Tumors of the Parapharyngeal Space Presenting with Obstructive Sleep Apnea: A Case Report and Literature Review
Abstract
1. Introduction
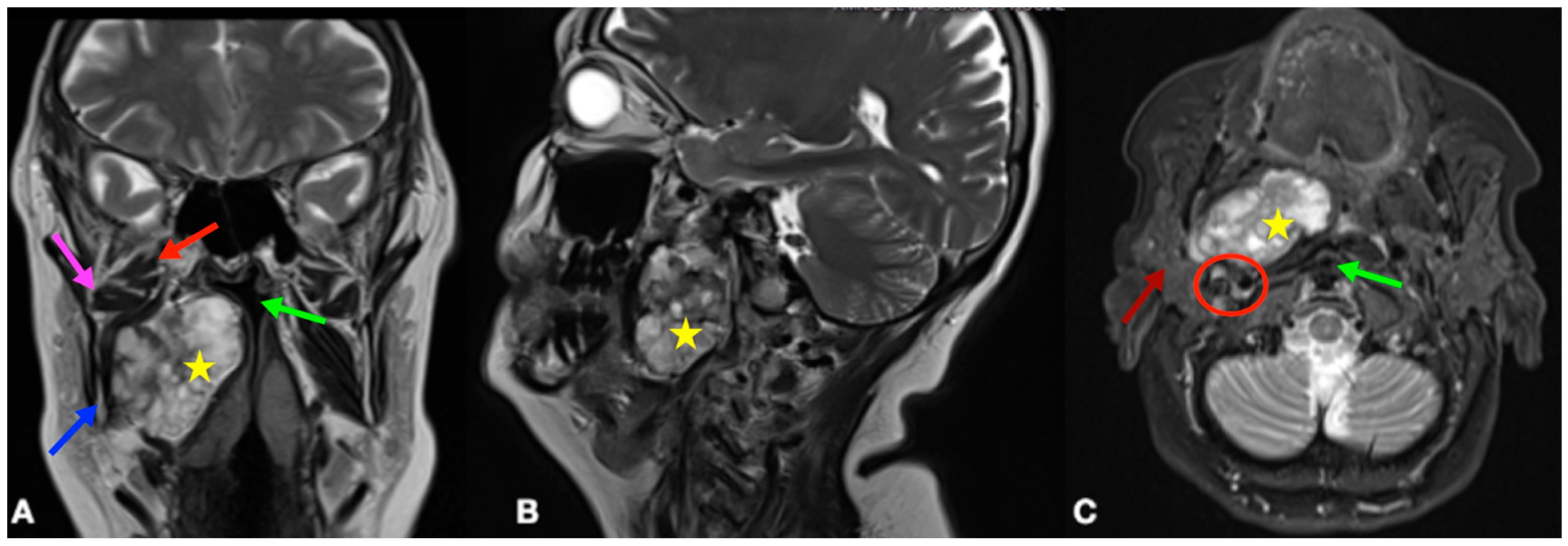
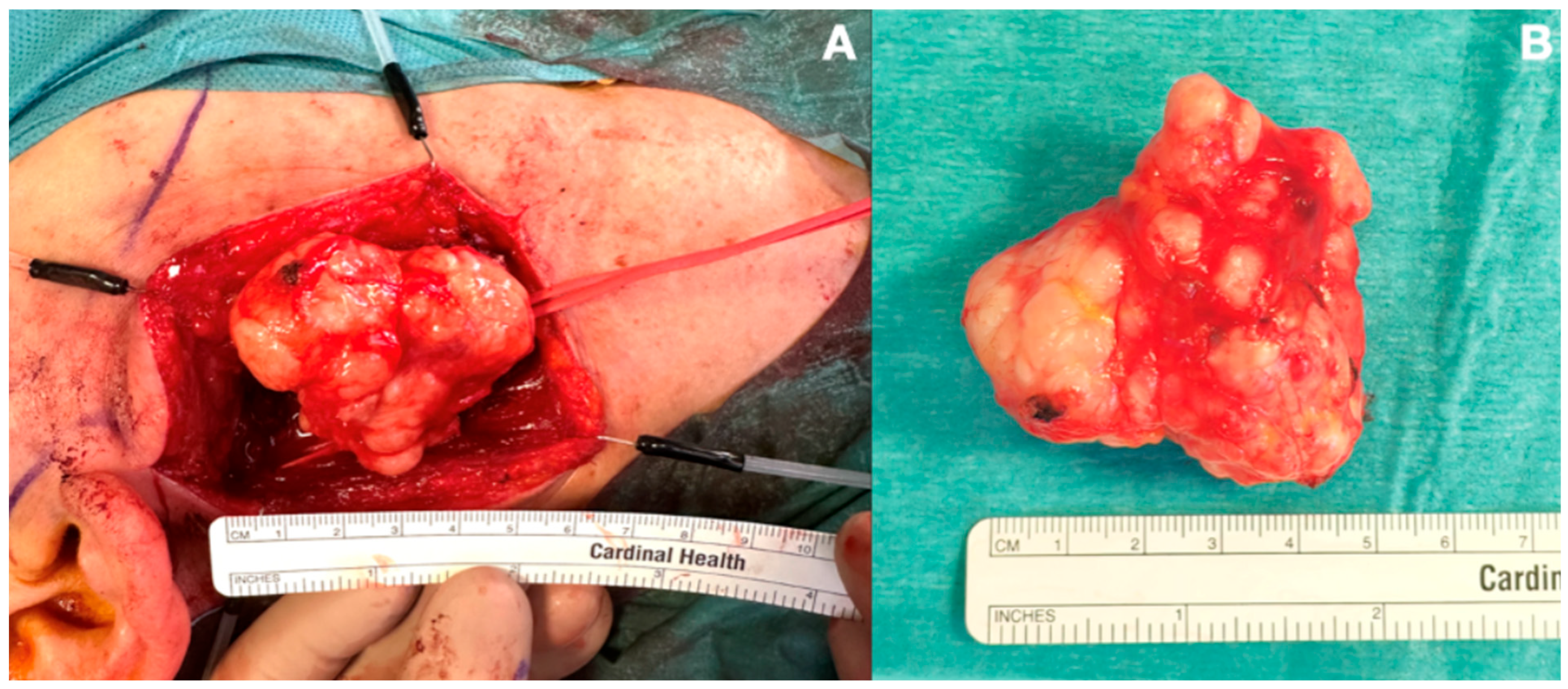
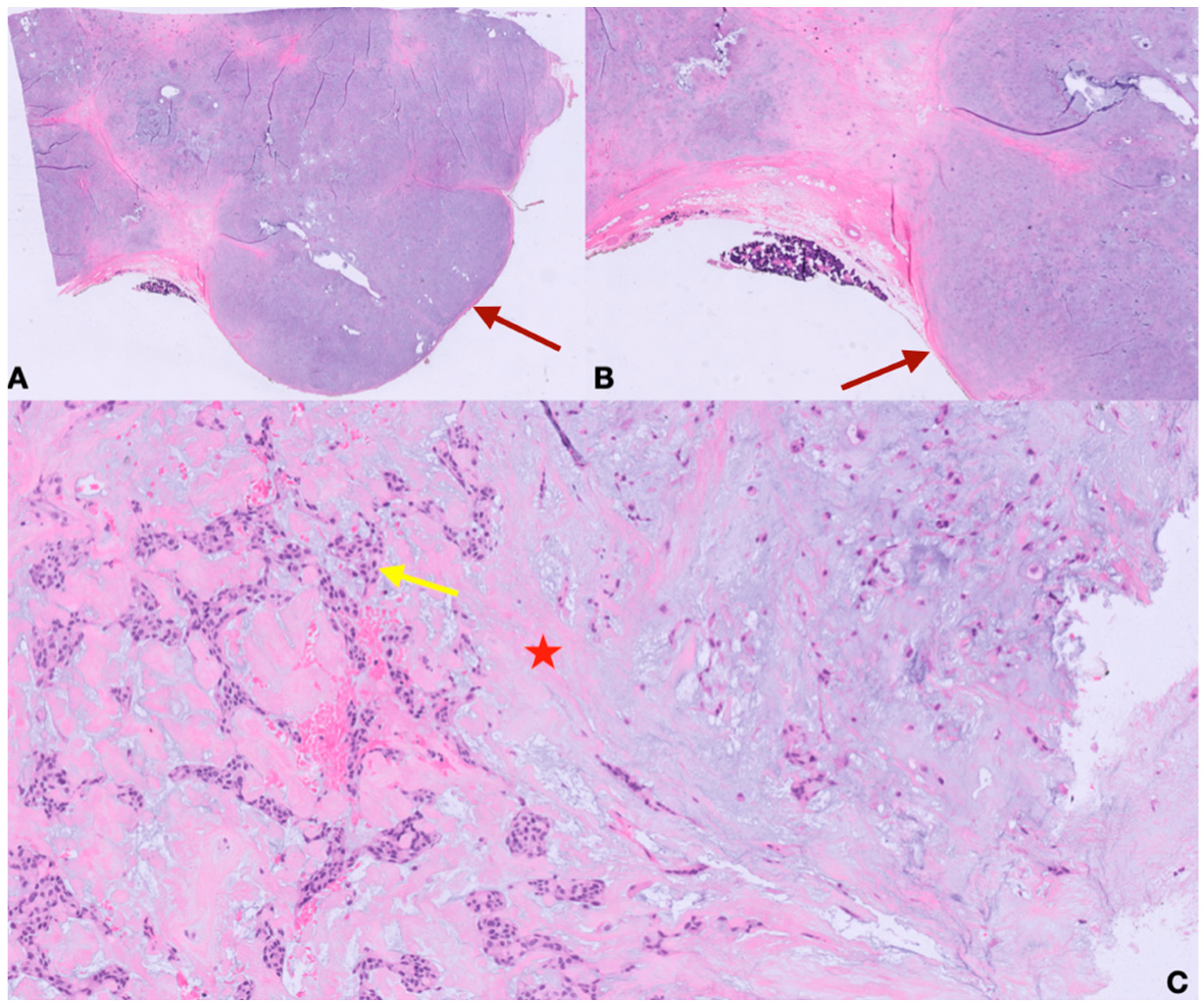
2. Case Presentation
3. Literature Review
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep. 2018, 10, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mosser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: THE HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population—A review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar] [PubMed]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Tasali, E.; Mokhlesi, B.; Cauter, E.V. Obstructive sleep apnea and type 2 diabetes: Interacting epidemics. Chest 2008, 133, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Shamsuzzaman, A.S.M.; Gersh, B.J.; Somers, V.K. Obstructive Sleep Apnea Implications for Cardiac and Vascular Disease. JAMA 2003, 290, 1906–1914. Available online: www.jama.com (accessed on 9 June 2025). [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1380–1400. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Hartfield, P.J.; Janczy, J.; Sharma, A.; Newsome, H.A.; Sparapani, R.A.; Rhee, J.S.; Woodson, B.T.; Garcia, G.J.M. Anatomical determinants of upper airway collapsibility in obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2023, 68, 101741. [Google Scholar] [CrossRef] [PubMed]
- López, F.; Suárez, C.; Poorten, V.V.; Mäkitie, A.; Nixon, I.J.; Strojan, P.; Hanna, E.Y.; Rodrigo, J.P.; Bree, R.d.; Quer, M.; et al. Contemporary management of primary parapharyngeal space tumors. Head Neck. 2019, 41, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Hees, T.v.; Weert, S.v.; Witte, B.; Leemans, C.R. Tumors of the parapharyngeal space: The VU University Medical Center experience over a 20-year period. Eur. Arch. Otorhinolaryngol. 2018, 275, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.C.; Giger, R.; Alwagdani, A.; Aldabal, N.; Stenzinger, A.; Heimgartner, S.; Nisa, L.; Borner, U. Primary neoplasms of the parapharyngeal space: Diagnostic and therapeutic pearls and pitfalls. Eur. Arch. Otorhinolaryngol. 2021, 278, 4933–4941. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.; CARE Group. