Tailoring Inflammatory Biomarker Assessment in Axial Spondyloarthritis: A Comparative Study of Erythrocyte Sedimentation Rate and C-Reactive Protein Across Disease Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Ethical Issues
2.2. Clinical and Laboratory Assessments
2.3. Outcome Composite Indices
2.4. Clinical Scenarios for Personalized Stratification
- -
- Disease activity: active (BASDAI ≥ 4 or ASDAS ≥ 2.1) vs. inactive/low activity [2].
- -
- Disease duration: early axSpA (≤2 years) vs. established disease (>2 years) [7].
- -
- Disease impact: high (ASAS-HI > 5) vs. low [8].
- -
- Severity: severe (RAPID3 > 6) vs. non-severe [9].
- -
- Therapy exposure: with vs. without current biologic therapy.
2.5. Statistical Analysis
3. Results
3.1. Summary of Study Population
3.2. Differences Based on HLA-B27
3.3. Correlation Between Inflammatory Markers and Outcome Measures
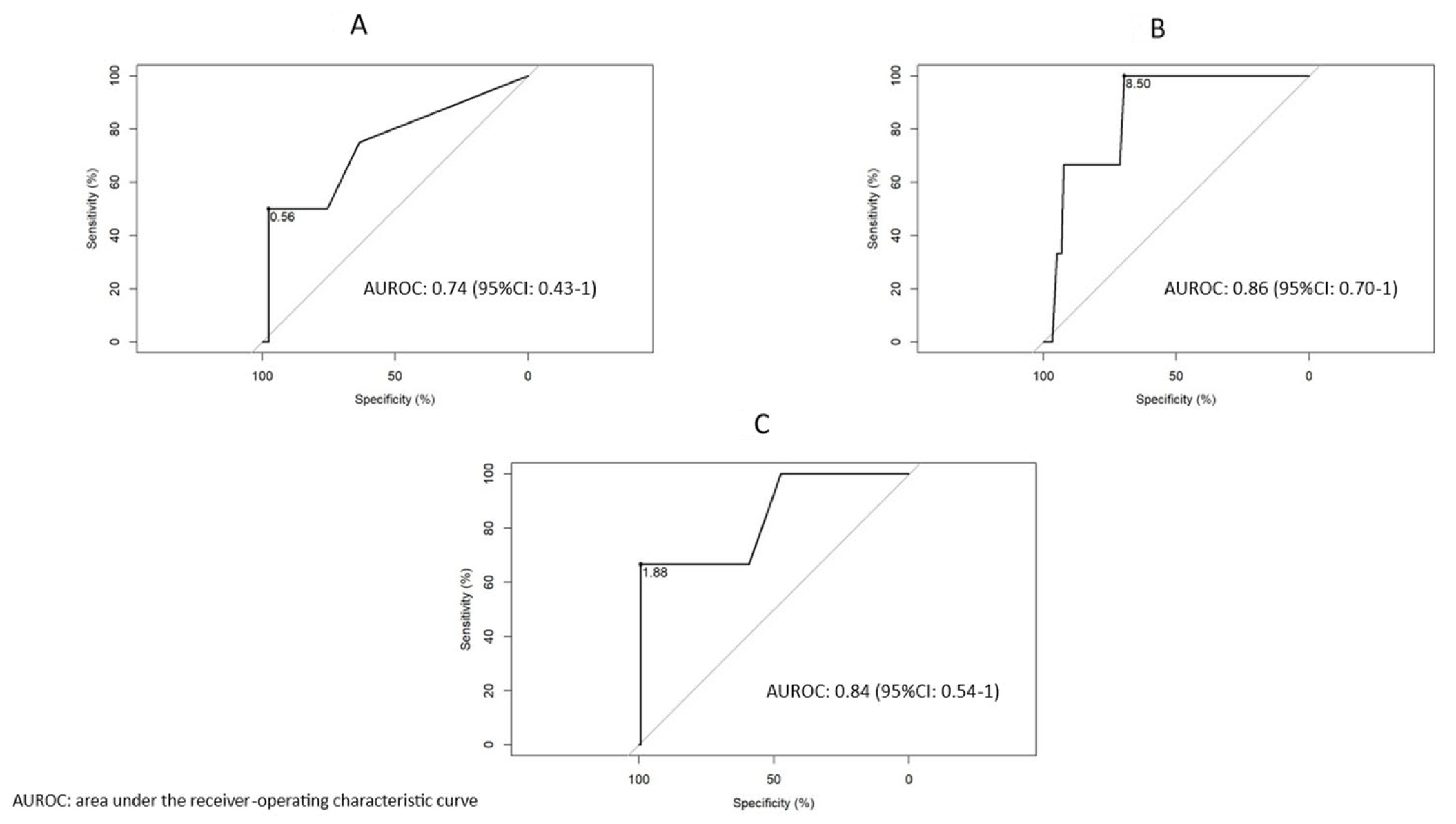
3.4. Biomarker Discrimination of Clinical Scenarios
3.5. Stratification by Disease Duration
3.6. Discriminatory Thresholds of ESR and CRP Based on Biologic Drug Exposure
3.7. ESR in Axial Spondyloarthritis: Highly Specific in Different Clinical Scenarios
3.8. CRP in Axial Spondyloarthritis: Highly Specific in Different Clinical Scenarios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanova, M.; Zimba, O.; Dimitrov, I.; Angelov, A.K.; Georgiev, T. Axial spondyloarthritis: An overview of the disease. Rheumatol. Int. 2024, 44, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, A.; Sanghavi, N.; Natu, A.; Danve, A. How to monitor disease activity of axial spondyloarthritis in clinical practice. Curr. Rheumatol. Rep. 2024, 26, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, S.; Nikiphorou, E.; Sepriano, A.; Ortolan, A.; Webers, C.; Baraliakos, X.; Landewé, R.B.M.; Van den Bosch, F.E.; Boteva, B.; Bremander, A.; et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 2023, 82, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, A.D.; Ceasovschih, A.; Șorodoc, V.; Pomîrleanu, C.; Lionte, C.; Șorodoc, L.; Ancuța, C. Practical Significance of Biomarkers in Axial Spondyloarthritis: Updates on Diagnosis, Disease Activity, and Prognosis. Int. J. Mol. Sci. 2022, 23, 11561. [Google Scholar] [CrossRef] [PubMed]
