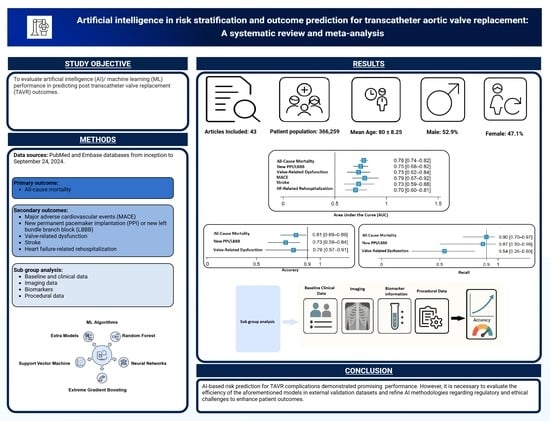
Artificial Intelligence in Risk Stratification and Outcome Prediction for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Screening
2.3. Data Extraction
2.4. Quality Assessment
2.5. Outcomes
2.6. Statistical Analysis
3. Results
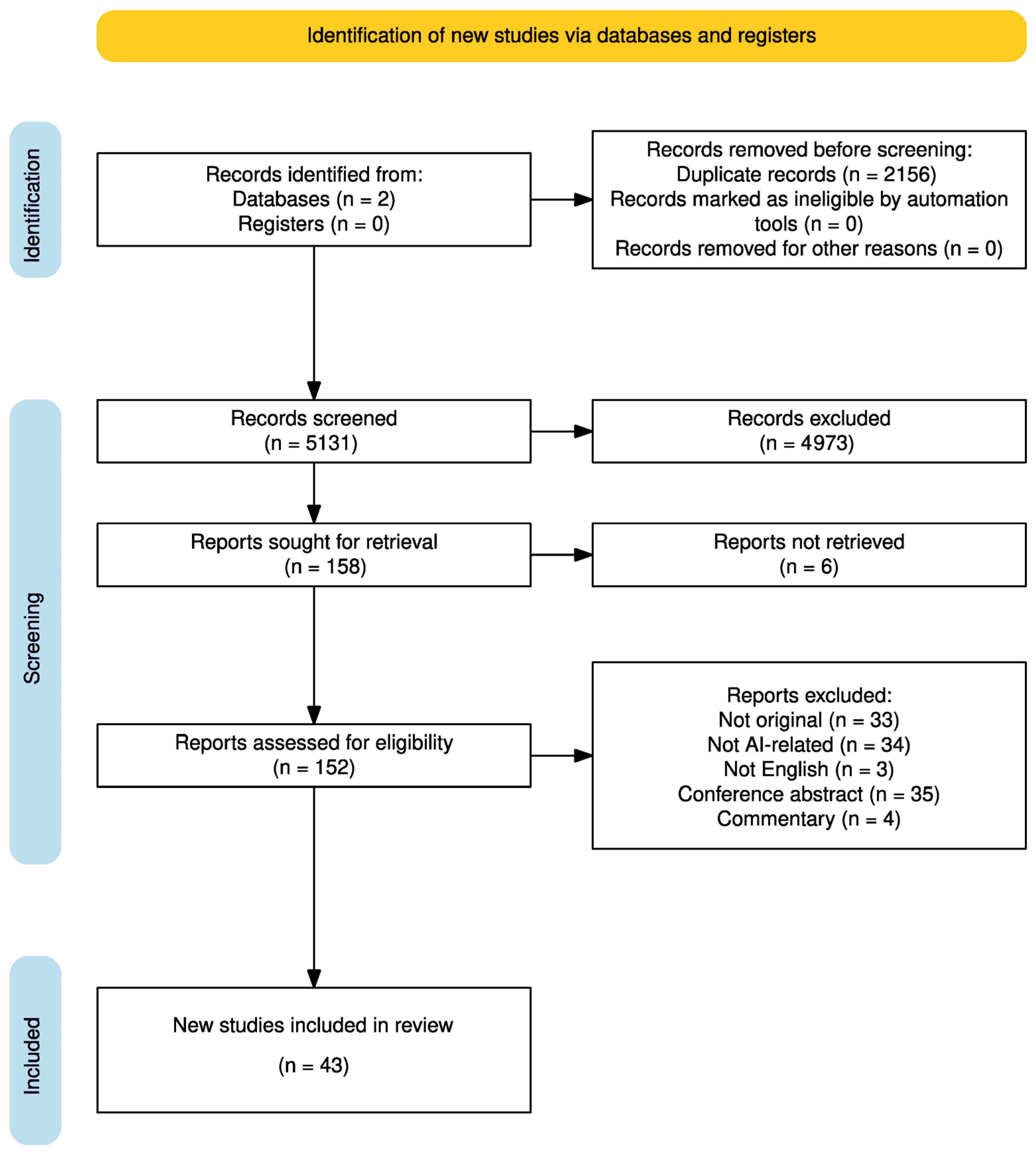
3.1. Study Selection and Characteristics
3.2. Risk of Bias Assessment
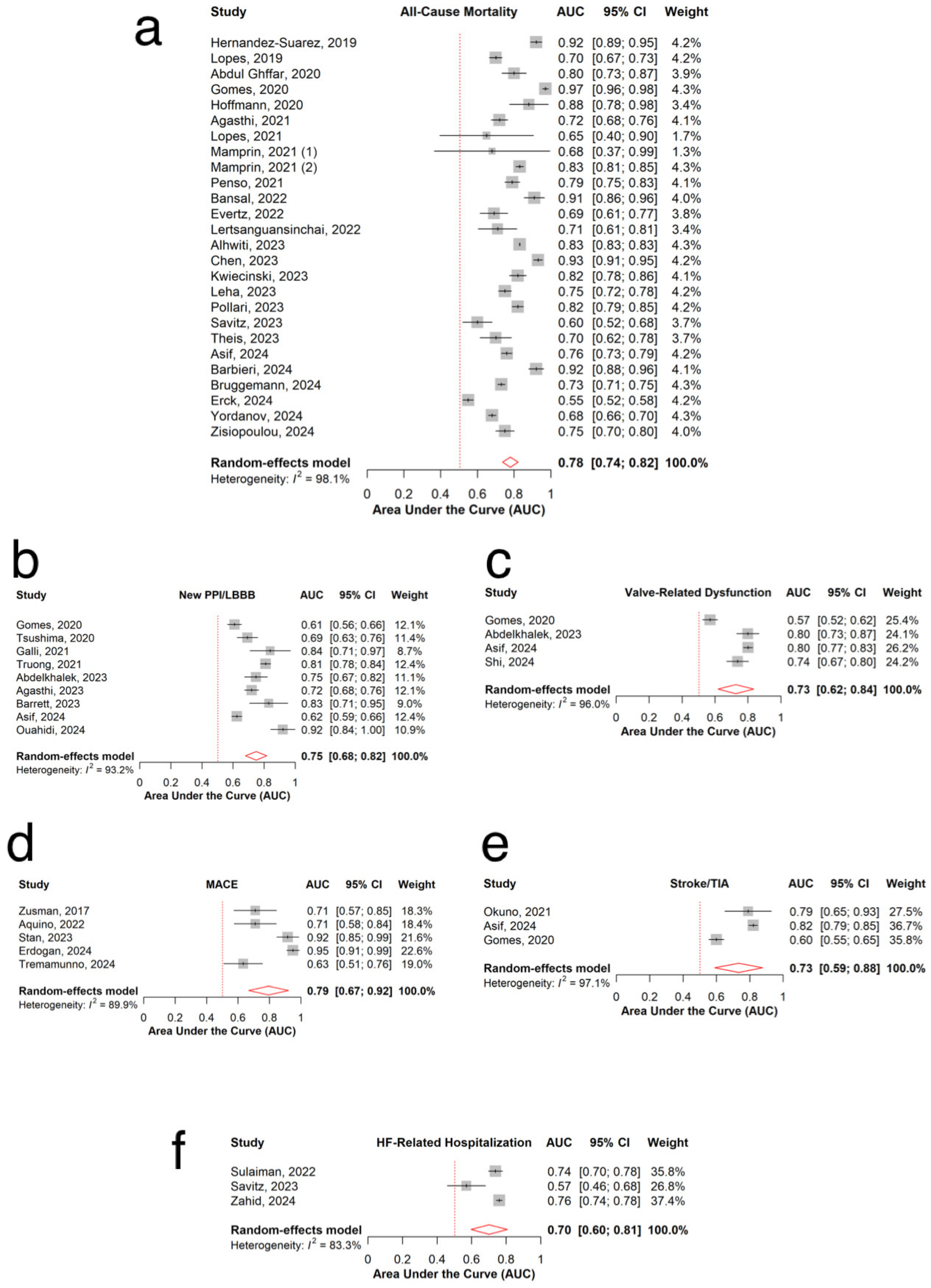
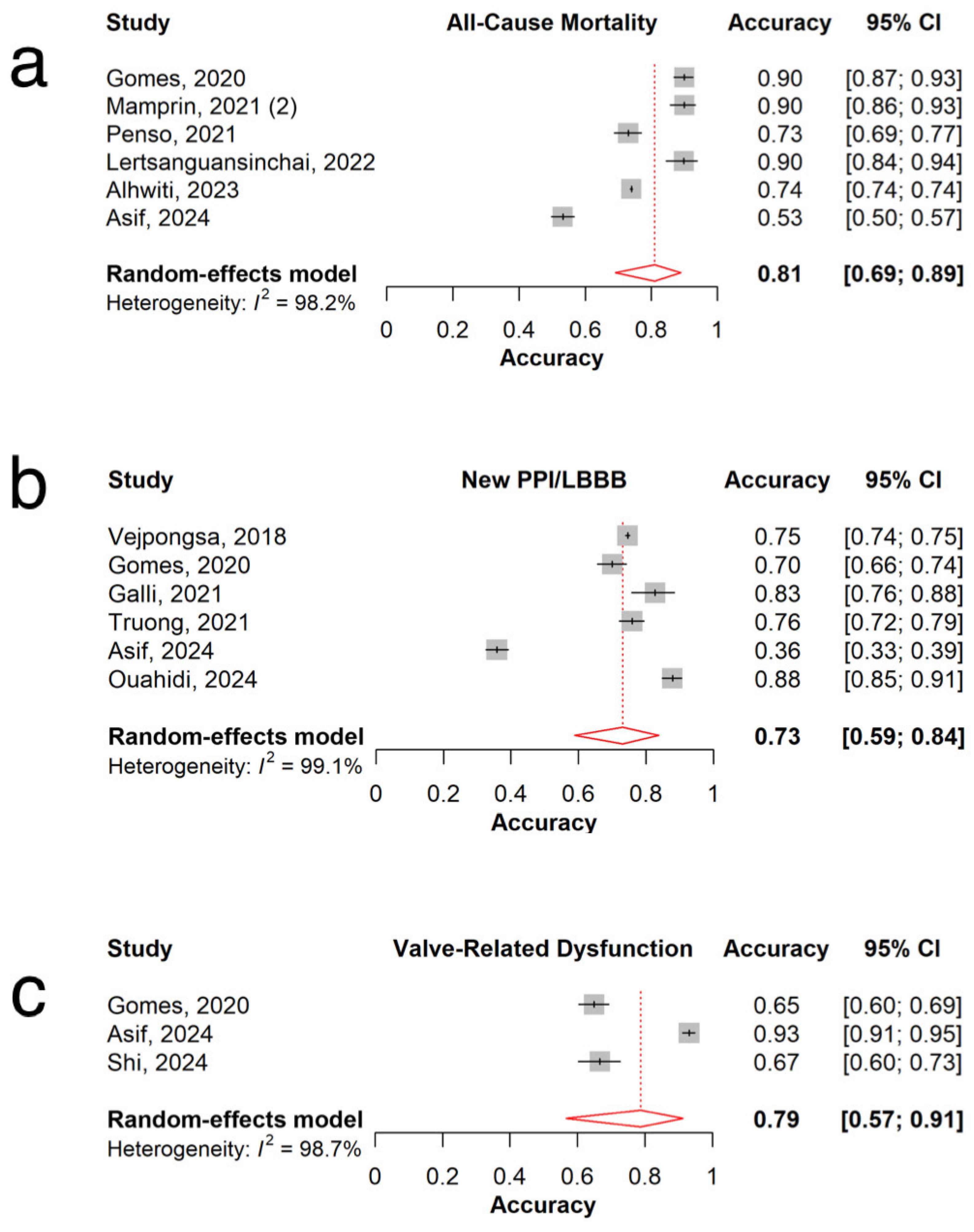
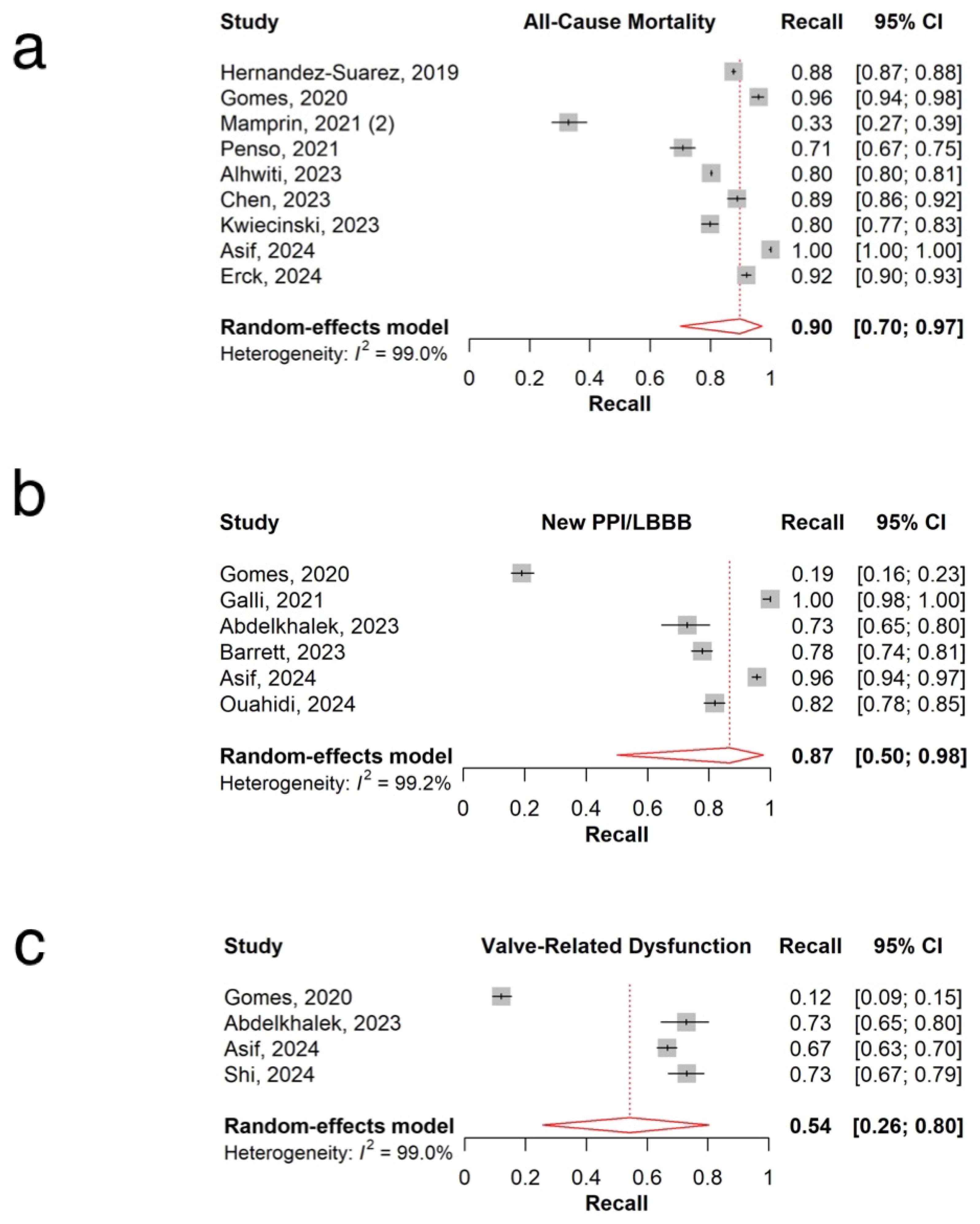
3.3. All-Cause Mortality
3.4. New Permanent Pacemaker Implantation or New Left Bundle Branch Block
3.5. Valve-Related Dysfunction
3.6. MACE
3.7. Stroke
3.8. Heart Failure-Related Re-Hospitalization
3.9. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Munoz, D.R. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Pol. Heart J. 2018, 76, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, Z.; Rehman, Z.U.; Ishtiaq, A.; Iftikhar, H.; Khokhar, M.M.; Khan, B.; Asad, A.; Nasir, H.; Athar, S.M.; Hassan, A.; et al. Comparative Outcomes of Transcatheter Versus Surgical Aortic Valve Replacement in Moderate-Risk Patients with Aortic Stenosis: A Systematic Review of Clinical Trials. Cureus 2024, 16, e70268. [Google Scholar] [CrossRef]
- Zou, Q.; Wei, Z.; Sun, S. Complications in transcatheter aortic valve replacement: A comprehensive analysis and management strategies. Curr. Probl. Cardiol. 2024, 49, 102478. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.H.L.; Verma, S.; Bhatt, D.L. Transcatheter Aortic Valve Replacement in Low-Risk Patients. Circulation 2019, 140, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, S.; Fallahtafti, P.; Sharifi, M.; Mohammadi, N.S.H.; Soleimani, H.; Moghadam, A.S.; Karimi, E.; Sattar, Y.; Jenab, Y.; Mehrani, M.; et al. Trends in Transcatheter Versus Surgical Aortic Valve Replacement Outcomes in Patients With Low-Surgical Risk: A Systematic Review and Meta-Analysis of Randomized Trials. J. Am. Heart Assoc. 2024, 13, e036179. [Google Scholar] [CrossRef]
- Tang, M.; Wang, D.; Xue, F.; Chen, Y.; Zhang, N.; Wang, J.; Zhao, P.; Zhou, T. Analysis of potential categories of quality of life of transcatheter aortic valve replacement patients and discussion of their influencing factors. BMC Cardiovasc. Disord. 2025, 25, 259. [Google Scholar] [CrossRef]
- Arnold Suzanne, V.; Zhang, Y.; Baron Suzanne, J.; McAndrew Thomas, C.; Alu Maria, C.; Kodali Susheel, K.; Kapadia, S.; Thourani Vinod, H.; Miller, D.C.; Mack Michael, J.; et al. Impact of Short-Term Complications on Mortality and Quality of Life After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 362–369. [Google Scholar] [CrossRef]
- AziziKia, H.; Mousavi, A.; Shojaei, S.; Shaker, F.; Salabat, D.; Bahri, R.A.; Dolama, R.H.; Radkhah, H. Predictive potential of pre-procedural cardiac and inflammatory biomarkers regarding mortality following transcatheter aortic valve implantation: A systematic review and meta-analysis. Heart Lung 2025, 69, 229–240. [Google Scholar] [CrossRef]
- Siddiqi, T.J.; Usman, M.S.; Khan, M.S.; Khan, M.A.A.; Riaz, H.; Khan, S.U.; Murad, M.H.; Kavinsky, C.J.; Doukky, R.; Kalra, A.; et al. Systematic review and meta-analysis of current risk models in predicting short-term mortality after transcatheter aortic valve replacement. EuroIntervention 2020, 15, 1497–1505. [Google Scholar] [CrossRef]
- Agasthi, P.; Ashraf, H.; Pujari, S.H.; Girardo, M.E.; Tseng, A.; Mookadam, F.; Venepally, N.R.; Buras, M.; Khetarpal, B.K.; Allam, M.; et al. Artificial Intelligence Trumps TAVI(2)-SCORE and CoreValve Score in Predicting 1-Year Mortality Post-Transcatheter Aortic Valve Replacement. Cardiovasc. Revasc. Med. 2021, 24, 33–41. [Google Scholar] [CrossRef]
- Sazzad, F.; Ler, A.A.L.; Furqan, M.S.; Tan, L.K.Z.; Leo, H.L.; Kuntjoro, I.; Tay, E.; Kofidis, T. Harnessing the power of artificial intelligence in predicting all-cause mortality in transcatheter aortic valve replacement: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2024, 11, 1343210. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Multimodal AI in Biomedicine: Pioneering the Future of Biomaterials, Diagnostics, and Personalized Healthcare. Nanomaterials 2025, 15, 895. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.-W.; et al. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar] [CrossRef] [PubMed]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and regulatory challenges of AI technologies in healthcare: A narrative review. Heliyon 2024, 10, e26297. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wolff, R.F.; Moons, K.G.M.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A Tool to Assess the Risk of Bias and Applicability of Prediction Model Studies. Ann. Intern. Med. 2019, 170, 51–58. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Okuno, T.; Overtchouk, P.; Asami, M.; Tomii, D.; Stortecky, S.; Praz, F.; Lanz, J.; Siontis, G.C.M.; Gräni, C.; Windecker, S.; et al. Deep learning-based prediction of early cerebrovascular events after transcatheter aortic valve replacement. Sci. Rep. 2021, 11, 18754. [Google Scholar] [CrossRef]