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob. Adv. Health Med. 2013, 2, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Marion, F.; Videlaine, A.; Piot, B.; Merlet, F.L.; Longis, J.; Bertin, H. A giant parapharyngeal lipoma causing obstructive sleep apnea. J. Stomatol. Oral. Maxillofac. Surg. 2019, 120, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Wang, Q.Y.; Zhou, S.H.; Bao, Y.Y.; Wang, S.Q. Obstructive sleep apnea syndrome caused by uncommon tumors of the upper aerodigestive tract. Int. J. Clin. Exp. Pathol. 2014, 7, 6686–6693. [Google Scholar] [PubMed]
- Li, F.; Cha, X.; Wang, S.; Xie, Y.; Xu, Z.; Liu, H. Analysis of Diagnosis and Treatment Strategies for Parapharyngeal Space Tumors With Follicular Dendritic Sarcoma. J. Craniofac. Surg. 2024, 35, 2394–2396. [Google Scholar] [CrossRef] [PubMed]
- Loudghiri, M.; Arrih, B.S.; Oukessou, Y.; Rouadi, S.; Abada, R.; Mahtar, M. Management of a rare case of parapharyngeal lipoma presentation of case. Int. J. Surg. Case Rep. 2023, 106, 108145. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Ran, Q.; Wang, L. Parapharyngeal liposarcoma: A case report. Diagn. Pathol. 2013, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Pirovino, C.A.; Giger, R.; Landis, B.N. Sleep apnea: Do not forget to inspect the throat! Clin. Case Rep. 2018, 7, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Luczak, K.; Dorobisz, K.; Krecicki, T.; Janczak, D.; Chabowski, M.; Zatonski, T. The Lipomatosis of the Parapharyngeal and Retropharyngeal Space: A Case Report. Srp. Arh. Celok. Lek. 2015, 143, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Salvinelli, F.; Mallio, C.A.; Frari, V.; Vincenzi, B.; Bressi, F.; Quattrocchi, C.C. Upper airway study should always come before any sleep study in OSAS evaluation: A giant parapharyngeal lipoma behind OSAS. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 106–109. [Google Scholar] [PubMed]
