Portal vein thrombosis | 1.15 (0.92–1.43) | 0.229 |
| MELD score | 0.99 (0.98–1.01) | 0.352 |
| Dialysis | 1.23 (0.98–1.55) | 0.123 |
| Life-supporting device | 1.20 (0.84–1.70) | 0.311 |
| Mechanical ventilation | 1.19 (0.78–1.83) | 0.423 |
| Albumin (g/dL) | 0.99 (0.90–1.09) | 0.815 |
| Total bilirubin (mg/dL) | 1.01 (1.00–1.02) | 0.105 |
| Creatinine (mg/dL) | 1.02 (0.97–1.07) | 0.551 |
| Prothrombin time (INR) | 1.02 (0.96–1.08) | 0.484 |
| Hazard Ratio | p-Value | |
|---|---|---|
| Female-to-male transplantation | ||
| vs. Female-to-female | 1.00 (0.88–1.14) | 0.981 |
| vs. Male-to-female | 0.98 (0.85–1.13) | 0.758 |
| vs. Male-to-male | 0.97 (0.86–1.08) | 0.551 |
| Donor age (y) | 1.01 (1.01–1.01) | <0.001 |
| Donor body mass index (kg/m2) | 1.00 (0.99–1.00) | 0.507 |
| Cause of donor death (vs. anoxia) | ||
| Cerebrovascular/stroke | 1.09 (0.97–1.21) | 0.141 |
| Others | 0.92 (0.80–1.06) | 0.238 |
| Cold ischemia time (h) | 1.02 (1.01–1.04) | 0.002 |
| Transplant era (vs. 2000–2007) | <0.001 | |
| 2008–2015 | 0.76 (0.67–0.84) | <0.001 |
| 2016–2023 | 0.60 (0.52–0.69) | <0.001 |
| Age (y) | 1.00 (1.00–1.01) | 0.522 |
| Body mass index (kg/m2) | 1.00 (0.99–1.01) | 0.693 |
| Diabetes | 1.28 (1.16–1.42) | <0.001 |
| Primary liver disease (vs. viral) | ||
| Alcoholic | 1.00 (0.89–1.14) | 0.960 |
| MASH | 0.86 (0.74–1.01) | 0.071 |
| Others | 0.89 (0.79–1.00) | 0.056 |
| Hepatic encephalopathy III-IV | 1.07 (0.94–1.22) | 0.321 |
| Refractory ascites | 1.02 (0.93–1.12) | 0.713 |
| Previous upper abdominal surgery | 1.24 (1.13–1.36) | <0.001 |
| Portal vein thrombosis | 1.25 (1.10–1.43) | <0.001 |
| MELD score | 1.01 (1.00–1.02) | 0.237 |
| Dialysis | 1.16 (0.99–1.37) | 0.067 |
| Life-supporting device | 1.17 (0.89–1.52) | 0.261 |
| Mechanical ventilation | 1.59 (1.17–2.15) | 0.003 |
| Albumin (g/dL) | 0.90 (0.84–0.95) | <0.001 |
| Total bilirubin (mg/dL) | 1.00 (0.99–1.01) | 0.856 |
| Creatinine (mg/dL) | 1.06 (1.02–1.10) | 0.005 |
| Prothrombin time (INR) | 0.97 (0.93–1.02) | 0.277 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Agopian, V.A.; Steggerda, J.A.; Kim, I.K.; Sanford, A.; Lee, Y.-T.; Kwon, J.-H.; Rhu, J.S.; Kim, G.S.; Yang, J.-D. Impact of Donor Age on Graft Failure After Deceased Donor Liver Transplantation by Donor-Recipient Sex Combinations: An Analysis of the UNOS OPTN Database. J. Pers. Med. 2025, 15, 357. https://doi.org/10.3390/jpm15080357
Han S, Agopian VA, Steggerda JA, Kim IK, Sanford A, Lee Y-T, Kwon J-H, Rhu JS, Kim GS, Yang J-D. Impact of Donor Age on Graft Failure After Deceased Donor Liver Transplantation by Donor-Recipient Sex Combinations: An Analysis of the UNOS OPTN Database. Journal of Personalized Medicine. 2025; 15(8):357. https://doi.org/10.3390/jpm15080357
Chicago/Turabian StyleHan, Sangbin, Vatche A. Agopian, Justin A. Steggerda, Irene K. Kim, Alison Sanford, Yi-Te Lee, Ji-Hye Kwon, Jin Soo Rhu, Gaab Soo Kim, and Ju-Dong Yang. 2025. "Impact of Donor Age on Graft Failure After Deceased Donor Liver Transplantation by Donor-Recipient Sex Combinations: An Analysis of the UNOS OPTN Database" Journal of Personalized Medicine 15, no. 8: 357. https://doi.org/10.3390/jpm15080357
APA StyleHan, S., Agopian, V. A., Steggerda, J. A., Kim, I. K., Sanford, A., Lee, Y.-T., Kwon, J.-H., Rhu, J. S., Kim, G. S., & Yang, J.-D. (2025). Impact of Donor Age on Graft Failure After Deceased Donor Liver Transplantation by Donor-Recipient Sex Combinations: An Analysis of the UNOS OPTN Database. Journal of Personalized Medicine, 15(8), 357. https://doi.org/10.3390/jpm15080357