- El Ouahidi, A.; El Ouahidi, Y.; Nicol, P.-P.; Hannachi, S.; Benic, C.; Mansourati, J.; Pasdeloup, B.; Didier, R. Machine learning for pacemaker implantation prediction after TAVI using multimodal imaging data. Sci. Rep. 2024, 14, 25008. [Google Scholar] [CrossRef]
- Penso, M.; Pepi, M.; Fusini, L.; Muratori, M.; Cefalù, C.; Mantegazza, V.; Gripari, P.; Ali, S.G.; Fabbiocchi, F.; Bartorelli, A.L.; et al. Predicting Long-Term Mortality in TAVI Patients Using Machine Learning Techniques. J. Cardiovasc. Dev. Dis. 2021, 8, 44. [Google Scholar] [CrossRef]
- Pollari, F.; Hitzl, W.; Rottmann, M.; Vogt, F.; Ledwon, M.; Langhammer, C.; Eckner, D.; Jessl, J.; Bertsch, T.; Pauschinger, M.; et al. A Machine Learning Model for the Accurate Prediction of 1-Year Survival in TAVI Patients: A Retrospective Observational Cohort Study. J. Clin. Med. 2023, 12, 5481. [Google Scholar] [CrossRef]
- Savitz, S.T.; Leong, T.; Sung, S.H.; Kitzman, D.W.; McNulty, E.; Mishell, J.; Rassi, A.; Ambrosy, A.P.; Go, A.S. Predicting short-term outcomes after transcatheter aortic valve replacement for aortic stenosis. Am. Heart J. 2023, 256, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Feng, D.; Hu, X.; Wang, C.; Niu, G.; Zhao, Z.; Zhang, H.; Wang, M.; Wu, Y. Prediction of hypoattenuating leaflet thickening in patients undergoing transcatheter aortic valves replacement based on clinical factors and 4D-computed tomography morphological characteristics: A retrospective cross-sectional study. Int. J. Cardiol. 2024, 410, 132219. [Google Scholar] [CrossRef]
- Stan, A.; Călburean, P.A.; Drinkal, R.K.; Harpa, M.; Elkahlout, A.; Nicolae, V.C.; Tomșa, F.; Hadadi, L.; Brînzaniuc, K.; Suciu, H.; et al. Inflammatory Status Assessment by Machine Learning Techniques to Predict Outcomes in Patients with Symptomatic Aortic Stenosis Treated by Transcatheter Aortic Valve Replacement. Diagnostics 2023, 13, 2907. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.; Kawsara, A.; Mahayni, A.A.; El Sabbagh, A.; Singh, M.; Crestanello, J.; Gulati, R.; Alkhouli, M. Development and Validation of a Machine Learning Score for Readmissions After Transcatheter Aortic Valve Implantation. JACC Adv. 2022, 1, 100060. [Google Scholar] [CrossRef]
- Theis, M.; Block, W.; Luetkens, J.A.; Attenberger, U.I.; Nowak, S.; Sprinkart, A.M. Direct deep learning-based survival prediction from pre-interventional CT prior to transcatheter aortic valve replacement. Eur. J. Radiol. 2023, 168, 111150. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.T.; Beyerbach, D.; Mazur, W.; Wigle, M.; Bateman, E.; Pallerla, A.; Ngo, T.N.M.; Shreenivas, S.; Tretter, J.T.; Palmer, C.; et al. Machine learning method for predicting pacemaker implantation following transcatheter aortic valve replacement. Pacing Clin. Electrophysiol. 2021, 44, 334–340. [Google Scholar] [CrossRef]
- Tsushima, T.; Nadeem, F.; Al-Kindi, S.; Clevenger, J.R.; Bansal, E.J.; Wheat, H.L.; Kalra, A.; Attizzani, G.F.; Elgudin, Y.; Markowitz, A.; et al. Risk Prediction Model for Cardiac Implantable Electronic Device Implantation After Transcatheter Aortic Valve Replacement. JACC Clin. Electrophysiol. 2020, 6, 295–303. [Google Scholar] [CrossRef]
- Vejpongsa, P.; Zhang, X.; Bhise, V.; Kitkungvan, D.; Shivamurthy, P.; Anderson, H.V.; Balan, P.; Nguyen, T.C.; Estrera, A.L.; Dougherty, A.H.; et al. Risk Prediction Model for Permanent Pacemaker Implantation after Transcatheter Aortic Valve Replacement. Struct. Heart 2018, 2, 328–335. [Google Scholar] [CrossRef]
- Zahid, S.; Agrawal, A.; Salman, F.; Khan, M.Z.; Ullah, W.; Teebi, A.; Khan, S.U.; Sulaiman, S.; Balla, S. Development and Validation of a Machine Learning Risk-Prediction Model for 30-Day Readmission for Heart Failure Following Transcatheter Aortic Valve Replacement (TAVR-HF Score). Curr. Probl. Cardiol. 2024, 49, 102143. [Google Scholar] [CrossRef]
- Zisiopoulou, M.; Berkowitsch, A.; Redlich, L.; Walther, T.; Fichtlscherer, S.; Leistner, D.M. Personalised preinterventional risk stratification of mortality, length of stay and hospitalisation costs in transcatheter aortic valve implantation using a machine learning algorithm: A pilot trial. Open Heart 2024, 11, e002540. [Google Scholar] [CrossRef]
- Zusman, O.; Kornowski, R.; Witberg, G.; Lador, A.; Orvin, K.; Levi, A.; Assali, A.; Vaknin-Assa, H.; Sharony, R.; Shapira, Y.; et al. Transcatheter Aortic Valve Implantation Futility Risk Model Development and Validation Among Treated Patients With Aortic Stenosis. Am. J. Cardiol. 2017, 120, 2241–2246. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, M.; Bahadormanesh, N.; Ganame, J.; Keshavarz-Motamed, Z. Incremental prognostic value of intensity-weighted regional calcification scoring using contrast CT imaging in TAVR. Eur. Heart J. Imaging Methods Pract. 2023, 1, qyad027. [Google Scholar] [CrossRef]