- Lorenzin, M.; Ometto, F.; Ortolan, A.; Felicetti, M.; Favero, M.; Doria, A.; Ramonda, R. An update on serum biomarkers to assess axial spondyloarthritis and to guide treatment decision. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20934277. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classifcation criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Compán, V.; Benavent, D.; Capelusnik, D.; van der Heijde, D.; Landewé, R.; Poddubnyy, D.; van Tubergen, A.; Baraliakos, X.; Van den Bosch, F.E.; van Gaalen, F.A.; et al. ASAS consensus definition of early axial spondyloarthritis. Ann. Rheum. Dis. 2024, 83, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Kiltz, U.; van der Heijde, D.; Boonen, A.; Akkoc, N.; Bautista-Molano, W.; Burgos-Vargas, R.; Wei, J.C.; Chiowchanwisawakit, P.; Dougados, M.; Duruoz, M.T.; et al. Measurement properties of the ASAS Health Index: Results of a global study in patients with axial and peripheral spondyloarthritis. Ann. Rheum. Dis. 2018, 77, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Pincus, T.; Askanase, A.D.; Swearingen, C.J. A Multi-dimensional Health Assessment Questionnaire (MDHAQ) and Routine Assessment of Patient Index Data (RAPID3) scores are informative in patients with all rheumatic diseases. Rheum. Dis. Clin. North Am. 2009, 35, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Turina, M.C.; Yeremenko, N.; Van Gaalen, F.; van Oosterhout, M.; Berg, I.J.; Ramonda, R.; Lebre, C.M.; Landewé, R.; Baeten, D. Serum inflammatory biomarkers fail to identify early axial spondyloarthritis: Results from the SpondyloArthritis Caught Early (SPACE) cohort. RMD Open 2017, 3, e000319. [Google Scholar] [CrossRef] [PubMed]
- Reveille, J.D. Biomarkers in axial spondyloarthritis and low back pain: A comprehensive review. Clin. Rheumatol. 2022, 41, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Prajzlerová, K.; Grobelná, K.; Pavelka, K.; Šenolt, L.; Filková, M. An update on biomarkers in axial spondyloarthritis. Autoimmun. Rev. 2016, 15, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Sinnappan, S.; Forte, A.; Ermann, J. Axial Spondyloarthritis Treatment Recommendations and Disease Activity Monitoring in Clinical Practice: Results of an Online Survey. J. Rheumatol. 