- Alobid, I.; Benítez, P.; Berenguer, J.; Bernal-Sprekelsen, M.; Mullol, J. Parapharyngeal angiolipoma causing obstructive sleep apnoea syndrome. Acta Otolaryngol. 2004, 124, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Pellanda, A.; Zagury, S.; Pasche, P. Parapharyngeal lipoma causing obstructive sleep apnea syndrome. Otolaryngol. Head Neck Surg. 2003, 128, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Giddings, C.E.; Bray, D.; Rimmer, J.; Williamson, P. Pleomorphic adenoma and severe obstructive sleep apnoea. J. Laryngol. Otol. 2005, 119, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.J.; Patterson, A.R.; Brady, G.; Whitfield, P.H. Resolution of obstructive sleep apnoea after resection of a pleomorphic salivary adenoma. Br. J. Oral. Maxillofac. Surg. 2008, 46, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Morariu, I.; Dias, A.; Curran, A. Giant parotid pleomorphic adenoma in parapharyngeal space causing severe obstructive sleep apnoea. Ir. Med. J. 2012, 105, 184–185. [Google Scholar] [PubMed]
- Mulla, O.; Agada, F.; Dawson, D.; Sood, S. Deep lobe parotid pleomorphic adenoma presenting as obstructive sleep apnoea. BMJ. Case Rep. 2013, 2013, bcr2013008655. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Wang, J.T.; Levin, B.; Mosatyn, A.; Carsten, E.P.; Faruque, R. Parapharyngeal pleomorphic adenoma as a cause of severe obstructive sleep apnoea. ANZ J. Surg. 2014, 84, 883–884. [Google Scholar] [CrossRef] [PubMed]
- Walshe, P.; Smith, D.; Coakeley, D.; Dunne, B.; Timon, C. Sleep apnoea of unusual origin. J. Laryngol. Otol. 2002, 116, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Strohl, M.P.; El-Sayed, I.H. Contemporary Management of Parapharyngeal Tumors. Curr. Oncol. Rep. 2019, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Atturo, F.; Tombolini, M.; Colangeli, R.; Simone, M.; Seta, D.D.; Campora, L.d.; Tassone, D.; Camaioni, A. Parapharyngeal space tumors: A twenty-year single-center retrospective analysis on the effectiveness of transcervical and transoral approaches on local control and disease-specific survival. Am. J. Otolaryngol. 2023, 44, 103741. [Google Scholar] [CrossRef] [PubMed]
- Bozza, F.; Vigili, M.G.; Ruscito, P.; Marzetti, A.; Marzetti, F. Surgical management of parapharyngeal space tumours: Results of 10-year follow-up. Acta Otorhinolaryngol. Ital. 2009, 29, 10–15. [Google Scholar] [PubMed]