- Agasthi, P.; Ashraf, H.; Pujari, S.H.; Girardo, M.; Tseng, A.; Mookadam, F.; Venepally, N.; Buras, M.R.; Abraham, B.; Khetarpal, B.K.; et al. Prediction of permanent pacemaker implantation after transcatheter aortic valve replacement: The role of machine learning. World J. Cardiol. 2023, 15, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Alhwiti, T.; Aldrugh, S.; Megahed, F.M. Predicting in-hospital mortality after transcatheter aortic valve replacement using administrative data and machine learning. Sci. Rep. 2023, 13, 10252. [Google Scholar] [CrossRef]
- Aquino, G.J.; Abadia, A.F.; Schoepf, U.J.; Emrich, T.; Yacoub, B.; Kabakus, I.; Violette, A.; Wiley, C.; Moreno, A.; Sahbaee, P.; et al. Coronary CT Fractional Flow Reserve before Transcatheter Aortic Valve Replacement: Clinical Outcomes. Radiology 2022, 302, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Asif, N.; Ayoade, P.; Razzouk, J.; Bohen, D.; Tooker, M.; Gladstone, L.; Hoff, J.; Mohsen, A.; Arnold, S.; Rabkin, D.G. Multilayer Perceptron Neural Network Analysis of Fluoroscopic Working Angle on Transcatheter Aortic Valve Implantation Complications. Cureus 2024, 16, e59144. [Google Scholar] [CrossRef]
- Bansal, A.; Mentias, A.; Jaber, W.; Zmaili, M.A.; Svensson, L.G.; Krishnaswamy, A.; Reed, G.W.; Puri, R.; Kapadia, S.R.; Xu, B. Machine-Learning Risk Model for Predicting In-Hospital Mortality for Patients With Infective Endocarditis After Transcatheter Aortic Valve Replacement. Cardiovasc. Revasc. Med. 2022, 39, 121–122. [Google Scholar] [CrossRef]
- Barbieri, F.; Pfeifer, B.E.; Senoner, T.; Dobner, S.; Spitaler, P.; Semsroth, S.; Lambert, T.; Zweiker, D.; Neururer, S.B.; Scherr, D.; et al. A Neuronal Network-Based Score Predicting Survival in Patients Undergoing Aortic Valve Intervention: The ABC-AS Score. J. Clin. Med. 2024, 13, 3691. [Google Scholar] [CrossRef]
- Barrett, C.D.; Nickel, A.; Rosenberg, M.A.; Ream, K.; Tzou, W.S.; Aleong, R.; Tumolo, A.; Garg, L.; Zipse, M.; West, J.J.; et al. PRIME score for prediction of permanent pacemaker implantation after transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 2023, 102, 1357–1363. [Google Scholar] [CrossRef]
- Brüggemann, D.; Kuzo, N.; Anwer, S.; Kebernik, J.; Eberhard, M.; Alkadhi, H.; Tanner, F.C.; Konukoglu, E. Predicting mortality after transcatheter aortic valve replacement using preprocedural CT. Sci. Rep. 2024, 14, 12526. [Google Scholar] [CrossRef] [PubMed]
- van Erck, D.; Moeskops, P.; Schoufour, J.D.; Weijs, P.J.M.; Scholte op Reimer, W.J.M.; van Mourik, M.S.; Planken, R.N.; Vis, M.M.; Baan, J.; Išgum, I.; et al. Low muscle quality on a procedural computed tomography scan assessed with deep learning as a practical useful predictor of mortality in patients with severe aortic valve stenosis. Clin. Nutr. ESPEN 2024, 63, 142–147. [Google Scholar] [CrossRef]
- Erdogan, A.; Genc, O.; Inan, D.; Yildirim, A.; Ibisoglu, E.; Guler, Y.; Genc, D.; Guler, A.; Karagoz, A.; Kurt, I.H.; et al. Prediction of Major Adverse Cardiac Events After Transcatheter Aortic Valve Implantation: A Machine Learning Approach with GRACE Score. Sisli Etfal Hastan. Tip. Bul. 2024, 58, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Evertz, R.; Lange, T.; Backhaus, S.J.; Schulz, A.; Beuthner, B.E.; Topci, R.; Toischer, K.; Puls, M.; Kowallick, J.T.; Hasenfuß, G.; et al. Artificial Intelligence Enabled Fully Automated CMR Function Quantification for Optimized Risk Stratification in Patients Undergoing Transcatheter Aortic Valve Replacement. J. Interv. Cardiol. 2022, 2022, 1368878. [Google Scholar] [CrossRef]
- Galli, V.; Loncaric, F.; Rocatello, G.; Astudillo, P.; Sanchis, L.; Regueiro, A.; De Backer, O.; Swaans, M.; Bosmans, J.; Ribeiro, J.M.; et al. Towards patient-specific prediction of conduction abnormalities induced by transcatheter aortic valve implantation: A combined mechanistic modelling and machine learning approach. Eur. Heart J. Digit. Health 2021, 2, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.; Pilz, M.; Reich, C.; Leuschner, F.; Konstandin, M.; Katus, H.A.; Meder, B. Machine learning-based risk prediction of intrahospital clinical outcomes in patients undergoing TAVI. Clin. Res. Cardiol. 2021, 110, 343–356. [Google Scholar] [CrossRef]
- Hernandez-Suarez, D.F.; Kim, Y.; Villablanca, P.; Gupta, T.; Wiley, J.; Nieves-Rodriguez, B.G.; Rodriguez-Maldonado, J.; Feliu Maldonado, R.; da Luz Sant’Ana, I.; Sanina, C.; et al. Machine Learning Prediction Models for In-Hospital Mortality After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 1328–1338. [Google Scholar] [CrossRef]
- Hoffmann, J.; Mas-Peiro, S.; Berkowitsch, A.; Boeckling, F.; Rasper, T.; Pieszko, K.; De Rosa, R.; Hiczkiewicz, J.; Burchardt, P.; Fichtlscherer, S.; et al. Inflammatory signatures are associated with increased mortality after transfemoral transcatheter aortic valve implantation. ESC Heart Fail. 2020, 7, 2597–2610. [Google Scholar] [CrossRef]
- Kwiecinski, J.; Dabrowski, M.; Nombela-Franco, L.; Grodecki, K.; Pieszko, K.; Chmielak, Z.; Pylko, A.; Hennessey, B.; Kalinczuk, L.; Tirado-Conte, G.; et al. Machine learning for prediction of all-cause mortality after transcatheter aortic valve implantation. Eur. Heart J. Qual. Care Clin. Outcomes 2023, 9, 768–777. [Google Scholar] [CrossRef]
