Comparing Multigene Molecular Testing Results of MRI-Target Versus Systematic Prostate Needle Biopsies of Candidates for and Under Active Surveillance
Abstract
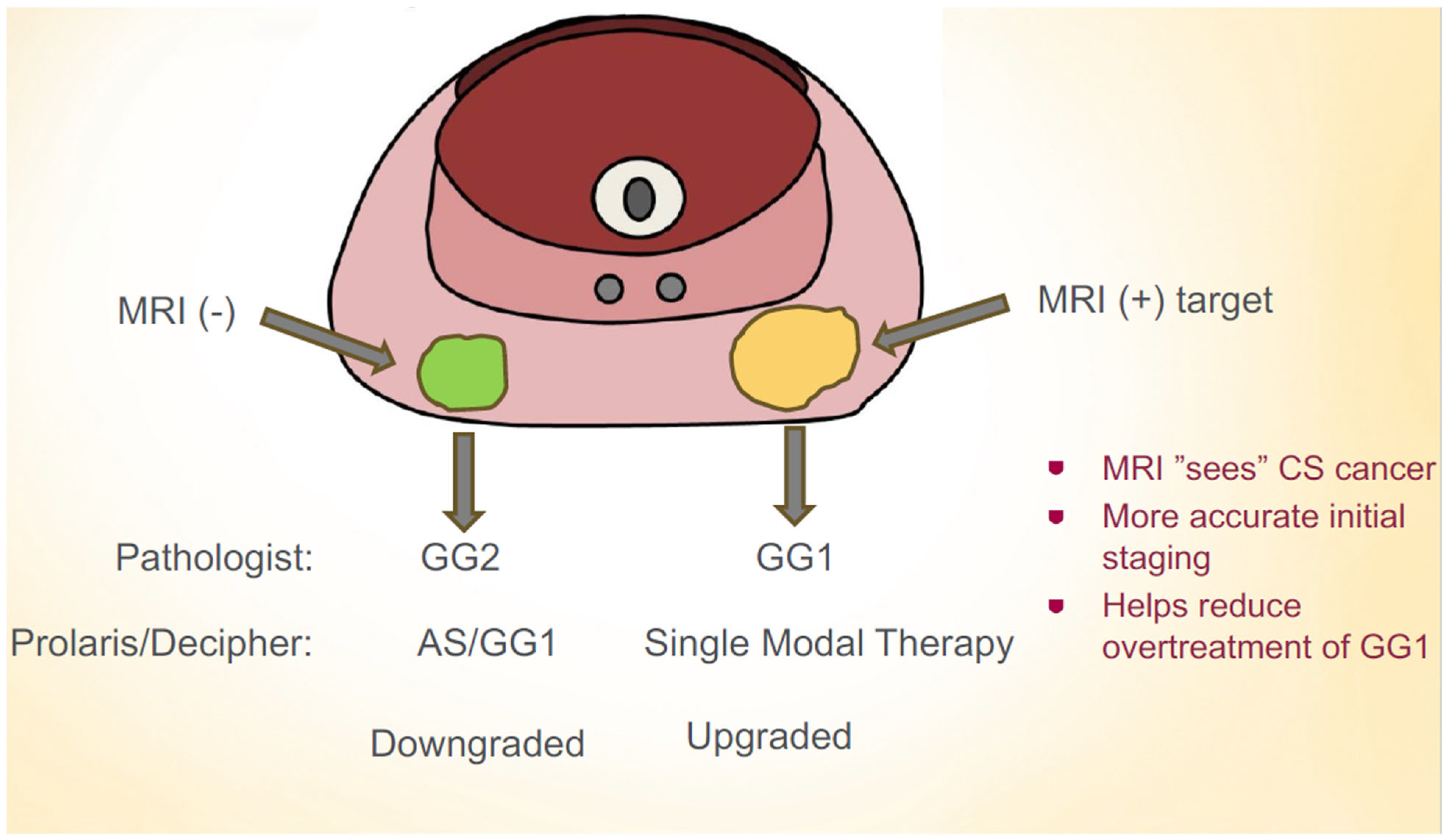
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Meeks, W.; Fang, R.; Gaylis, F.D.; Catalona, W.J.; Makarov, D.V. Time Trends and Variation in the Use of Active Surveillance for Management of Low-risk Prostate Cancer in the US. JAMA Netw. Open 2023, 6, e231439. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.M.; Laviana, A.A.; Penson, D.F.; Lin, D.W.; Bill-Axelson, A.; Carlsson, S.V.; Newcomb, L.F.; Trock, B.J.; Carter, H.B.; Carroll, P.R.; et al. Prostate cancer mortality and metastasis under different biopsy frequencies in North American active surveillance cohorts. Cancer 2020, 126, 583–592. [Google Scholar] [CrossRef]
- Daskivich, T.J.; Chamie, K.; Kwan, L.; Labo, J.; Palvolgyi, R.; Dash, A.; Greenfield, S.; Litwin, M.S. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer 2011, 117, 2058–2066. [Google Scholar] [CrossRef]
- Shill, D.K.; Roobol, M.J.; Ehdaie, B.; Vickers, A.J.; Carlsson, S.V. Active surveillance for prostate cancer. Transl. Androl. Urol. 2021, 10, 2809–2819. [Google Scholar] [CrossRef]
- Mahal, B.A.; Butler, S.; Franco, I.; Spratt, D.E.; Rebbeck, T.R.; D’Amico, A.V.; Nguyen, P.L. Use of Active Surveillance or Watchful Waiting for Low-Risk Prostate Cancer and Management Trends Across Risk Groups in the United States, 2010–2015. JAMA 2019, 321, 704–706. [Google Scholar] [CrossRef]
- Baboudjian, M.; Breda, A.; Rajwa, P.; Gallioli, A.; Gondran-Tellier, B.; Sanguedolce, F.; Verri, P.; Diana, P.; Territo, A.; Bastide, C.; et al. Active Surveillance for Intermediate-risk Prostate Cancer: A Systematic Review, Meta-analysis, and Metaregression. Eur. Urol. Oncol. 2022, 5, 617–627. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef]
- Eggener, S.E.; Rumble, R.B.; Armstrong, A.J.; Morgan, T.M.; Crispino, T.; Cornford, P.; van der Kwast, T.; Grignon, D.J.; Rai, A.J.; Agarwal, N.; et al. Molecular Biomarkers in Localized Prostate Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1474–1494. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko Antti, S.; Borghi, M.; Panebianco, V.; Mynderse Lance, A.; Vaarala Markku, H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley Richard, G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.T.; Barocas, D.; Carlsson, S.; Coakley, F.; Eggener, S.; Etzioni, R.; Fine, S.W.; Han, M.; Kim, S.K.; Kirkby, E.; et al. Early Detection of Prostate Cancer: AUA/SUO Guideline Part I: Prostate Cancer Screening. J. Urol. 2023, 210, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Berney, D.M.; Fisher, G.; Mesher, D.; Møller, H.; Reid, J.E.; Perry, M.; Park, J.; Younus, A.; Gutin, A.; et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br. J. Cancer 2012, 106, 1095–1099. [Google Scholar] [CrossRef]
- Norris, J.M.; Simpson, B.S.; Parry, M.A.; Allen, C.; Ball, R.; Freeman, A.; Kelly, D.; Kim, H.L.; Kirkham, A.; You, S.; et al. Genetic Landscape of Prostate Cancer Conspicuity on Multiparametric Magnetic Resonance Imaging: A Systematic Review and Bioinformatic Analysis. Eur. Urol. Open Sci. 2020, 20, 37–47. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Bitting, R.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; Desai, N.; Dorff, T.; et al. NCCN Guidelines® Insights: Prostate Cancer, Version 3.2024: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2024, 22, 140–150. [Google Scholar] [CrossRef]
- Boutros, P.C.; Fraser, M.; Harding, N.J.; de Borja, R.; Trudel, D.; Lalonde, E.; Meng, A.; Hennings-Yeomans, P.H.; McPherson, A.; Sabelnykova, V.Y.; et al. Spatial genomic heterogeneity within localized, multifocal prostate cancer. Nat. Genet. 2015, 47, 736–745. [Google Scholar] [CrossRef]
- Wei, L.; Wang, J.; Lampert, E.; Schlanger, S.; DePriest, A.D.; Hu, Q.; Gomez, E.C.; Murakam, M.; Glenn, S.T.; Conroy, J.; et al. Intratumoral and Intertumoral Genomic Heterogeneity of Multifocal Localized Prostate Cancer Impacts Molecular Classifications and Genomic Prognosticators. Eur. Urol. 2017, 71, 183–192. [Google Scholar] [CrossRef]
- Houlahan, K.E.; Salmasi, A.; Sadun, T.Y.; Pooli, A.; Felker, E.R.; Livingstone, J.; Huang, V.; Raman, S.S.; Ahuja, P.; Sisk, A.E., Jr.; et al. Molecular Hallmarks of Multiparametric Magnetic Resonance Imaging Visibility in Prostate Cancer. Eur. Urol. 2019, 76, 18–23. [Google Scholar] [CrossRef]
- Dianat, S.S.; Carter, H.B.; Pienta, K.J.; Schaeffer, E.M.; Landis, P.K.; Epstein, J.I.; Trock, B.J.; Macura, K.J. Magnetic resonance-invisible versus magnetic resonance-visible prostate cancer in active surveillance: A preliminary report on disease outcomes. Urology 2015, 85, 147–153. [Google Scholar] [CrossRef]