- Tuan, H.X.; Tri, C.M.; Huy, N.A.; Duc, N.M. A giant parapharyngeal space lipoma. Radiol. Case Rep. 2022, 18, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, D.; Ferrari, M.; Paderno, A.; Taboni, S.; Rampinelli, V.; Barbara, F.; Schreiber, A.; Mattavelli, D.; Tomasoni, M.; Farina, D.; et al. Selection of the surgical approach for lesions with parapharyngeal space involvement: A single-center experience on 153 cases. Oral Oncol. 2020, 109, 104872. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Costantino, A.; Mercante, G.; Di Maio, P.; Iocca, O.; Spriano, G. Trans-oral robotic surgery in the management of parapharyngeal space tumors: A systematic review. Oral Oncol. 2020, 103, 104581. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Strojan, P.; Beitler, J.J.; Langendijk, J.A.; Suarez, C.; Lee, A.W.; Rinaldo, A.; Rodrigo, J.P.; Smee, R.; Eisbruch, A.; et al. Radiotherapy for parapharyngeal space tumors. Am. J. Otolaryngol. 2019, 40, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Bosi, M.; De Vito, A.; Kotecha, B.; Viglietta, L.; Braghiroli, A.; Steier, J.; Pengo, M.; Sorrenti, G.; Gobbi, R.; Vicini, C.; et al. Phenotyping the pathophysiology of obstructive sleep apnea using polygraphy/polysomnography: A review of the literature. Sleep Breath. 2018, 22, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Stål, P. Myopathy of the upper airway in snoring and obstructive sleep apnea. Laryngoscope Investig. Otolaryngol. 2022, 7, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.K.; Lin, Y.C.; Lu, C.M.; Kao, Y.H. Snoring Index and Neck Circumference as Predictors of Adult Obstructive Sleep Apnea. Healthcare 2022, 10, 2543. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.N.; Yoo, J.; Song, I.S.; Joo, J.W.; Yoo, J.H.; Kim, T.H.; Lee, H.M.; Lee, S.H.; Lee, S.H. Does Snoring Time Always Reflect the Severity of Obstructive Sleep Apnea? Ann. Otol. Rhinol. Laryngol. 2017, 126, 693–696. [Google Scholar] [CrossRef] [PubMed]
| Timepoint | AHI (Supine/Not Supine) | ODI | Nadir SpO2 | CT90 | Mean Nadir SpO2 | Snoring Rate | ESS |
|---|---|---|---|---|---|---|---|
| Preoperative | 77.1/h (77.3/76.8) | 73.3/h | 68.0% | 24.7% | 87.4% | 29.3% | 12/24 |
| 2 months | 43.5/h (43.5/43.5) | 42.5/h | 72.0% | 34.2% | 87.5% | 58.2% | 8/24 |
| 4 months | 13.7/h (23.2/8.5) | 14.6/h | 81.0% | 17.9% | 87.1% | 63.4% | 6/24 |