2024, 51, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, S.; González Del Pozo, P.; Alvarez, P.; Calleja, N.; Burger, S.; Queiro, R. C-reactive protein thresholds for discriminating active disease in axial spondyloarthritis: Should we lower them? Clin. Exp. Rheumatol. 2025, 43, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Loredo, M.; González Del Pozo, P.; Alvarez, P.; Calleja, N.; Queiro, R. C-reactive protein thresholds for discriminating active disease in psoriatic arthritis may be different in early versus established disease. Clin. Exp. Rheumatol. 2025, 43, 467–471. [Google Scholar] [CrossRef] [PubMed]


| Total N: 330 | Women N: 127 | Men N: 203 | p-Valor | |
|---|---|---|---|---|
| Age, years, mean (SD) | 47.8 (12.9) | 47.3 (13.0) | 48.1 (12.9) | NS |
| Dis. duration, years, median [Q1, Q3] | 8.0 [4.0–16.0] | 6.0 [3.0–12.0] | 10.0 [5.0–20.0] | <0.001 |
| Education, n (%) | ||||
| Primary | 87 (26.4) | 38 (29.9) | 49 (24.1) | NS |
| Secondary | 157 (47.6) | 52 (40.9) | 105 (51.7) | NS |
| University | 86 (26.0) | 37 (29.2) | 49 (24.2) | NS |
| Associated conditions, n (%) | ||||
| Enthesitis | 26 (7.9) | 7 (5.5) | 19 (9.4) | NS |
| Uveitis | 52 (15.8) | 19 (15.0) | 33 (16.3) | NS |
| IBD | 34 (10.3) | 16 (12.6) | 18 (8.9) | NS |
| Comorbidities, n (%) | ||||
| Diabetes | 15 (4.5) | 5 (3.9) | 10 (4.9) | NS |
| HBP | 47 (14.2) | 14 (11.0) | 33 (16.3) | NS |
| Obesity | 39 (11.8) | 16 (12.6) | 23 (11.3) | NS |
| Smoking | 115 (34.8) | 41 (32.3) | 74 (36.5) | NS |
| Therapy, n (%) | ||||
| NSAIDs | 233 (70.6) | 94 (74.0) | 139 (68.5) | NS |
| Biologics | 209 (63.3) | 77 (60.6) | 132 (65.0) | NS |
| csDMARDs | 57 (17.3) | 22 (17.5) | 35 (17.2) | NS |
| Laboratory parameters | ||||
| CRP (mg/dL), median [Q1, Q3] | 0.20 [0.10–0.40] | 0.20 [0.10–0.50] | 0.10 [0.10–0.40] | NS |
| ESR (mm/h), median [Q1, Q3] | 5.0 [2.0–10.8] | 8.0 [3.0–17.0] | 4.0 [2.0–8.0] | <0.001 |
| HLA-B27, n (%) | 233 (70.6) | 80 (63.0) | 147 (72.4) | 0.07 |
| Structural damage, n (%) | ||||
| r-axSpA | 264 (80) | 62 (48.8) | 202 (99.5) | <0.001 |
| nr-axSpA | 66 (20) | 65 (51.2) | 1 (0.5) | <0.001 |
| High-grade sacroiliitis | 287 (87.0) | 98 (77.2) | 189 (93.1) | <0.001 |
| Syndesmophytes | 93 (28.2) | 18 (14.2) | 75 (36.9) | <0.001 |
| mSASSS, median [Q1, Q3] | 6.0 [2.0–15.0] | 4.0 [0.0–8.0] | 8.0 [4.0–20.0] | <0.001 |
| Treatment | ||||