- Lertsanguansinchai, P.; Chokesuwattanaskul, R.; Petchlorlian, A.; Suttirut, P.; Buddhari, W. Machine learning-based predictive risk models for 30-day and 1-year mortality in severe aortic stenosis patients undergoing transcatheter aortic valve implantation. Int. J. Cardiol. 2023, 374, 20–26. [Google Scholar] [CrossRef]
- Lopes, R.R.; Mamprin, M.; Zelis, J.M.; Tonino, P.A.L.; van Mourik, M.S.; Vis, M.M.; Zinger, S.; de Mol, B.A.J.M.; de With, P.H.N.; Marquering, H.A. Local and Distributed Machine Learning for Inter-hospital Data Utilization: An Application for TAVI Outcome Prediction. Front. Cardiovasc. Med. 2021, 8, 787246. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.R.; van Mourik, M.S.; Schaft, E.V.; Ramos, L.A.; Baan, J., Jr.; Vendrik, J.; de Mol, B.; Vis, M.M.; Marquering, H.A. Value of machine learning in predicting TAVI outcomes. Neth. Heart J. 2019, 27, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Mamprin, M.; Zelis, J.M.; Tonino, P.A.L.; Zinger, S.; de With, P.H.N. Decision Trees for Predicting Mortality in Transcatheter Aortic Valve Implantation. Bioengineering 2021, 8, 22. [Google Scholar] [CrossRef]
- Abdul Ghffar, Y.; Osman, M.; Shrestha, S.; Shaukat, F.; Kagiyama, N.; Alkhouli, M.; Raybuck, B.; Badhwar, V.; Sengupta, P.P. Usefulness of Semisupervised Machine-Learning-Based Phenogrouping to Improve Risk Assessment for Patients Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2020, 136, 122–130. [Google Scholar] [CrossRef]
- Tremamunno, G.; Vecsey-Nagy, M.; Schoepf, U.J.; Zsarnoczay, E.; Aquino, G.J.; Kravchenko, D.; Laghi, A.; Jacob, A.; Sharma, P.; Rapaka, S.; et al. Artificial Intelligence Improves Prediction of Major Adverse Cardiovascular Events in Patients Undergoing Transcatheter Aortic Valve Replacement Planning CT. Acad. Radiol. 2024, 32, 702–711. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Kai Chan, J.S.; Liu, T.; Hothi, S.S.; Roever, L.; Rajan, R.; Kei Wong, I.C.; Zhang, Q.; Tse, G.; et al. Development of an Electronic Frailty Index for Predicting Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement using Machine Learning. Ann. Clin. Cardiol. 2023, 5, 17–26. [Google Scholar] [CrossRef]
- Mamprin, M.; Lopes, R.R.; Zelis, J.M.; Tonino, P.A.L.; van Mourik, M.S.; Vis, M.M.; Zinger, S.; de Mol, B.; de With, P.H.N. Machine Learning for Predicting Mortality in Transcatheter Aortic Valve Implantation: An Inter-Center Cross Validation Study. J. Cardiovasc. Dev. Dis. 2021, 8, 65. [Google Scholar] [CrossRef]
- Leha, A.; Huber, C.; Friede, T.; Bauer, T.; Beckmann, A.; Bekeredjian, R.; Bleiziffer, S.; Herrmann, E.; Möllmann, H.; Walther, T.; et al. Development and validation of explainable machine learning models for risk of mortality in transcatheter aortic valve implantation: TAVI risk machine scores. Eur. Heart J. Digit. Health 2023, 4, 225–235. [Google Scholar] [CrossRef]
- Yordanov, T.R.; Ravelli, A.C.J.; Amiri, S.; Vis, M.; Houterman, S.; Van der Voort, S.R.; Abu-Hanna, A. Performance of federated learning-based models in the Dutch TAVI population was comparable to central strategies and outperformed local strategies. Front. Cardiovasc. Med. 2024, 11, 1399138. [Google Scholar] [CrossRef]
- McBane, R.D., 2nd; Wysokinski, W.E.; Le-Rademacher, J.G.; Zemla, T.; Ashrani, A.; Tafur, A.; Perepu, U.; Anderson, D.; Gundabolu, K.; Kuzma, C.; et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J. Thromb. Haemost. 2020, 18, 411–421. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Hu, P.; Lin, N.; Wu, Z. Research and Prediction of Factors Related to High Degree Atrioventricular Block after TAVI Surgery Based on Logistic Regression Model. Heart Surg. Forum 2023, 26, E531–E536. [Google Scholar] [CrossRef] [PubMed]
- Kurmanaliyev, A.; Sutiene, K.; Braukylienė, R.; Aldujeli, A.; Jurenas, M.; Kregzdyte, R.; Braukyla, L.; Zhumagaliyev, R.; Aitaliyev, S.; Zhanabayev, N. An Integrative Machine Learning Model for Predicting Early Safety Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation. Medicina 2025, 61, 374. [Google Scholar] [CrossRef]
- Sulaiman, R.; Faisal, M.A.A.; Hasan, M.; Chowdhury, M.E.; Bensaali, F.; Alnabti, A.; Yalcin, H.C. Machine learning for predicting outcomes of transcatheter aortic valve implantation: A systematic review. Int. J. Med. Inform. 2025, 197, 105840. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, S.; Xing, A.; Zheng, L.; Shen, L.; Tu, B.; Yao, Y. Machine learning-based long-term outcome prediction in patients undergoing percutaneous coronary intervention. Cardiovasc. Diagn. Ther. 2021, 11, 736–743. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, J.; Huang, Q.; Cui, R.; Chen, J. In-hospital major adverse cardiovascular events after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction: A retrospective study under the China chest pain center (standard center) treatment system. BMC Cardiovasc. Disord. 2023, 23, 198. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, S.; Poorhosseini, H.; Jenab, Y.; Alidoosti, M.; Sadeghian, M.; Mehrani, M.; Tabrizi, Z.; Hashemi, P. Comparison of machine-learning models for the prediction of 1-year adverse outcomes of patients undergoing primary percutaneous coronary intervention for acute ST-elevation myocardial infarction. Clin. Cardiol. 2024, 47, e24157. [Google Scholar] [CrossRef]