| Author | Design | Population | Gender (M/F) | Age (Years) | BMI (kg/m2) | Origin (Side) | Size (cm) | Surgical Approach | Histopathology Report | Preoperative AHI | Postoperative AHI | Follow-Up, Duration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marion F et al. 2019 [14] | Case report | 1 | 1/0 | 69 | 26 | Parapharyngeal space (R) | 12 × 10 × 3.3 | Transcervical | Lipoma | 34/h | 2/h | NED, 8 months |
| Zhu SJ et al. 2014 [15] | Case series | 2 | 2/0 | (1) 33 (2) 34 | (1) 22 (2) 19 | (1) Deep lobe parotid (L) (2) Deep lobe parotid (L) | (1) 3 × 4.5 (2) 4 × 5 | Transoral | (1,2) Pleomorphic adenoma | (1) 60/h (2) 24/h | NR | (1) Recurrence, 9 years (2) NED, 2 years |
| Li F et al. 2024 [16] | Case report | 1 | 1/0 | 22 | NR | Parapharyngeal space (L) | 3.2 × 2.1 × 4.5 | Transoral | Follicular dendritic cell sarcoma | NR | NR | NED, unspecified |
| Loudghiri M et al. 2023 [17] | Case report | 1 | 1/0 | 44 | NR | Parapharyngeal space (L) | 6 × 9.2 × 4 | Combined transoral–transcervical | Lipoma | NR | NR | NED, 1 month |
| Li H et al. 2013 [18] | Case report | 1 | 1/0 | 30 | NR | Parapharyngeal space (L) | 7 × 7 × 6 | Transcervical | Liposarcoma | NR | NR | NED, 6 months |
| Pirovino CA et al. 2018 [19] | Case report | 1 | 1/0 | 54 | 30.9 | Parapharyngeal space (R) | 6 × 4 × 2 | Transoral | Lipoma | 42/h | 11/h | NED, 4 months |
| Luczak K et al. 2015 [20] | Case report | 1 | 1/0 | 75 | NR | Para- and retropharyngeal space (R) | 8.5 × 5.8 × 7.2 | Transcervical | Lipoma | NR | NR | NED, 14 months |
| Casale M et al. 2012 [21] | Case report | 1 | 1/0 | 70 | 38 | Para- and retropharyngeal space (L) | 9 × 6 | Transcervical | Lipoma | 65/h | 31/h | NED, 1 month |
| Alobid I et al. 2004 [22] | Case report | 1 | 1/0 | 47 | NR | Parapharyngeal space (L) | 3.5 × 3 × 8 | Cervical–transparotid | Angiolipoma | 72/h | <10/h | NED, 54 months |
| Pellanda A et al. 2003 [23] | Case report | 1 | 1/0 | 53 | 25.3 | Parapharyngeal space (R) | 9 × 4 × 5 | NR | Lipoma | 32.35/h | 8.9/h | NED, 2 months |
| Giddings CE et al. 2005 [24] | Case series | 2 | 1/1 | (1) 35 (2) 67 | (1) NR (2) 36 | (1) Deep lobe parotid (L) (2) Deep lobe parotid (R) | (1) NR (2) 4 (transverse diameter) | (1) Total parotidectomy (2) Total parotidectomy with mandibular condylotomy | (1,2) Pleomorphic adenoma | (1) NR (2) 93.2 | (1) NR (2) 29.5 | (1) Recurrence, 2 years (2) NED, 2 years |