- Abd-Alazeez, M.; Ahmed, H.U.; Arya, M.; Allen, C.; Dikaios, N.; Freeman, A.; Emberton, M.; Kirkham, A. Can multiparametric magnetic resonance imaging predict upgrading of transrectal ultrasound biopsy results at more definitive histology? Urol. Oncol. 2014, 32, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Bangma, C.; Doan, P.; Zhu, L.; Remmers, S.; Nieboer, D.; Helleman, J.; Roobol, M.J.; Sugimoto, M.; Chung, B.H.; Lee, L.S.; et al. Has Active Surveillance for Prostate Cancer Become Safer? Lessons Learned from a Global Clinical Registry. Eur. Urol. Oncol. 2025, 8, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Myriad Genetics Inc. Understanding My Prolaris® Report. Available online: https://myriad.com/urology/understanding-my-prolaris-results/#:~:text=Men%20with%20more%20aggressive%20tumors,radiation%20therapy%20and%20hormonal%20therapy (accessed on 27 May 2025).
- Wibmer, A.G.; Robertson, N.L.; Hricak, H.; Zheng, J.; Capanu, M.; Stone, S.; Ehdaie, B.; Brawer, M.K.; Vargas, H.A. Extracapsular extension on MRI indicates a more aggressive cell cycle progression genotype of prostate cancer. Abdom. Radiol. 2019, 44, 2864–2873. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, A.; Yerram, N.K.; O’Connor, L.; Brisbane, W.; Sharma, V.; Chuang, R.; Jayadevan, R.; Ahdoot, M.; Daneshvar, M.; Priester, A.; et al. Magnetic Resonance Imaging-Guided Biopsy in Active Surveillance of Prostate Cancer. J. Urol. 2022, 207, 823–831. [Google Scholar] [CrossRef]
- Bhanji, Y.; Mamawala, M.K.; Fletcher, S.A.; Landis, P.; Patel, H.D.; Macura, K.J.; Pavlovich, C.P. Is Confirmatory Biopsy Still Necessary for Active Surveillance of Men With Grade Group 1 Prostate Cancer in the Era of Multiparametric MRI? J. Urol. 2025, 213, 20–26. [Google Scholar] [CrossRef]
- Crawford, E.D.; Scholz, M.C.; Kar, A.J.; Fegan, J.E.; Haregewoin, A.; Kaldate, R.R.; Brawer, M.K. Cell cycle progression score and treatment decisions in prostate cancer: Results from an ongoing registry. Curr. Med. Res. Opin. 2014, 30, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
| Variable | Target Lesion (n = 86) | Systematic Biopsy (n = 178) | p-Value |
|---|---|---|---|
| Age (years) | 65.7 ± 10.7 | 65.5 ± 7.7 | 0.45 |
| PSA (ng/mL) | 6.8 ± 3.5 | 6.7 ± 3.8 | 0.84 |
| PSA Density (ng/mL/cm3) | 0.16 ± 0.13 | 0.16 ± 0.12 | 0.81 |
| Gleason Grade Group | |||
| -Grade 1 (%) | 67 (77.90%) | 115 (64.60%) | |
| -Grade 2 (%) | 19 (22.10%) | 63 (35.40%) | |
| ProlarisTM Score (Mean ± SD) | 3.23 ± 0.62 | 3.14 ± 0.72 | 0.18 |
| ProlarisTM 10-y DSM (Mean ± SD) | 2.57% ± 1.37 | 2.54% ± 1.35 | 0.42 |
| ProlarisTM 10-y Metastasis (Mean ± SD) | 1.44% ± 1.38 | 1.29% ± 1.13 | 0.21 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 75.60% | 77.50% | 0.74 |