- Chen, P.; Wang, B.; Zhao, L.; Ma, S.; Wang, Y.; Zhu, Y.; Zeng, X.; Bai, Z.; Shi, B. Machine learning for predicting intrahospital mortality in ST-elevation myocardial infarction patients with type 2 diabetes mellitus. BMC Cardiovasc. Disord. 2023, 23, 585. [Google Scholar] [CrossRef]
- Ghavidel, A.A.; Javadikasgari, H.; Maleki, M.; Karbassi, A.; Omrani, G.; Noohi, F. Two new mathematical models for prediction of early mortality risk in coronary artery bypass graft surgery. J. Thorac. Cardiovasc. Surg. 2014, 148, 1291–1298.e1291. [Google Scholar] [CrossRef]
- Mejia, O.A.V.; Antunes, M.J.; Goncharov, M.; Dallan, L.R.P.; Veronese, E.; Lapenna, G.A.; Lisboa, L.A.F.; Dallan, L.A.O.; Brandão, C.M.A.; Zubelli, J.; et al. Predictive performance of six mortality risk scores and the development of a novel model in a prospective cohort of patients undergoing valve surgery secondary to rheumatic fever. PLoS ONE 2018, 13, e0199277. [Google Scholar] [CrossRef]
- Zea-Vera, R.; Ryan, C.T.; Navarro, S.M.; Havelka, J.; Wall, M.J.; Coselli, J.S.; Rosengart, T.K.; Chatterjee, S.; Ghanta, R.K. Development of a Machine Learning Model to Predict Outcomes and Cost After Cardiac Surgery. Ann. Thorac. Surg. 2023, 115, 1533–1542. [Google Scholar] [CrossRef]
- Khalaji, A.; Behnoush, A.H.; Jameie, M.; Sharifi, A.; Sheikhy, A.; Fallahzadeh, A.; Sadeghian, S.; Pashang, M.; Bagheri, J.; Ahmadi Tafti, S.H.; et al. Machine learning algorithms for predicting mortality after coronary artery bypass grafting. Front. Cardiovasc. Med. 2022, 9, 977747. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Xu, K.; Bai, Y.; Lv, M.; Shan, L.; Li, W.; Zhang, X.; Li, Z.; Wang, Z.; Zhao, X.; et al. Machine-learning predictions for acute kidney injuries after coronary artery bypass grafting: A real-life muticenter retrospective cohort study. BMC Med. Inform. Decis. Mak. 2023, 23, 270. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Goyal, A.; Miller, J.K.; Gleason, T.G.; Dubrawksi, A. Performance of a machine learning algorithm in predicting outcomes of aortic valve replacement. Ann. Thorac. Surg. 2021, 111, 503–510. [Google Scholar] [CrossRef]
- Chong, C.F.; Li, Y.C.; Wang, T.L.; Chang, H. Stratification of adverse outcomes by preoperative risk factors in coronary artery bypass graft patients: An artificial neural network prediction model. AMIA Annu. Symp. Proc. 2003, 2003, 160–164. [Google Scholar] [PubMed]
- Benjamin, M.M.; Rabbat, M.G. Artificial Intelligence in Transcatheter Aortic Valve Replacement: Its Current Role and Ongoing Challenges. Diagnostics 2024, 14, 261. [Google Scholar] [CrossRef]
- Paranjape, K.; Schinkel, M.; Nannan Panday, R.; Car, J.; Nanayakkara, P. Introducing Artificial Intelligence Training in Medical Education. JMIR Med. Educ. 2019, 5, e16048. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.S.; Hong, J.H.; Woo, H.; Lee, C.; Yoon, J.H.; Lee, K.-B.; Chung, S.; Yoon, D.S.; Lee, J.H. Artificial Intelligence in Bacterial Diagnostics and Antimicrobial Susceptibility Testing: Current Advances and Future Prospects. Biosens. Bioelectron. 2025, 280, 117399. [Google Scholar] [CrossRef]
- Lessmann, N.; van Ginneken, B.; Zreik, M.; de Jong, P.A.; de Vos, B.D.; Viergever, M.A.; Isgum, I. Automatic Calcium Scoring in Low-Dose Chest CT Using Deep Neural Networks With Dilated Convolutions. IEEE Trans. Med. Imaging 2018, 37, 615–625. [Google Scholar] [CrossRef]
- Gouravani, M.; Shahrabi Farahani, M.; Salehi, M.A.; Shojaei, S.; Mirakhori, S.; Harandi, H.; Mohammadi, S.; Saleh, R.R. Diagnostic performance of artificial intelligence in detection of renal cell carcinoma: A systematic review and meta-analysis. BMC Cancer 2025, 25, 155. [Google Scholar] [CrossRef]
- Munafò, R.; Saitta, S.; Ingallina, G.; Denti, P.; Maisano, F.; Agricola, E.; Redaelli, A.; Votta, E. A Deep Learning-Based Fully Automated Pipeline for Regurgitant Mitral Valve Anatomy Analysis From 3D Echocardiography. IEEE Access 2024, 12, 5295–5308. [Google Scholar] [CrossRef]
- Kim, J.-C.; Chung, K. Recurrent neural network-based multimodal deep learning for estimating missing values in healthcare. Appl. Sci. 2022, 12, 7477. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.; Kim, S.-H.; Woo, J. Intraoperative hypotension prediction model based on systematic feature engineering and machine learning. Sensors 2022, 22, 3108. [Google Scholar] [CrossRef] [PubMed]