| Adams AJ et al. 2008 [25] | Case report | 1 | 1/0 | 54 | NR | Parapharyngeal space (R) | 6 × 5 × 3.5 | Lip-split with mandibulotomy | Pleomorphic adenoma with chondroid metaplasia | NR | NR | NED, unspecified |
| Morariu M et al. 2012 [26] | Case report | 1 | 1/0 | 42 | NR | Deep lobe parotid (R) | 7 × 6 × 4 | Cervical–transparotid | Pleomorphic adenoma | NR | NR | NED, 6 months |
| Mulla O et al. 2013 [27] | Case report | 1 | 1/0 | 60 | 26 | Deep lobe parotid (R) | 6.7 × 3 × 6.8 | NR | Pleomorphic adenoma | NR | NR | NED, 6 months |
| Wang AY et al. 2014 [28] | Case report | 1 | 1/0 | 26 | NR | Deep lobe parotid (R) | 7 × 5.5 × 3.8 | Transcervical | Pleomorphic adenoma | NR | NR | NED, unspecified |
| Walshe P et al. 2002 [29] | Case report | 1 | 1/0 | 72 | NR | Parapharyngeal space (L) | NR | Transcervical | Shwannoma | 10 | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerri, L.; Giombi, F.; Cerasuolo, M.; Pace, G.M.; Losurdo, A.; Lunardi, G.; Grecchi, F.; Volpini, E.; Malvezzi, L. Tumors of the Parapharyngeal Space Presenting with Obstructive Sleep Apnea: A Case Report and Literature Review. J. Pers. Med. 2025, 15, 331. https://doi.org/10.3390/jpm15080331
Cerri L, Giombi F, Cerasuolo M, Pace GM, Losurdo A, Lunardi G, Grecchi F, Volpini E, Malvezzi L. Tumors of the Parapharyngeal Space Presenting with Obstructive Sleep Apnea: A Case Report and Literature Review. Journal of Personalized Medicine. 2025; 15(8):331. https://doi.org/10.3390/jpm15080331
Chicago/Turabian StyleCerri, Luca, Francesco Giombi, Michele Cerasuolo, Gian Marco Pace, Anna Losurdo, Giuseppe Lunardi, Francesco Grecchi, Elena Volpini, and Luca Malvezzi. 2025. "Tumors of the Parapharyngeal Space Presenting with Obstructive Sleep Apnea: A Case Report and Literature Review" Journal of Personalized Medicine 15, no. 8: 331. https://doi.org/10.3390/jpm15080331
APA StyleCerri, L., Giombi, F., Cerasuolo, M., Pace, G. M., Losurdo, A., Lunardi, G., Grecchi, F., Volpini, E., & Malvezzi, L. (2025). Tumors of the Parapharyngeal Space Presenting with Obstructive Sleep Apnea: A Case Report and Literature Review. Journal of Personalized Medicine, 15(8), 331. https://doi.org/10.3390/jpm15080331