| Anti-TNFα | 167 (50.6) | 67 (52.7) | 100 (49.3) | NS |
| Anti-IL17 | 42 (12.7) | 15 (11.8) | 27 (13.3) | NS |
| Anti-JAK | 22 (6.7) | 8 (6.3) | 14 (6.9) | NS |
| Outcome measures | ||||
| BASDAI, mean (SD) | 3.6 (2.4) | 4.4 (2.4) | 3.2 (2.3) | <0.001 |
| ASDAS, mean (SD) | 2.1 (0.8) | 2.3 (0.8) | 1.9 (0.8) | <0.001 |
| BASFI, mean (SD) | 3.2 (2.4) | 3.5 (2.4) | 2.9 (2.4) | 0.031 |
| ASAS HI, mean (SD) | 5.4 (4.0) | 6.1 (3.9) | 4.9 (3.9) | 0.026 |
| RAPID3, mean (SD) | 9.4 (6.6) | 11.5 (6.2) | 8.2 (6.6) | 0.005 |
| BASDAI_categories, n (%) | ||||
| ≥4 | 146 (44.2) | 73 (57.5) | 73 (36.0) | <0.001 |
| ASDAS_categories, n (%) | ||||
| ≥2.1 | 147 (44.7) | 71 (55.9) | 76 (37.6) | 0.002 |
| ASAS-HI, n: 200 (%) | ||||
| >5 | 94 (47) | 46/79 (58.2) | 48/121 (39.7) | 0.012 |
| RAPID3, n: 131 (%) | ||||
| >6 | 87 (66.4) | 40/49 (81.6) | 47/82 (57.3) | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiro, R.; Alonso, S.; Burger, S.; Pardo, E.; Braña, I.; Loredo, M.; Alperi, M. Tailoring Inflammatory Biomarker Assessment in Axial Spondyloarthritis: A Comparative Study of Erythrocyte Sedimentation Rate and C-Reactive Protein Across Disease Profiles. J. Pers. Med. 2025, 15, 329. https://doi.org/10.3390/jpm15080329
Queiro R, Alonso S, Burger S, Pardo E, Braña I, Loredo M, Alperi M. Tailoring Inflammatory Biomarker Assessment in Axial Spondyloarthritis: A Comparative Study of Erythrocyte Sedimentation Rate and C-Reactive Protein Across Disease Profiles. Journal of Personalized Medicine. 2025; 15(8):329. https://doi.org/10.3390/jpm15080329
Chicago/Turabian StyleQueiro, Rubén, Sara Alonso, Stefanie Burger, Estefanía Pardo, Ignacio Braña, Marta Loredo, and Mercedes Alperi. 2025. "Tailoring Inflammatory Biomarker Assessment in Axial Spondyloarthritis: A Comparative Study of Erythrocyte Sedimentation Rate and C-Reactive Protein Across Disease Profiles" Journal of Personalized Medicine 15, no. 8: 329. https://doi.org/10.3390/jpm15080329
APA StyleQueiro, R., Alonso, S., Burger, S., Pardo, E., Braña, I., Loredo, M., & Alperi, M. (2025). Tailoring Inflammatory Biomarker Assessment in Axial Spondyloarthritis: A Comparative Study of Erythrocyte Sedimentation Rate and C-Reactive Protein Across Disease Profiles. Journal of Personalized Medicine, 15(8), 329. https://doi.org/10.3390/jpm15080329