- Boehm, K.M.; Aherne, E.A.; Ellenson, L.; Nikolovski, I.; Alghamdi, M.; Vázquez-García, I.; Zamarin, D.; Long Roche, K.; Liu, Y.; Patel, D.; et al. Multimodal data integration using machine learning improves risk stratification of high-grade serous ovarian cancer. Nat. Cancer 2022, 3, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Jaltotage, B.; Lu, J.; Dwivedi, G. Use of Artificial Intelligence Including Multimodal Systems to Improve the Management of Cardiovascular Disease. Can. J. Cardiol. 2024, 40, 1804–1812. [Google Scholar] [CrossRef]
- Abimannan, S.; El-Alfy, E.-S.; Chang, Y.-S.; Hussain, S.; Shukla, S.; Satheesh, D. Ensemble Multifeatured Deep Learning Models and Applications: A Survey. IEEE Access 2023, 11, 107194–107217. [Google Scholar] [CrossRef]
- Ozturk, C.; Pak, D.H.; Rosalia, L.; Goswami, D.; Robakowski, M.E.; McKay, R.; Nguyen, C.T.; Duncan, J.S.; Roche, E.T. AI-Powered Multimodal Modeling of Personalized Hemodynamics in Aortic Stenosis. Adv. Sci. 2025, 12, 2404755. [Google Scholar] [CrossRef]
- Li, Y.; El Habib Daho, M.; Conze, P.-H.; Zeghlache, R.; Le Boité, H.; Tadayoni, R.; Cochener, B.; Lamard, M.; Quellec, G. A review of deep learning-based information fusion techniques for multimodal medical image classification. Comput. Biol. Med. 2024, 177, 108635. [Google Scholar] [CrossRef]
- Hosseinpour Aghaei, B.; Taiefi Nasrabadi, N.; Pirali, Y.; Shojaei, S.S.R. The Prevalence of Fasciola (Digenea: Fasciolidae) Species in Livestock and Humans in Iran, A Systematic Review. Arch. Razi Inst. 2025, 80, 263–269. [Google Scholar]
- Ali, S.; Akhlaq, F.; Imran, A.S.; Kastrati, Z.; Daudpota, S.M.; Moosa, M. The enlightening role of explainable artificial intelligence in medical & healthcare domains: A systematic literature review. Comput. Biol. Med. 2023, 166, 107555. [Google Scholar] [CrossRef]
- Jacob, C.; Brasier, N.; Laurenzi, E.; Heuss, S.; Mougiakakou, S.G.; Cöltekin, A.; Peter, M.K. AI for IMPACTS Framework for Evaluating the Long-Term Real-World Impacts of AI-Powered Clinician Tools: Systematic Review and Narrative Synthesis. J. Med. Internet Res. 2025, 27, e67485. [Google Scholar] [CrossRef] [PubMed]
- Ong Ly, C.; Unnikrishnan, B.; Tadic, T.; Patel, T.; Duhamel, J.; Kandel, S.; Moayedi, Y.; Brudno, M.; Hope, A.; Ross, H.; et al. Shortcut learning in medical AI hinders generalization: Method for estimating AI model generalization without external data. NPJ Digit. Med. 2024, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Bertl, M.; Lamo, Y.; Leucker, M.; Margaria, T.; Mohammadi, E.; Mukhiya, S.K.; Pechmann, L.; Piho, G.; Rabbi, F. Challenges for AI in Healthcare Systems. In Bridging the Gap between AI and Reality, Proceedings of the International Conference on Bridging the Gap between AI and Reality, Crete, Greece, 23–28 October 2023; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
| Author Year | Country | Study Design | Overall Dataset Size | % of Male Participants | Mean ± SD Age of Participants | Outcomes | Algorithm | Architecture | Model Development | Validation Method |
|---|---|---|---|---|---|---|---|---|---|---|
| Asif 2024 [37] | US | Retrospective cohort | 834 | N/A | N/A | Valve-related dysfunction | MLP | Initial architecture search performed using Hyperopt, a Python library built for automatic model selection and hyperparameter optimization | Baseline and clinical data | N/A |
| New PPI/LBBB | ||||||||||
| Stroke | ||||||||||
| All-cause mortality | ||||||||||
| Barbieri 2024 [39] | Austria | Post hoc analysis of a retrospective study | 3079 | 52.19 | N/A | All-cause mortality | ABC-AS score | N/A | Baseline and clinical data + biomarkers | N/A |
| Bruggemann 2024 [41] | Switzerland | Retrospective analysis | 1449 | 52.42 | N/A | All-cause mortality | DNN | CT images 3D deep neural network | Imaging data | Cross-validation |
| Erck 2024 [42] | Netherlands | Retrospective cohort | 1199 | 47.0 | 80 ± 7 | All-cause mortality | Adjusted model (intermuscular adipose tissue with deep learning model) | CT images | Baseline and clinical data + imaging data + procedural data | N/A |
| Erdogan 2024 [43] | Turkey | Retrospective cohort | 453 | 40.8 | 76.1 ± 6.6 | MACE | XGBoost | N/A | Baseline and clinical data + imaging data + biomarkers | N/A |
| Ouahidi 2024 [19] | France | Retrospective cohort | 520 | 51.8 | 84.3 ± 5.5 | New PPI/LBBB | SVM | N/A | Baseline and clinical data + imaging data + procedural data | Cross-validation |
| Shi 2024 [23] | China | Retrospective cohort | 234 | 55.6 | 74.34 ± 7.62 | Valve-related dysfunction | LASSO | N/A | Baseline and clinical data + imaging data | Cross-validation |
| Tremamunno 2024 [55] | USA | Retrospective cohort | 648 | 58.9 | 77 ± 9.3 | MACE | cVAE | CT images | Imaging data | N/A |
| Yordanov 2024 [59] | Netherlands | Retrospective cohort | 16,661 | 50.8 | 79.6 | All-cause mortality | Central | N/A | Baseline and clinical data + biomarkers + procedural data | Cross-validation |
| Zahid 2024 [30] | US | Retrospective cohort | 92,363 | N/A | N/A | HF-related hospitalization | LR | N/A | Baseline and clinical data | N/A |
| Zisiopoulou 2024 [31] | Germany | Prospective cohort | 284 | 51.76 | 81.03 ± 4.75 | All-cause mortality | LR | N/A | N/A | N/A |