| -Single Modal Treatment (%) | 24.40% | 22.50% |
| Variable | GG1 Target (n = 67) | GG2 Non-Target (n = 63) | p-Value |
|---|---|---|---|
| Age (years) | 64.7 ± 11.1 | 67.7 ± 8.1 | 0.083 |
| PSA (ng/mL) | 6.9 ± 3.7 | 6.5 ± 2.7 | 0.44 |
| PSA Density (ng/mL/cm3) | 0.16 ± 0.14 | 0.17 ± 0.13 | 0.85 |
| ProlarisTM Score (Mean ± SD) | 3.18 ± 0.66 | 3.4 ± 0.71 | 0.023 |
| ProlarisTM 10-y DSM (Mean ± SD) | 2.3% ± 1.3 | 3.4% ± 1.6 | <0.01 |
| ProlarisTM 10-y Metastasis (Mean ± SD) | 1.2% ± 1.3 | 1.9% ± 1.4 | 0.013 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 82.10% | 57.90% | <0.01 |
| -Single Modal Treatment (%) | 17.90% | 42.10% |
| Variable | GG1 Target (n = 67) | GG1 Non-Target (n = 115) | p-Value |
|---|---|---|---|
| Age (years) | 64.7 ± 11.1 | 65.9 ± 7.5 | 0.38 |
| PSA (ng/mL) | 6.9 ± 3.7 | 6.9 ± 4.3 | 0.91 |
| PSA Density (ng/mL/cm3) | 0.16 ± 0.14 | 0.15 ± 0.12 | 0.47 |
| ProlarisTM Score (Mean ± SD) | 3.18 ± 0.66 | 2.99 ± 0.68 | 0.03 |
| ProlarisTM 10-y DSM (Mean ± SD) | 2.35% ± 1.3 | 2.05% ± 0.85 | 0.034 |
| ProlarisTM 10-y Metastasis (Mean ± SD) | 1.18%± 1.27 | 0.87% ± 0.58 | 0.037 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 82.10% | 87.80% | 0.29 |
| -Single Modal Treatment (%) | 17.90% | 12.20% | |
| MRI-target GG2 vs. GG2 | |||
| Variable | GG2 Target (n = 19) | GG2 Non-Target (n = 63) | p-Value |
| Age (years) | 69.1 ± 8.4 | 67.7 ± 8.1 | 1.52 |
| PSA (ng/mL) | 6.4 ± 2.6 | 6.5 ± 2.7 | 0.93 |
| PSA Density (ng/mL/cm3) | 0.15 ± 0.1 | 0.17 ± 0.13 | 0.53 |
| ProlarisTM Score (Mean ± SD) | 3.38 ± 0.43 | 3.42 ± 0.71 | 0.4 |
| ProlarisTM 10-y DSM (Mean ± SD) | 3.38% ± 1.40 | 3.39% ± 1.61 | 0.49 |
| ProlarisTM 10-y Metastasis (Mean ± SD) | 2.33% ± 1.38 | 1.86%± 1.42 | 0.14 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 52.60% | 57.90% | 0.79 |
| -Single Modal Treatment (%) | 47.40% | 42.10% |
| Variable | Target Lesion (n = 57) | Systematic Biopsy (n = 125) | p-Value |
|---|---|---|---|
| Age (years) | 65.5 ± 12.0 | 66.5 ± 8.1 | 0.49 |
| PSA (ng/mL) | 6.9 ± 3.7 | 6.9 ± 3.7 | 1 |
| PSA Density (ng/mL/cm3) | 0.16 ± 0.14 | 0.1 ± 0.14 | 0.99 |
| Gleason Grade Group | |||
| -Grade 1 (%) | 51 (89.5%) | 84 (67.2%) | |
| -Grade 2 (%) | 6 (10.5%) | 41 (33.8%) | |
| ProlarisTM Score (Mean ± SD) | 3.20 ± 0.64 | 3.20 ± 0.71 | 0.66 |
| ProlarisTM 10-y DSM (Mean ± SD) | 2.49% ± 1.40 | 2.52% ± 1.29 | 0.89 |
| ProlarisTM 10-y Metastasis (Mean ± SD) | 1.08% ± 1.34 | 0.98% ± 1.10 | 0.59 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 75.4% | 75.2% | 0.8 |