| Abdelkhalek 2023 [33] | Canada | Retrospective cohort | 133 | 57.9 | 81.33 ± 7.49 | New PPI/LBBB | MMLR | CT images | Imaging data | N/A |
| Valve-related dysfunction | ||||||||||
| Agasthi 2023 [34] | US | Retrospective cohort | 657 | 42.6 | 80.7 ± 8.2 | New PPI/LBBB | GBM | N/A | Baseline and clinical data + imaging data | Cross-validation |
| Alhwiti 2023 [35] | US | Retrospective cohort | 54,739 | 53.9 | 79.65 ± 8.5 | All-cause mortality | GBM | N/A | Baseline and clinical data | Cross-validation |
| Barrett 2023 [40] | US | Retrospective cohort | 606 | N/A | N/A | New PPI/LBBB | PRIME | N/A | Baseline and clinical data + procedural data | N/A |
| Chen 2023 [56] | UK | Retrospective cohort | 450 | 51.0 | 82.43 ± 5.21 | All-cause mortality | GBST | N/A | N/A | Cross-validation |
| Kwiecinski 2023 [49]. | Multinational | Retrospective cohort | 823 | 46.0 | 82 ± 5 | All-cause mortality | XGBoost | N/A | Baseline and clinical data + imaging data + biomarkers + procedural data | Cross-validation |
| Leha 2023 [58] | Germany | Retrospective cohort | 28,147 | 46.8 | 81 ± 6.1 | All-cause mortality | RF | N/A | Procedural data | Cross-validation |
| Pollari 2023 [21] | Germany | Retrospective cohort | 629 | 45.0 | 81.9 (53.8–94.5) | All-cause mortality | Bayes | N/A | Baseline and clinical data + imaging data + biomarkers | Cross-validation |
| Savitz 2023 [22] | US | Retrospective cohort | 1565 | 56.6 | 81 ± 8.2 | HF-related hospitalization | GBM | N/A | Baseline and clinical data | Cross-validation |
| Stan 2023 [24] | Romania | Retrospective cohort | 338 | 60.3 | 76 (71–80) | MACE | XGBoost | N/A | Baseline and clinical data | Cross-validation |
| Theis 2023 [26] | Germany | Retrospective cohort | 760 | 51.0 | 81 ± 6 | All-cause mortality | CNN | N/A | Imaging data | Cross-validation |
| Aquino 2022 [36] | US | Retrospective cohort | 196 | 43.9 | 75 ± 11 | MACE | CT-FFR with CCTA | N/A | Imaging data | N/A |
| Bansal 2022 [38] | US | Retrospective cohort | 499 | 60.7 | 78.8 ± 9.9 | All-cause mortality | RF | N/A | Baseline and clinical data | Cross-validation |
| Evertz 2022 [44] | Germany | Prospective cohort | 142 | 62.0 | 80 (74–83) | All-cause mortality | Fully automated assessment of the volumetric parameters | Commercially available AI software provided by Neosoft (suiteHEART,) | Imaging data | N/A |
| Lertsanguansinchai 2022 [50] | Thailand | Retrospective cohort | 178 | 43.8 | 81.6 ± 8.3 | All-cause mortality | DT | N/A | Baseline and clinical data + imaging data | Cross-validation |
| Sulaiman 2022 [25] | US | Retrospective cohort | 117,398 | 54.8 | 79.5 ± 8.4 | HF-related hospitalization | LASSO | N/A | Baseline and clinical data + procedural data | Random split |
| Agasthi 2021 [10] | US | Retrospective cohort | 1055 | 58.2 | 80.9 ± 7.9 | All-cause mortality | GBM | N/A | Baseline and clinical data | Cross-validation |
| Galli 2021 [45] | Multinational | Retrospective cohort | 151 | N/A | N/A | New PPI/LBBB | K-nearest neighbors ML model | Multi-slice CT | Imaging data+ procedural data | Cross-validation |
| Lopes 2021 [51] | Netherlands | Retrospective cohort | 1791 | 55.66 | N/A | All-cause mortality | XGBoost | N/A | Baseline and clinical data + imaging data + biomarkers | Cross-validation |
| Mamprin 2021 (1) [57] | Netherlands | Inter center Cross-validation study | 1931 | 48.05 | N/A | All-cause mortality | CatBoost | N/A | Baseline and clinical data + imaging data + biomarkers | Cross-validation |
| Mamprin 2021 (2) [53]. | Netherlands | Retrospective analysis | 270 | 52.0 | 80.7 ± 6.2 | All-cause mortality | CatBoost | N/A | Baseline and clinical data + imaging data + biomarkers + procedural data | Cross-validation |
| Okuno 2021 [18] | France | Prospective cohort | 2279 | 52.0 | 83.2 years (interquartile range [IQR] 79.4–86) | MACE | Encoder–Decoder NN | N/A | Imaging data | Random split |
| Penso 2021 [20] | Italy | Retrospective cohort | 471 | 36.3 | 81 ± 6 | All-cause mortality | MLP | N/A | Baseline and clinical data + imaging data | Cross-validation |
| Truong 2021 [27] | US | Retrospective cohort | 557 | 52.0 | 80 ± 9 | New PPI/LBBB | RF | N/A | Baseline and clinical data + imaging data | Random split |
| Gomes 2020 [46] | Germany | Retrospective analysis | 451 | N/A | N/A | Valve-related dysfunction | XGBoost | N/A | Baseline and clinical data + procedural data | Cross-validation |
| All-cause mortality | ||||||||||
| Stroke/TIA | ||||||||||
| New PPI/LBBB | SVM | |||||||||
| Abdul Ghffar 2020 [54] | US | Retrospective cohort | 143 | 50.0 | 79.39 (75.07, 84.36) | All-cause mortality | N/A | N/A | Baseline and clinical data | Cross-validation |
| Tsushima 2020 [28] | US | Retrospective cohort | 888 | N/A | N/A | New PPI/LBBB | LR | N/A | Baseline and clinical data + procedural data | Random split |
| Hernandez-Suarez 2019 [47] | US | Retrospective cohort | 10,883 | 52.3 | 81 ± 8.5 | All-cause mortality | LR | N/A | Baseline and clinical data + procedural data | N/A |