| -Single Modal Treatment (%) | 24.6% | 24.8% |
| Variable | GG1 Target (n = 51) | GG2 Systematic (n = 41) | p-Value |
|---|---|---|---|
| Age (years) | 64.7 ± 12.3 | 67.7 ± 8.7 | 0.19 |
| PSA (ng/mL) | 7.0 ± 3.8 | 7.1 ± 2.9 | 0.94 |
| PSA Density (ng/mL/cm3) | 0.17 ± 0.14 | 0.16 ± 0.16 | 0.56 |
| ProlarisTM Score (Mean ± SD) | 3.17 ± 0.66 | 3.42 ± 0.70 | 0.073 |
| ProlarisTM 10-y DSM (Mean ± SD) | 2.39% ± 1.4 | 3.41% ± 1.47 | <0.01 |
| ProlarisTM 10-y Metastasis (Mean ± SD) | 0.98% ± 1.36 | 1.71% ± 1 | 0.02 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 81.4% | 53.7% | <0.01 |
| -Single Modal Treatment (%) | 19.6% | 46.3% |
| Variable | GG1 Target (n = 51) | GG1 Non-Target (n = 84) | p-Value |
|---|---|---|---|
| Age (years) | 64.7 ± 12.3 | 65.9 ± 7.8 | 0.5 |
| PSA (ng/mL) | 7.0 ± 3.0 | 6.8 ± 4.1 | 0.8 |
| PSA Density (ng/mL/cm3) | 0.17 ± 0.14 | 0.16 ± 0.13 | 0.66 |
| ProlarisTM Score (Mean ± SD) | 3.20 ± 0.66 | 3.0 ± 0.69 | 0.21 |
| ProlarisTM 10-y DSM (Mean ± SD) | 2.39% ± 1.4 | 2.08 ± 0.92 | 0.12 |
| ProlarisTM 10-y Metastasis (Mean ± SD) | 0.98 ± 1.36 | 0.63 ± 0.66 | 0.06 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 80.4% | 85.7% | 0.74 |
| -Single Modal Treatment (%) | 19.6% | 14.3% |
| Variable | Target Lesion (n = 29) | Systematic Biopsy (n = 52) | p-Value |
|---|---|---|---|
| Age (years) | 66.0 ± 7.8 | 66.5 ± 6.8 | 0.78 |
| PSA (ng/mL) | 6.6 ± 3.1 | 6.3 ± 4.0 | 0.78 |
| PSA Density (ng/mL/cm3) | 0.15 ± 0.11 | 0.13 ± 0.06 | 0.24 |
| Gleason Grade Group | |||
| -Grade 1 (%) | 16 (55.2) | 31 (59.6) | |
| -Grade 2 (%) | 13 (44.8) | 21 (40.4) | |
| ProlarisTM Score (Mean ± SD) | 3.3 ± 0.6 | 3.1 ± 0.7 | 0.19 |
| ProlarisTM 10-y DSM (Mean ± SD) | 2.7% ± 1.3 | 2.4% ± 0.99 | 0.23 |
| ProlarisTM 10-y Metastasis (Mean ± SD) | 1.14 ± 1.4 | 0.81 ± 0.86 | 0.21 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 75.9% | 82.7% | 0.56 |
| -Single Modal Treatment (%) | 24.1% | 17.3% |
| Variable | GG1 Target (n = 67) | GG2 Non-Target (n = 63) | p-Value |
|---|---|---|---|
| Age (years) | 64.6 ± 6.6 | 67.4 ± 6.9 | 0.23 |
| PSA (ng/mL) | 6.6 ± 3.3 | 5.4 ± 1.9 | 0.19 |
| PSA Density (ng/mL/cm3) | 0.15 ± 0.11 | 0.13 ± 0.05 | 0.65 |
| ProlarisTM Score (Mean ± SD) | 3.2% ± 0.66 | 3.3% ± 0.63 | 0.64 |
| ProlarisTM 10-y DSM (Mean ± SD) | 2.2% ± 0.8 | 3.0% ± 1.1 | 0.02 |
| ProlarisTM 10-y Metastasis (Mean ± SD) | 0.6% ± 0.5 | 1.31% ± 1.0 | 0.01 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 87.5% | 67.7% | 0.25 |
| -Single Modal Treatment (%) | 12.5% | 33.3% |
| Variable | GG1 Target (n = 16) | GG1 Non-Target (n = 31) | p-Value |
|---|---|---|---|
| Age (years) | 64.6 ± 6.6 | 65.9 ± 6.7 | 0.53 |
| PSA (ng/mL) | 6.6 ± 3.3 | 6.9 ± 5.0 | 0.83 |
| PSA Density (ng/mL/cm3) | 0.15 ± 0.11 | 0.12 ± 0.07 | 0.41 |
| ProlarisTM Score (Mean ± SD) | 3.2 ± 0.66 | 2.99 ± 0.68 | 0.13 |
| ProlarisTM 10-y DSM (Mean ± SD) | 2.35% ± 1.3 | 2.9% ± 0.7 | 0.33 |
| ProlarisTM 10-y Metastasis (Mean± SD) | 2.2% ± 0.8 | 1.98% ± 0.65 | 0.28 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 87.5% | 93.1% | 0.6 |
| -Single Modal Treatment (%) | 12.5% | 6.9% | |
| GG2 Target (n = 13) | GG2 Non-Target (n = 21) | p-Value | |
| Age (years) | 67.8 ± 9.1 | 6.4 ± 6.9 | 0.88 |
| PSA (ng/mL) | 6.6 ± 2.9 | 5.4 ± 1.9 | 0.15 |
| PSA Density (ng/mL/cm3) | 0.16 ± 0.11 | 0.13 ± 0.05 | 0.41 |
| ProlarisTM Score (Mean ± SD) | 3.3 ± 0.5 | 3.3 ± 0.6 | 0.98 |
| ProlarisTM 10-y DSM (Mean ± SD) | 3.4% ± 1.5 | 3.01% ± 1.1 | 0.43 |
| ProlarisTM 10-y Metastasis (Mean ± SD) | 1.77% ± 1.85 | 1.31% ± 1.1 | 0.37 |
| Recommended Treatment | |||
| -Active Surveillance (AS) (%) | 39.5% | 67.7% | 1 |
| -Single Modal Treatment (%) | 61.5% | 33.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanzotti, N.J.; Du, C.; Hall, J.; Saba, J.; Picken, M.M.; Gupta, G.N. Comparing Multigene Molecular Testing Results of MRI-Target Versus Systematic Prostate Needle Biopsies of Candidates for and Under Active Surveillance. J. Pers. Med. 2025, 15, 279. https://doi.org/10.3390/jpm15070279
Lanzotti NJ, Du C, Hall J, Saba J, Picken MM, Gupta GN. Comparing Multigene Molecular Testing Results of MRI-Target Versus Systematic Prostate Needle Biopsies of Candidates for and Under Active Surveillance. Journal of Personalized Medicine. 2025; 15(7):279. https://doi.org/10.3390/jpm15070279
Chicago/Turabian StyleLanzotti, Nicholas J., Chris Du, Julia Hall, Joseph Saba, Maria M. Picken, and Gopal N. Gupta. 2025. "Comparing Multigene Molecular Testing Results of MRI-Target Versus Systematic Prostate Needle Biopsies of Candidates for and Under Active Surveillance" Journal of Personalized Medicine 15, no. 7: 279. https://doi.org/10.3390/jpm15070279
APA StyleLanzotti, N. J., Du, C., Hall, J., Saba, J., Picken, M. M., & Gupta, G. N. (2025). Comparing Multigene Molecular Testing Results of MRI-Target Versus Systematic Prostate Needle Biopsies of Candidates for and Under Active Surveillance. Journal of Personalized Medicine, 15(7), 279. https://doi.org/10.3390/jpm15070279