| Hoffmann 2020 [48] | Germany | Prospective cohort | 129 | 58.9 | 82.67 ± 5.25 | All-cause mortality | Gradient-boosted trees (linear predictor score) | N/A | Baseline and clinical data + biomarkers | N/A |
| Lopes 2019 [52] | Netherlands | Retrospective analysis | 1478 | 45.0 | 82.43 ± 6.23 | All-cause mortality | RF | N/A | Baseline and clinical data + imaging data + biomarkers | N/A |
| Vejpongsa 2018 [29] | US | Retrospective cohort | 18,400 | N/A | N/A | New PPI/LBBB | LR | N/A | Baseline and clinical data + procedural data | N/A |
| Zusman 2017 [32] | Israel | Retrospective cohort | 435 | 43.0 | 82.67 ± 5.21 | MACE | LR | N/A | Baseline and clinical data + imaging data + biomarkers | Cross-validation |
| Outcome Category | AUC | Accuracy | Recall | ||||
|---|---|---|---|---|---|---|---|
| Estimate (95% CI) | Heterogeneity (I2) | Estimate (95% CI) | Heterogeneity (I2) | Estimate (95% CI) | Heterogeneity (I2) | ||
| Clinical outcomes | All-Cause Mortality | 0.78 (0.74, 0.82) | 98.1% | 0.81 (0.69, 0.89) | 98.2% | 0.90 (0.70, 0.97) | 99% |
| MACE | 0.79 (0.67, 0.92) | 89.9% | N/A | N/A | N/A | N/A | |
| Stroke/TIA | 0.73 (0.59, 0.88) | 97.1% | N/A | N/A | N/A | N/A | |
| Heart Failure-Related Hospitalization | 0.7 (0.60, 0.81) | 83.3% | N/A | N/A | N/A | N/A | |
| Procedural | Pacemaker and Conduction Abnormalities | 0.75 (0.68, 0.82) | 93.2% | 0.73 (0.59, 0.84) | 99.1% | 0.87 (0.50, 0.98) | 99.2% |
| Valve-Related Dysfunction | 0.73 (0.62, 0.84) | 96% | 0.79 (0.57, 0.91) | 98.7% | 0.54 (0.26, 0.80) | 99% | |
| Clinical Outcomes | Subgroup | Pooled Estimate | 95% CI | Heterogeneity (I2) | Clinical Outcomes | Subgroup | Pooled Estimate | 95% CI | Heterogeneity (I2) |
|---|---|---|---|---|---|---|---|---|---|
| All-Cause Mortality (AUC) | Baseline and Clinical Data | 0.77 | (0.69, 0.85) | 94.1% | Pacemaker and Conduction Abnormalities (AUC) | Baseline and Clinical Data | 0.61 | (0.56, 0.66) | N/A |
| Imaging Data | 0.73 | (0.71, 0.74) | 0% | Imaging Data | 0.75 | (0.67, 0.82) | N/A | ||
| Procedural Data | 0.75 | (0.72, 0.78) | N/A | Baseline and Clinical Data + Imaging Data | 0.77 | (0.68, 0.85) | 90.1% | ||
| Baseline and Clinical Data + Imaging Data | 0.77 | (0.69, 0.84) | 48.7% | Baseline and Clinical Data + Procedural Data | 0.75 | (0.62, 0.88) | 73% | ||
| Baseline and Clinical Data + Biomarkers | 0.91 | (0.88, 0.95) | 0% | Imaging Data + Procedural Data | 0.84 | (0.71, 0.97) | N/A | ||
| Baseline and Clinical Data + Procedural Data | 0.95 | (0.90, 1.00) | 88.3% | Baseline and Clinical Data + Imaging Data + Procedural Data | 0.92 | (0.84, 1.00) | N/A | ||
| Baseline and Clinical Data + Imaging Data + Biomarkers | 0.78 | (0.66, 0.89) | 17.4% | Valve-Related Dysfunction | Imaging Data | 0.8 | (0.73, 0.87) | N/A | |
| Baseline and Clinical Data + Imaging Data + Procedural Data | 0.55 | (0.52, 0.58) | N/A | Baseline and Clinical Data + Imaging Data | 0.74 | (0.67, 0.80) | N/A | ||
| Baseline and Clinical Data + Biomarkers + Procedural Data | 0.68 | (0.66, 0.70) | N/A | Baseline and Clinical Data + Procedural Data | 0.57 | (0.52, 0.62) | N/A | ||
| Baseline and Clinical Data + Imaging Data + Biomarkers + Procedural Data | 0.78 | (0.70,0.87) | 95.1% | Heart Failure-Related Hospitalization (AUC) | Baseline and Clinical Data | 0.67 | (0.49, 0.86) | 91.2% | |
| MACE (AUC) | Imaging Data | 0.67 | (0.58, 0.76) | 0% | Baseline and Clinical Data + Procedural Data | 0.74 | (0.70, 0.78) | ||
| Baseline and Clinical Data + Imaging Data + Biomarkers | 0.84 | (0.60, 1.00) | 91% | ||||||
| Baseline and Clinical Data | 0.92 | (0.85, 0.99) | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shojaei, S.; Mousavi, A.; Kazemian, S.; Armani, S.; Maleki, S.; Fallahtafti, P.; Arashlow, F.T.; Daryabari, Y.; Naderian, M.; Alkhouli, M.; et al. Artificial Intelligence in Risk Stratification and Outcome Prediction for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. J. Pers. Med. 2025, 15, 302. https://doi.org/10.3390/jpm15070302
Shojaei S, Mousavi A, Kazemian S, Armani S, Maleki S, Fallahtafti P, Arashlow FT, Daryabari Y, Naderian M, Alkhouli M, et al. Artificial Intelligence in Risk Stratification and Outcome Prediction for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2025; 15(7):302. https://doi.org/10.3390/jpm15070302
Chicago/Turabian StyleShojaei, Shayan, Asma Mousavi, Sina Kazemian, Shiva Armani, Saba Maleki, Parisa Fallahtafti, Farzin Tahmasbi Arashlow, Yasaman Daryabari, Mohammadreza Naderian, Mohamad Alkhouli, and et al. 2025. "Artificial Intelligence in Risk Stratification and Outcome Prediction for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 15, no. 7: 302. https://doi.org/10.3390/jpm15070302
APA StyleShojaei, S., Mousavi, A., Kazemian, S., Armani, S., Maleki, S., Fallahtafti, P., Arashlow, F. T., Daryabari, Y., Naderian, M., Alkhouli, M., Rana, J. S., Mehrani, M., Jenab, Y., & Hosseini, K. (2025). Artificial Intelligence in Risk Stratification and Outcome Prediction for Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 15(7), 302. https://doi.org/10.3390/jpm15070302